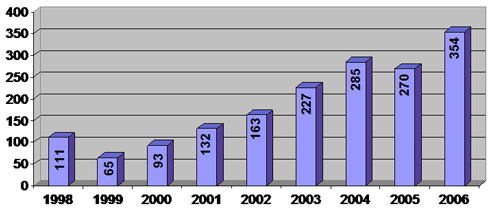
Human Cell & Tissue and Cellular & Tissue-based Products (HCT/P)
Inspections Performed in Fiscal Years 1998 to 2006
Inspection Conclusion by Fiscal Year1
2003 |
2004 |
2005 |
2006 |
|
|---|---|---|---|---|
Inspections Classified NAI * |
160 |
233 |
217 |
249 |
Inspections Classified VAI |
53 |
43 |
46 |
96 |
Inspections Classified OAI |
5 |
7 |
0 |
10 |
Avg. hours per inspection |
33.1 |
33.7 |
32.2 |
44.7 |
*NAI = No Action Indicated, meaning no objectionable conditions or practices were found during the inspection (or the significance of the documented objectionable conditions found does not justify further action).
VAI = Voluntary Action Indicated, meaning objectionable conditions were found and documented but the agency is not prepared to take or recommend regulatory action.
OAI = Official Action Indicated, meaning objectionable conditions were found and regulatory action should be recommended.
(See also, ORA Field Management Directive No. 86, Establishment Inspection Report (EIR) - Inspection Conclusions and District Decisions, June 7, 2007.
QUESTIONS?
- Compliance: Hang Dinh - 301-827-6220 or hang.dinh@fda.hhs.gov
- Scientific: Dr. Ruth Solomon - 301-827-6107 or ruth.solomonr@fda.hhs.gov
1Sum of inspection classifications does not equal total number of inspections performed by fiscal year due to data constraints