|
 |
 |
 |
 | |||||
| |||||||||
|
|
|
|
|
Guidance for Industry (PDF version of this document) DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding this draft document should be submitted within 60 days of publication in the Federal Register of the notice announcing the availability of the draft guidance. Submit comments to the Division of Dockets Management (HFA-305), Food and Drug Administration, 5630 Fishers Lane, rm. 1061, Rockville, MD 20852. All comments should be identified with the docket number listed in the notice of availability that publishes in the Federal Register. For questions regarding this draft document contact (CDER) Howard Chazin at 301-796-0700, or (CBER) Leonard Wilson at 301-827-0373. U.S. Department of Health and Human Services June 2007 Procedural Guidance for Industry Additional copies are available from: Office of Training and Communications or Office of Communication, Training, and
U.S. Department of Health and Human Services June 2007 TABLE OF CONTENTS Guidance for Industry 1.
I. INTRODUCTIONThis guidance is intended to clarify for industry where to include the integrated summary of effectiveness (ISE) and integrated summary of safety (ISS) when submitting applications in the common technical document (CTD) format. The guidance applies to applicants submitting new drug applications (NDAs) or biologics license applications (BLAs) to the Food and Drug Administration (FDA) in the CTD or the electronic common technical document (eCTD) format. The word summary in the terms integrated summary of effectiveness and integrated summary of safety has caused confusion for companies submitting applications in the CTD format, as it suggests a reference to the abbreviated overview documents that are placed in Module 2 of an application in the CTD format. However, the ISE and ISS are not summaries but rather detailed integrated analyses of all relevant data from the clinical study reports that belong in Module 5.2. The FDA considers the ISE and ISS critical components of the clinical efficacy and safety portions of a marketing or licensing application. Therefore, the ISE and ISS are required in applications submitted to the FDA in accordance with the regulations for NDA submissions (21 CFR 314.50(d)(5)(v) and 21 CFR 314.50(d)(5)(vi)(a), respectively). Although there are no corresponding regulations requiring an ISE or ISS for BLA submissions, applicants are encouraged to provide these analyses. This guidance focuses on where to place ISE and ISS documents within the structure of the CTD or eCTD. It does not outline in detail the content for the ISE and ISS. The content will be addressed in future guidances. FDA’s guidance documents, including this guidance, do not establish legally enforceable responsibilities. Instead, guidances describe the Agency’s current thinking on a topic and should be viewed only as recommendations, unless specific regulatory or statutory requirements are cited. The use of the word should in Agency guidances means that something is suggested or recommended, but not required. II. BACKGROUNDIn July 1988, the FDA published guidance on the Format and Content of the Clinical and Statistical Sections of an Application (Clin-Stat guidance). The Clin-Stat guidance describes an appropriate format for organizing the clinical and statistical sections of an application and presenting the clinical and statistical information and accompanying statistical documentation of a clinical trial. In 2001, the broad outline of the ISE and ISS, as described in the Clin-Stat guidance, was partially updated by the ICH guidance M4 Common Technical Document for the Registration of Pharmaceuticals for Human Use, which is published in four parts (general organization, quality, safety, and efficacy). However, since the ISE and ISS are unique to the United States, their contents are not addressed in detail in M4E: The CTD — Efficacy, which covers the Clinical Overview and Clinical Summary sections of Module 2 and the Clinical Study Reports in Module 5. Specifically, ICH M4E does not specify the location of the full reports of the ISE and ISS. Therefore, when finalized, this guidance will describe the appropriate location of the ISE and ISS, whose contents are described in the Clin-Stat guidance, sections II.G. and H., respectively. III. ISE-AND ISS-RELATED DIFFERENCES BETWEEN MODULE 2 AND MODULE 5 OF THE CTD AND eCTDThe purpose of the CTD or eCTD is to provide a common organization for the contents of a marketing or licensing application. In some cases, ICH guidance has led to substantial or complete harmonization of content areas such as the study report, but that is not the case for all parts of all applications. Therefore, applications can still vary based on regional requirements or applicant preference, and applications using the CTD or eCTD format (Module 2 to Module 5) will not be identical for all ICH regions. The CTD/eCTD Module 2 contains several clinical sections that are summaries. These sections include section 2.7.3, Summary of Clinical Efficacy, and section 2.7.4, Summary of Clinical Safety. Generally, the Module 2 clinical summary sections (hereafter clinical summary sections) follow the outline of the ISE and ISS described in ICH M4E; however, they do not describe the needed level of detail for an ISE or an ISS. In addition, these clinical summary sections are subject to space limitations in the eCTD of about 400 pages, whereas a typical ISS alone often can be substantially larger. A common problem with the CTD-formatted applications is that the applicants incorrectly assume that the clinical summary sections satisfy the regulatory requirement for the ISE and ISS. This assumption can result in a determination by the FDA that an application is incomplete and may result in a refusal-to-file action for the application. 