|
 |
 |
 |
 | |||||
| |||||||||
|
|
|
|
|
CDER Report to the Nation: 2005 Print version
1 Drug ReviewIndexHighlightsMany Americans benefited from last year’s timely reviews of new prescription medicines, over-the-counter medicines and the generic equivalents for both. When we review a medicine, we use the best science available to determine if a medicine’s benefits outweigh its risks for its intended use. An internal study showed that about half of our professional staff time is spent on safety assessment. We oversee the development of new medicines in the United States, and our paramount concern is the safety of patient volunteers in clinical trials. Highlights for 2005 include: n 80 new medicines. We approved 78 drugs and two biologics (22 priority and 58 standard reviews). n 20 truly new medicines. We approved 18 drugs and two new biologics that had never been marketed before in any form in this country (15 priority and 5 standard reviews). n 141 new treatment options. We approved new or expanded uses for 126 already approved drugs and 15 already approved biologics (36 priority and 105 standard reviews). n 5 over-the-counter drugs. Our approvals included five new medicines to be sold over the counter without a prescription, and four of them can be used by children. We approved three new uses for existing OTCs, all of which can be used by children. n 10 “orphan” medicines. Our approvals included nine drugs and one biologic for patient populations of 200,000 or fewer. n 344 generic drugs. We gave final approval to 344 generic versions of existing drugs and tentative approval to another 108. We received 777 marketing applications for generic drugs. n User fee goals. We exceeded all our performance goals for the fiscal year 2004 receipt cohort, the latest year for which we have full statistics. We are on track for exceeding most user-fee performance goals for the fiscal year 2005 cohort. n 652 clinical trial inspections. We conducted foreign and domestic inspections that help protect volunteers in clinical trials from research risks and validate the quality and integrity of data submitted to us. New drug review consolidated at White Oak campusMost of our review operations, except for generics, were successfully consolidated in a new facility in White Oak, Md. We took advantage of the move to reorganize the Office of New Drugs in a way that created logical groupings in the same divisions, created divisions with better balanced workloads and resource allocation and completed the integration of biologics reviewers and indications within our review divisions. Within OND, we renamed two offices—the Office of Oncology Drug Products and the Office of Antimicrobial Products. We created a new Office of Nonprescription Products with two divisions. Review of psychiatric and neurological products was separated into two divisions. More information is at http://www.fda.gov/cder/cderorg/ond_reorg.htm. New Drug and Biologic ReviewDefinitionsn New drug applications. NDAs are the formal submissions of data that sponsors send us when they are seeking approval to market a “new drug” in the United States. Some NDAs are NMEs; however, “new drugs” can also include an active substance previously sold in a different form. n New molecular entities. NMEs contain an active substance that has never before been approved for marketing in any form in the United States. Because of high interest in truly new medicines, we report approvals of NMEs and “new BLAs.” The charts for all NDAs and all BLAs include NMEs and new BLAs. n Biologic license application. BLAs are the formal submissions of data that sponsors send us when they are seeking approval to market a biologic in the United States. A “new BLA” is a biologic that has never been approved for marketing in the United States. n Review and approval times. Review time represents the time that we spend examining the application. Approval time represents our review time plus industry’s response time to our requests for additional information. n Priority approvals. These products represent significant improvements compared with marketed products. We have a goal of reviewing 90 percent of these applications within six months. n Standard approvals. These products have therapeutic qualities similar to those of