|
 |

|
 |
 |
|||||
|
|||||||||
|
|
|
|
|
CDER Report to the Nation: 2003 Adobe Acrobat version of this document 3 International ActivitiesIndex
International Conference on HarmonizationHarmonization-making the drug regulatory processes more efficient and uniform-is an issue that is important not only to Americans, but to drug regulatory agencies and pharmaceutical companies throughout the world. The International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use has worked to bring together government regulators and drug industry experts from innovator trade associations in the European Union, Japan and the United States. We are leading the FDA's collaboration with the ICH. This work is making new drugs available with minimum delays not only to American consumers but also to patients in other parts of the world. The drug regulatory systems in all three regions share the same fundamental concerns for the safety, efficacy and quality of drug products. Before ICH, many time-consuming and expensive technical tests had to be repeated in all three regions. The ICH goal is to minimize unnecessary duplicate testing during the research and development of new drugs. The ICH process results in guidance documents that create consistency in the requirements for product registration. Common Technical DocumentThe ICH Common Technical Document allows data in the same format to be submitted to drug review authorities in all three ICH regions. Specifications for electronic submission of the CTD, known as the eCTD, were completed in 2002. eCTD improves review efficiencyElectronic submissions using the eCTD specifications can be used to submit all applications and related submissions such as promotional materials and adverse events. Among other things, the eCTD allows reviewers to:
ICH guidance documentsAs of April 30, 2004, we had published:
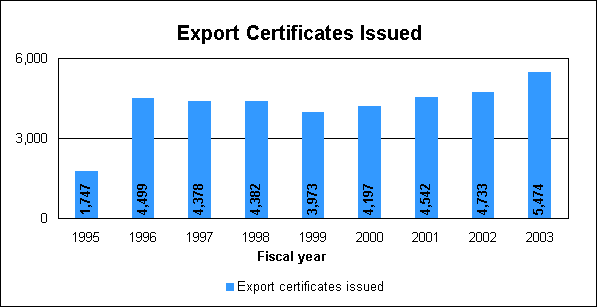
We publish ICH documents as guidances to industry on our Web site at http://www.fda.gov/cder/guidance/index.htm. International information-sharing agreementsIn an era of enhanced cooperation among regulators around the world, FDA entered new international agreements in which we play a critical role in implementing. We have a growing list of regulatory partners worldwide with whom we can pursue more open dialogue on emerging issues as well as exchange routine information on scientific review, policy development and enforcement. New information-sharing agreements with Canada, Switzerland and the European Union add to those already in effect with Japan and Australia. Japan and Australia. We routinely exchange recall information about products of interest to Japan and Australia and communicate emerging enforcement activities of mutual interest. We met several times with our counterparts regarding the exchange of site inspection information. With limited inspection resources of our own, we increasingly depend on foreign regulatory inspections and incorporate their inspection findings into a risk-based program for future inspection. European Agency for the Evaluation of Medicinals. This agreement establishes a basis for exchanging confidential information with the European Union agency primarily responsible for approving drugs. It will permit our review and compliance staff to share important information about pending approvals, post-marketing surveillance and enforcement actions concerning products and facilities under the oversight of the EMEA. Implementation will be phased in and includes activities designed to build understanding and mutual confidence in each another’s systems. Canada. This agreement provides for the exchange of information about pending approvals, post-marketing surveillance and enforcement actions. It expands on information-sharing activities that began years ago as well as those developed more recently with Mexico and Canada. Exchanges of emerging compliance issues and site-specific information have already begun. Switzerland. The working arrangement with Switzerland began several years ago. The present agreement addresses the need for protection of confidential information and provides for the exchange of information about marketing approval decisions, post-market surveillance, policy developments and compliance or enforcement activities of mutual interest. Progress is being made in implementing this arrangement, including the exchange of technical staff and training opportunities. Export CertificatesExport certificates issued in fiscal year 2003: 5,474
We promote goodwill and cooperation between the United States and foreign governments through the Export Certificate Program. These certificates enable American manufacturers to export their products to foreign customers and foreign governments. The demand for certificates by foreign governments remains high due to expanding world trade, ongoing international harmonization initiatives and international development agreements. The certificates attest that the drug products are subject to inspection by the FDA and are manufactured in compliance with current good manufacturing practices. Export certificates verify that drug products being exported:
Internet resourcesMore information about our international activities, including Spanish language materials, is at http://www.fda.gov/cder/audiences/iact/iachome.htm.
Date created: May 24, 2004
|