3. Although the clinical summary sections should be considered the appropriate locations for the clinical summary portions of a marketing application submitted to the United States (as required under 21 CFR 314.50(c)(2)(viii)), as discussed in section III.A, the clinical summary sections should not be considered the appropriate location for the ISE or ISS, with rare exceptions. The recommended appropriate location for the ISE or ISS is Module 5, as discussed in section III.B. Table 1 lists the various sections of the CTD that contain summary and integrated discussions of efficacy and safety. The listed sections are mapped to the corresponding U.S. regulations, where applicable, that apply to the content of those sections. Any questions about these matters should be discussed with the appropriate review division. Table 1: ISE- and ISS-Related Sections with Corresponding Regulations
An overview of efficacy and safety results can be included in sections 2.5.3, Overview of Efficacy, and 2.5.4, Overview of Safety. Usually, these overviews should be brief but thorough discussions of the study design, effectiveness, and general safety results. These overviews should contain mostly text with some tables and figures incorporated. More detailed summaries of efficacy and safety should be provided in sections 2.7.3, Summary of Clinical Efficacy, and 2.7.4, Summary of Clinical Safety. These summary documents should consist mostly of text, but with tables and figures incorporated as needed, too. The clear intent of sections 2.7.3 and 2.7.4 is to provide summaries of data, not a complete exposition. It is not necessary, or desirable, to provide all of the details appropriate to an ISE or ISS in these sections, as this would defeat the purpose of a summary. Rather, sections 2.7.3 and 2.7.4 should contain summarized information derived from the full ISE and ISS (i.e., the highlights of the appropriate sections of each document as outlined in ICH M4E). Only in unusual cases should the narrative parts of the full ISE or ISS and the summaries in sections 2.7.3 and 2.7.4 be the same (see section III.C.). The ISE and ISS will be more extensive than the summaries and should include not only text and incorporated tables and figures, but additional appendices of tables, figures, and datasets as well.
In general, Module 5, specifically section 5.3.5.3, Reports of Analyses of Data from More than One Study (Including Any Formal Integrated Analyses, Meta-Analyses, and Bridging Analyses), is the appropriate location for the ISE and ISS. This module is designed to contain more detailed in-depth analyses, and unlike Module 2, Module 5 has no space limitation. Module 5 is the appropriate section of the CTD for analyses containing large appendices of tables, figures, and datasets typically found in an ISE and ISS.
There may be situations in which sections 2.7.3, Summary of Clinical Efficacy, and 2.7.4, Summary of Clinical Safety, would be sufficiently detailed to serve as the narrative portion of the ISE and ISS, respectively, while still concise enough to meet the suggested size limitations for Module 2. This situation is rare but can occur if the application is small and consists of a single study or a number of small studies. In our experience, the narrative portion of the ISE often is more amenable for inclusion in Module 2 than is the ISS. In such situations, the ISE and ISS can be split across Module 2 and Module 5, with the narrative portion located in section 2.7.3 or 2.7.4 and the appendices of tables, figures, and datasets located in section 5.3.5.3. If the ISE or ISS is split across modules in this way, it is critical that a clear explanation of where the parts are located be included. This explanation should be placed both in Module 2 (section 2.7.3 or 2.7.4) and in Module 5 (section 5.3.5.3). IV. SPECIAL INSTRUCTIONS FOR THE eCTD FORMATBecause an increasing number of applications are being submitted electronically, it is important for review purposes that the location of information in an eCTD be consistent across applications. Therefore, the ISE and ISS should be placed in Module 5, section 5.3.5.3. If the narrative portion of the ISE or ISS is suitable for use in section 2.7.3 or 2.7.4, the narrative portion should be submitted only once and referenced in both Module 2 (section 2.7.3 or 2.7.4) and Module 5, section 5.3.5.3 (i.e., provide leaf elements in both locations). V. EXAMPLES OF NDA AND BLA SUBMISSIONSThe following examples describe real-life scenarios of NDA and BLA submissions and where the ISE and ISS information should be located appropriately in the CTD.