already marketed products. We have a goal of reviewing 90 percent of these applications within 10 months. n Actions and filings. An application is “filed” when we determine it is complete and accept it for review. We make a filing decision within 60 days of receiving an application. Approval is one of the actions that we can take once an application is filed. Other actions include seeking more information from the sponsor. There is no direct connection between applications filed in one year and actions in the same year. n Orphan drugs. We administer a program that provides incentives to develop drugs for use in patient populations of 200,000 or fewer. Sponsors of orphan drugs receive inducements that include seven-year marketing exclusivity, tax credit for the product-associated clinical research, research design assistance from FDA and grants of up to $200,000 a year. n Accelerated approval. This program helps make products for serious or life-threatening diseases available earlier in the development process. We base our approval on a promising effect of the drug that can be observed significantly sooner than a long-term clinical benefit. Sponsors perform additional studies to demonstrate long-term clinical benefit. n Fast track development. This program facilitates the development and expedites our review of new drugs and biologics that demonstrate the potential to address unmet medical needs for serious or life-threatening conditions. Fast track emphasizes our close, early communication with sponsors. n Median times. Our charts show review and approval times as “medians.” The value for the median time is the number that falls in the middle of the group after the numbers are ranked in order. It provides a truer picture of our performance than average time, which can be unduly influenced by a few very long or short times. Our guide to understanding median approval time statistics is available at http://www.fda.gov/cder/present/MedianAPtime/index.htm. Therapeutic BLAs included starting with 2004 dataBeginning with 2004, our charts incorporate data on the review of therapeutic biologics transferred to us in late 2003. These include:
NDA and BLA Review StatisticsApproval totals
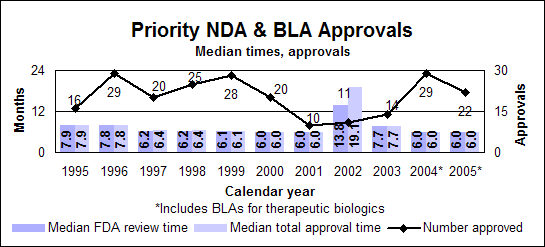
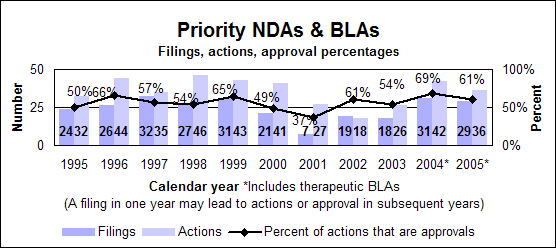
Priority new drugs and biologics
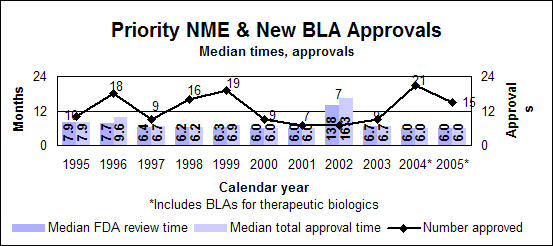
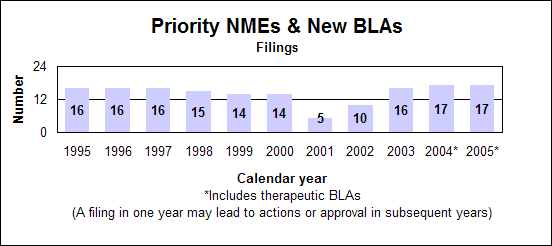
Click image for larger chart, click here for accessible text. Click image for larger chart, click here for accessible text. Priority new molecular entities and new biologics
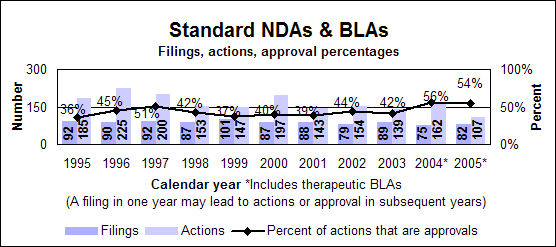
Click image for larger chart, click here for accessible text. Click image for larger chart, click here for accessible text. Standard drugs and biologics
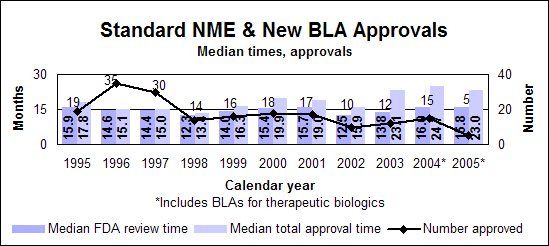
Click image for larger chart, click here for accessible text. Click image for larger chart, click here for accessible text. Standard new molecular entities and new biologics
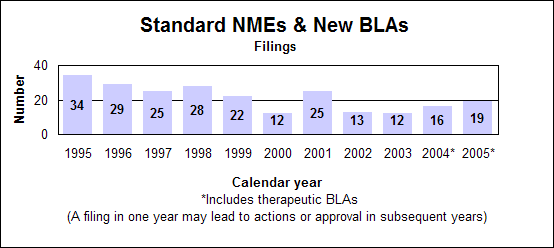
Click image for larger chart, click here for accessible text. Click image for larger chart, click here for accessible text. Listing of NMEs, new BLAs, other priority NDAsNMEs (P=priority, S=standard, O=orphan)
New BLAs
Other NDA priority approvals (T=tentative)
Drugs@FDADrugs@FDA—the most frequently used application on the FDA Web site—has official information about FDA approved brand-name and generic drugs such as:
To use Drugs@FDA, go to http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm Internet resources for drug review statisticsn Additional statistics. More review statistics are available at http://www.fda.gov/cder/rdmt/default.htm. n Data updated. You should be aware that these data may differ from those in previous issues of this report. We have revised data from previous years. Notable 2005 New ApprovalsLast year’s approvals benefited children, people with HIV infection, cancer, diabetes and other disorders. ChildrenEmtricitabine (Emtriva) is an oral solution of an antiretroviral medicine that can be used in combination with other antiretroviral agents for the treatment of HIV infection in children 3 months old and older. The drug, first approved as a capsule for adults in 2003, is an HIV nucleoside reverse transcriptase inhibitor that