 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
Respiratory Syncytial Virus Activity --- United States, 2000--01 SeasonRespiratory syncytial virus (RSV) has a worldwide distribution and can cause serious lower respiratory tract illness (LRTI). RSV is most commonly considered a pathogen among infants and young children; however, it can cause serious LRTI throughout life, especially among those with compromised respiratory, cardiac, or immune systems and the elderly (1--3). In temperate climates, RSV infections occur primarily during annual outbreaks, which peak during winter months (4). In the United States, RSV activity is monitored by the National Respiratory and Enteric Virus Surveillance System (NREVSS), a laboratory-based surveillance system. This report summarizes trends in RSV activity reported to NREVSS during July 2000--June 2001 and presents preliminary surveillance data from the weeks ending July 7 through December 8, 2001, indicating the onset of the 2001--02 RSV season. Health-care providers should consider RSV in the differential diagnosis of lower respiratory tract disease in persons of all ages, use isolation procedures to prevent nosocomial transmission, and consider use of immune globulin or monoclonal antibody prophylaxis in premature infants or infants and children with chronic lung disease (5). A total of 81 clinical and public health laboratories in 47 states and the District of Columbia report weekly to CDC the number of specimens tested and the number positive for several respiratory and enteric viruses by antigen detection and virus isolation methods. During July 2000--June 2001, 64 laboratories representing 41 states reported 138,984 tests for RSV; 18,605 (13.4%) were positive. Widespread* RSV activity began the week of November 11, 2000, and continued for 24 weeks until April 21, 2001. Activity peaked in late December in the southern region of the United States, and in late February in all other regions† (Figure 1). State-specific RSV season onset and conclusion dates varied widely, with a range of outbreak onsets during August 26--January 20, and a range of conclusions during January 29--May 26. Regional RSV outbreaks occurred earliest in the South (23 sites reporting; median weeks of onset and conclusion: October 21 and May 19, respectively), later in the Northeast (six sites; November 25 and May 5), and latest in the Midwest (20 sites; December 9 and May 26) and West (14 sites; October 21 and May 26). Although 94% of RSV detections were reported for the week ending October 30 through the week ending March 25, sporadic detections were reported throughout the year. During July--August 2001, laboratories in Arizona, California, Florida, Hawaii, Nevada, Ohio, Texas, Virginia, Washington, and West Virginia reported sporadic isolates of RSV. For the current reporting period (July 7 through December 13, 2001), 55 laboratories in 37 states reported results of testing for RSV. Since November 3, 2001, 25 participating laboratories have reported RSV (Figure 1). Reported by: National Respiratory and Enteric Virus Surveillance System collaborating laboratories. A LaMonte, MPH, D Shay, MD, L Anderson, MD, Respiratory and Enteric Viruses Br, Div of Viral and Rickettsial Diseases, National Center for Infectious Diseases, CDC. Editorial Note:For the July 2000--June 2001 surveillance period, the number of specimens that tested positive for RSV, median months of onset activity, and regional trends were similar to trends reported during previous years. The duration of the 2000--2001 RSV season also was consistent with that of previous years, including the characteristic earlier onset of RSV outbreaks reported by southern laboratories. RSV causes bronchiolitis and pneumonia in infants and young children; RSV causes an estimated 31 bronchiolitis associated hospitalizations per 1,000 children aged <1 year per year (6). The rate of RSV-associated hospitalizations is higher in certain populations, such as American Indian/Alaska Native children receiving care through the Indian Health Service (62 per 1,000 children aged <1 per year) (7). Because RSV infection confers only partial protection from subsequent infection, reinfections occur throughout life (1--3). As a result, health-care providers should consider RSV as a cause of acute respiratory disease in all age groups during community outbreaks. Persons with underlying cardiac or pulmonary disease, compromised immune systems, and the elderly are at increased risk for serious complications of RSV infection, including LRTI and death. The disease burden of RSV infections might be >50% of that associated with influenza (8). RSV infection among recipients of bone marrow transplants has been associated with mortality rates >50% (4). Rapid diagnostic techniques for clinicians vary in sensitivity and specificity. Some assays are sensitive for diagnosis in infants and young children but not in older children and adults. PCR-based assays are the most sensitive. No effective treatment for RSV-associated LRTI exists. Ribavirin initially was reported to be an effective treatment; however, subsequent trials could not substantiate a benefit from this therapy (9). NREVSS data can alert public health officials and health-care providers to the timing of seasonal RSV activity. Although no RSV vaccine is available, RSV immune globulin intravenous and a humanized murine anti-RSV monoclonal antibody are available as prophylaxis for some high-risk infants and young children (e.g., those born prematurely or with chronic lung disease) to prevent serious RSV disease (5). Contact isolation procedures are recommended for prevention and control of nosocomial transmission of RSV (10). The findings in this report are subject to at least three limitations. First, laboratory data indicate when RSV is circulating in a community; however, the correlation of these data to disease burden in the population is uncertain. Second, few laboratories represent some regions. Finally, diagnostic methods are not standardized among contributing laboratories, and the sensitivity and specificity of these methods probably vary among reporting laboratories. Additional information and updated data on RSV trends are available at http://www.cdc.gov/ncidod/dvrd/revb/nrevss/index.htm. References
* Widespread RSV activity is defined by NREVSS as the first of 2 consecutive weeks when 50% of participating laboratories report RSV detections or isolations, and when the mean percentage of specimens positive by antigen detection is >10%. † Northeast=Connecticut, Maine, Massachusetts, New Hampshire, New Jersey, New York, Pennsylvania, Rhode Island, and Vermont; Midwest=Illinois, Indiana, Iowa, Kansas, Michigan, Minnesota, Missouri, Nebraska, North Dakota, Ohio, South Dakota, and Wisconsin; South=Alabama, Arkansas, Delaware, District of Columbia, Florida, Georgia, Kentucky, Louisiana, Maryland, Mississippi, North Carolina, Oklahoma, South Carolina, Tennessee, Texas, Virginia, and West Virginia; West=Alaska, Arizona, California, Colorado, Hawaii, Idaho, Montana, Nevada, New Mexico, Oregon, Utah, Washington, and Wyoming. Figure 1 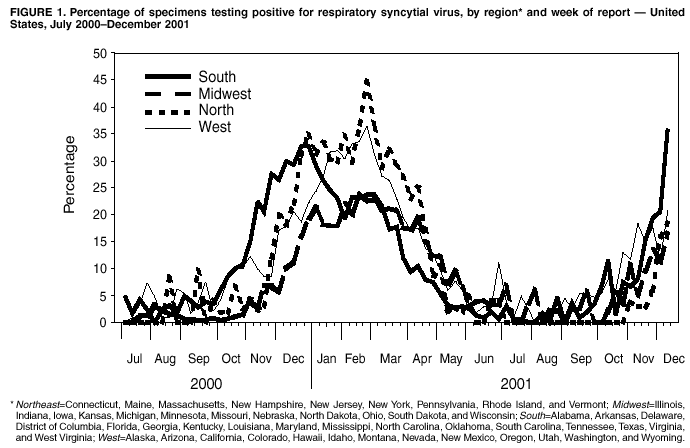 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 1/17/2002 |
|||||||||
This page last reviewed 1/17/2002
|