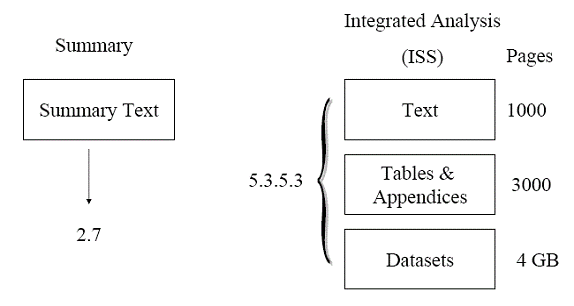
An applicant submits a BLA or BLA supplement with an ISS consisting of 1,000 pages of text (with incorporated tables and figures), 3,000 pages of appendices of supporting tables and figures, and 4 gigabytes (GB) of datasets used in the integrated safety analyses. The full ISS is placed in Module 5 (section 5.3.5.3). The text portion is summarized in a smaller 100-page document and placed in Module 2 (section 2.7.4) as the Summary of Clinical Safety. Figure 1 illustrates this example. Figure 1
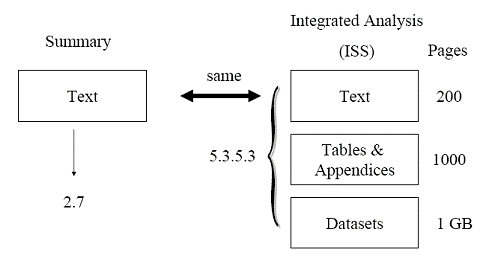
An applicant submits an NDA for an orphan drug. The ISS contains 100 pages of text (with incorporated tables and figures), 1,000 pages of appendices of supporting tables and figures, and 1 GB of datasets used in the integrated safety analyses. The full ISS is placed in Module 5 (section 5.3.5.3). The text portion of the ISS is repeated in Module 2 (section 2.7.4) as the Summary of Clinical Safety. Figure 2 illustrates this example. Figure 2 This submission is acceptable. The ISS is small so the text portion can also serve as the Summary of Clinical Safety in Module 2.
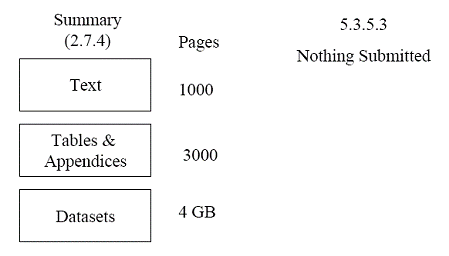
An applicant submits an NDA for a new molecular entity. The ISS contains 1,000 pages of text (with incorporated tables and figures), 3,000 pages of appendices of supporting tables and figures, and 4 GB of datasets. All are placed in Module 2, section 2.7.4. Nothing is placed in Module 5 (section 5.3.5.3). Figure 3 illustrates this example. Figure 3 This submission is unacceptable. Although the application contains an ISS, it is inappropriately placed in Module 2 and exceeds the suggested size limitation for section 2.7.4. The application does not contain a true clinical summary, which could result in a refuse-to-file action.
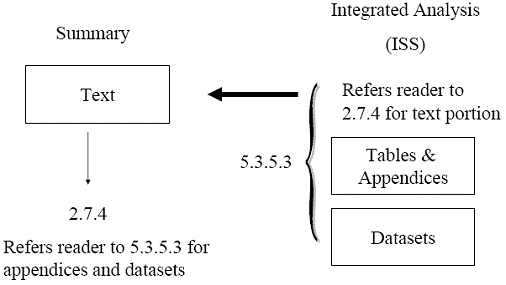
An applicant submits an NDA supplement. The ISS contains 100 pages of text (with incorporated tables and figures), 1,000 pages of appendices of supporting tables and figures, and 1 GB of datasets used in the integrated safety analyses. The ISS is split: the text portion is placed in Module 2 (section 2.7.4), and the appendices and datasets are placed in Module 5 (section 5.3.5.3). Section 2.7.4 refers the reader to section 5.3.5.3 for the appendices and datasets. Section 5.3.5.3 refers the reader to section 2.7.4 for the text portion of the ISS. Figure 4 illustrates this example. Figure 4 This submission is acceptable. The ISS is small, allowing the text portion of the ISS to also function as the Summary of Clinical Safety in Module 2. Each section of the split ISS refers the reader to the appropriate section where the remainder of the ISS is located. 1. This guidance has been prepared by the Office of New Drugs in the Center for Drug Evaluation and Research (CDER) in cooperation with the Center for Biologics Evaluation and Research (CBER) at the Food and Drug Administration. 2. For more information, see http://www.fda.gov/cder/regulatory/ersr/ISS_ISE_clarification.htm. 3. For more information, refer to the CDER guidance for industry Refusal to File (http://www.fda.gov/cder/guidance.index.htm) and the CBER Standard Operating Procedure and Policy 8404 Refusal to File Procedures for Biologic License Applications (http://www.fda.gov/cber/regsopp/regsopp.htm).
Date created: July 2, 2007 |