helps to block an enzyme needed for HIV to multiply. Related to Best Pharmaceuticals for Children Act. (priority) Mecasermin [rDNA origin] (Increlex) and Mecasermin rinfabate [rDNA origin] (Iplex) are for the long-term treatment of children who are very short for their age because their bodies do not make enough insulin-like growth factor-1. Both drugs contain human insulin-like growth factor-1 from genetically engineered bacteria, but mecasermin rinfabate also contains insulin-like growth factor binding protein-3 from genetically engineered bacteria. (2 NMEs, priorities, orphans) People with HIV infectionLopinavir/ritonavir (Kaletra) is a new formulation in a tablet form that may be prescribed for once-daily use in combination with other anti-HIV medicines for some patients who have not taken anti-HIV medications in the past. (priority) Tipranavir (Aptivus) is a protease inhibitor taken with 200 mg of ritonavir and two other anti-HIV medicines to treat adults with HIV infection. The drug blocks HIV protease, an enzyme needed for HIV to make more virus. Tipranavir helps reduce the amount of HIV in the blood and keep the immune system healthy so it can help fight infection. (NME, priority) Lamivudine/zidovudine/nevirapine is the first three-drug HIV regimen in one package approved for purchase under the President’s Emergency Plan for AIDS Relief. We gave it “tentative approval” in less than two weeks because patent or exclusivity provisions prevent its sale in the United States. It can also serve as a reference product for generic versions. (priority) People with cancerNelarabine (Arranon) is a chemotherapy drug for the treatment of patients with T-cell acute lymphoblastic leukemia and T-cell lymphoblastic lymphoma whose disease has not responded to or has relapsed following treatment with at least two chemotherapy regimens. (NME, priority, orphan) Sorafenib tosylate (Nexavar) is a chemotherapy agent indicated for the treatment of patients with advanced cancer of the kidney cells. (NME, priority, orphan) People with infectionsEntecavir (Baraclude), in tablets and oral solution, treats chronic infection with hepatitis B virus in adults who also have active liver damage. Entecavir, a nucleoside analogue, competes with a natural substance needed for viral replication. The tablet form was counted as an NME. We also provided priority approval to a separate application for the oral solution. (1 NME, both priorities) Micafungin sodium (Mycamine) is used to prevent fungal infections caused by Candida in patients who are undergoing a stem-cell transplantation. Micafungin inhibits synthesis of a component of the fungal cell wall. (NME, priority) Tigecycline (Tygacil) treats adults who have complicated skin or intra-abdominal infections caused by certain strains of bacteria. It belongs to the glycylcycline class of antibiotics. (NME, priority) People with eye diseaseFluocinolone acetonide intravitreal implant (Retisert) treats chronic non-infectious inflammation of the tissue in the rear of the eye. The drug product is surgically implanted into the affected eye and slowly releases an inflammation-controlling steroid over approximately the next 30 months. (orphan) Nepafenac (Nevanac) treats the pain and inflammation associated with cataract surgery. Nepafenac is a nonsteroidal anti-inflammatory and analgesic prodrug. After topical ocular dosing, nepafenac penetrates the cornea and is converted by eye tissue to amfenac, a nonsteroidal anti-inflammatory drug. (NME, priority) People with arthritisAbatacept (Orencia) treats adults with moderate to severe rheumatoid arthritis who have not been helped by other medicines. Abatacept may be used alone or with other arthritis medicines except those known as TNF antagonists. Abatacept modulates parts of the immune system implicated in causing rheumatoid arthritis. (biologic, priority) People with other rare disordersDeferasirox (Exjade) is an iron chelating agent for the treatment of chronic iron overload due to blood transfusions in patients 2 years of age and older. (NME, priority, orphan) Galsulfase (Naglazyme) is an enzyme replacement therapy for patients with mucopolysaccharidosis VI. Also known as MPS VI or Maroteaux-Lamy syndrome, the inherited condition results when the body fails to make sufficient enzymes to break down certain complex carbohydrates that then accumulate and cause widespread cellular, tissue and organ dysfunction. The biotechnology product provides an injectable enzyme that helps the body break down the appropriate proteins. (biologic, priority, orphan) Lenalidomide (Revlimid) treats people with transfusion-dependent anemia due to low or intermediate-1 risk myelodysplastic syndromes that are associated with a specific genetic abnormality. The syndromes result when the bone marrow does not make enough normal blood cells. Patients may need blood and platelet transfusions and antibiotic therapy for infections. In clinical trials, most patients treated with the drug became independent of transfusions by three months, and the transfusion-free period lasted for an average of 44 weeks. Because the drug is structurally similar to thalidomide, which is known to cause severe birth defects, it is sold under a risk management plan designed to prevent fetal exposure. (NME, priority, orphan) Hospitalized people with dangerously low sodiumConivaptan hydrochloride (Vaprisol) is an injectable medicine and the first indicated to treat hospitalized patients with a potentially life-threatening condition that occurs when the body’s blood sodium level falls significantly below normal. Known as euvolemic hyponatremia, the condition is the most common electrolyte disorder in clinical medicine and one of the most difficult to treat. It often results from elevated levels of antidiuretic hormone, a hormone that regulates water and salt balance in the body. The drug blocks the activity of this hormone, resulting in increased urine output without loss of valuable electrolytes such as sodium and potassium. (NME) People with pulmonary hypertensionSildenafil citrate (Revatio) treats pulmonary arterial hypertension to improve exercise ability. This serious condition, which can lead to fatal heart failure, is caused by continuous high blood pressure in the artery carrying blood from the heart to the lung. (priority) People with diabetesPramlintide acetate (Symlin) is an injectable medicine for adult patients with either type 1 or type 2 diabetes. It slows down the movement of food through the stomach. This affects how fast sugar enters the blood after eating. It is always used with insulin to help lower blood sugar during the three hours after meals. The synthetic drug is similar to a naturally occurring human hormone that contributes to blood sugar control after eating a meal. (NME) Exenatide (Byetta) is used to help improve blood sugar control in patients with type 2 diabetes who have not achieved adequate control on metformin, a sulfonylurea or a combination of metformin and a sulfonylurea. The drug, chemically different from other diabetes treatments, enhances insulin secretion, suppresses inappropriately elevated secretion of a hormone that raises blood sugar and slows emptying of the stomach. (NME) Insulin detemir [recombinant DNA origin] (Levemir) can be injected once or twice a day under the skin by patients with type 1 or type 2 diabetes who require a long-acting insulin for the control of high levels of blood sugar. Because the medicine, made by recombinant DNA technology, differs slightly from human insulin, it is released more slowly to target tissues. (NME) People with insomniaRamelteon (Rozerem) treats insomnia in adults where the problem is trouble falling asleep. The drug is active at the body’s melatonin receptors, which are thought to be involved in the maintenance of the circadian rhythm underlying the normal sleep-wake cycle. (NME) Treatment and diagnostic aidsHyaluronidase (Hydase) and Hyaluronidase human (Hylenex recombinant) help increase the absorption and dispersion of other injected drugs and improve resorption of X-ray contrast agents. The first drug is a purified enzyme derived from cow tissue, and the second is a purified preparation of the human enzyme hyaluronidase produced by genetically engineered hamster cells. (2 NMEs, priorities) Other priority approvalSodium benzoate/sodium phenylacetate (Ammonul) was approved to be manufactured by a different company. The drug, first approved in 1987 and discontinued by its original manufacturer, helps treat acutely elevated levels of ammonia and associated brain swelling in patients with deficiencies in enzymes of the urea cycle. (priority, orphan) New or Expanded Use ReviewApplications for a new or expanded use, often representing important new treatment options, are formally called “efficacy supplements” to the original new drug application. We have a goal of reviewing standard supplements in 10 months and priority supplements in six months. New or expanded use review statisticsApproval totals
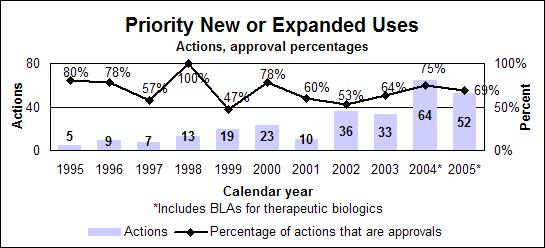
Priority new or expanded uses (efficacy supplements)
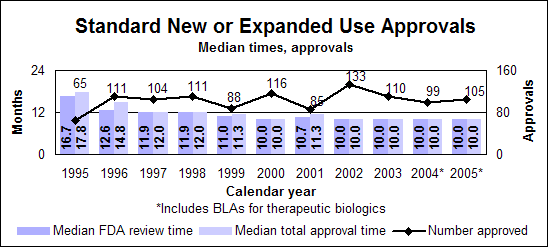
Click image for larger chart, click here for accessible text. Click image for larger chart, click here for accessible text. Standard new or expanded uses (efficacy supplements)
Click image for larger chart, click here for accessible text. Click image for larger chart, click here for accessible text. Notable 2005 new or expanded use approvalsPeople infected with both hepatitis and HIVPeginterferon alfa-2a (Pegasys), when used alone or in combination with Ribavirin, had its indication expanded to include treatment of adult chronic hepatitis C patients co-infected with HIV, who have clinically stable HIV disease. (priority, biologic) Ribavirin (Copegus), when used in combination with peginterferon alfa-2a, can now be used to treat chronic hepatitis C in adult patients co-infected with HIV. (priority) People with cancerBortezomib (Velcade) can be used to treat multiple myeloma patients who have received as least one prior therapy instead of the two prior therapies indicated in the 2003 approval. (priority) Erlotinib hydrochloride (Tarceva), a non-small-cell lung cancer treatment, can be used in combination with gemcitabine for the first-line treatment of patients with locally advanced pancreatic cancer that cannot be removed surgically or that has spread. (priority) Temozolomide (Temodar) can be used along with radiotherapy to treat patients with newly diagnosed high grade brain tumors and then as maintenance treatment. (priority) Letrozole (Femara) can be used to help treat postmenopausal women with hormone-receptor-positive early breast cancer. The drug, an inhibitor of estrogen synthesis, was originally approved for treating the late stage of the disease. (priority) People with heart diseaseCandesartan cilexetil (Atacand), a high-blood pressure treatment first approved in 1998, received two new indications. It can be used to treat heart failure in patients with left ventricular systolic dysfunction to reduce cardiovascular death and to reduce hospitalizations for heart failure and to treat heart failure to reduce the risk of death from cardiovascular causes and to reduce hospitalizations for heart failure. (priority) Perindopril erbumine (Aceon), a high-blood pressure treatment, can be used in patients with stable coronary artery disease to reduce the risk of cardiovascular mortality or non-fatal myocardial infarction. (priority) People with other conditionsFluocinolone acetonide (Derma-Smoothe/FS) ear drops can be used to treat chronic eczematous external inflammation of the ear. (priority) Infliximab (Remicade), a treatment for the inflammation of the gastrointestinal tract known as Crohn’s disease, has a new indication for the treatment of patients who have moderately to severely active ulcerative inflammation of the colon and who have had an inadequate response to conventional therapy. (priority, biologic) Ropinirole hydrochloride (Requip), a Parkinson’s disease therapy, can be used for restless legs syndrome, which is characterized by an urge to move the legs usually accompanied or caused by uncomfortable and unpleasant leg sensations. (priority) Pediatric new or expanded usesDrugs with new or expanded uses in children approved under priority review required by the Best Pharmaceuticals for Children Act. Antimicrobial ResistanceDrug-resistant bacteria continue to be a major threat to the public health. We continued our antimicrobial resistance education campaign partnership with the Centers for Disease Control and Prevention and jointly released two new print public service announcements—one English and one Spanish. In addition to the print public service announcements, a Spanish-language brochure also was produced.
Date created: August 18, 2006 |