 |
Pre-Assigned Application number
Requesting a Pre-Assigned Application number:
- Before you request a pre-assigned eCTD application number, apply for a secure email with the FDA by contacting Wendy Lee. If using a US Agent to request the number, please be sure that the agent has established secure e-mail.
- Send one email per application number request to cderappnumrequest@fda.hhs.gov. Please note that at this time the Electronic Submission Gateway (ESG) cannot accept requests for pre-assigned numbers.
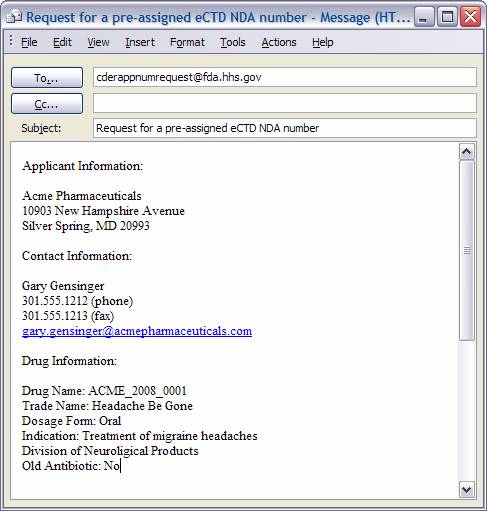
Include the following information in your e-mail:
- Subject: Request for a Pre-Assigned eCTD <insert Application Type> Number
- Text:
- Name of Applicant that will be on form (FDA 1571 or 356h) or transmittal letter (Master File)
- Applicant Address (street, city, state, zip code)
- Name of US Contact, Phone Number, Fax Number, Email Address
- Name of drug or Subject of Master File <insert Established Drug Name (if applicable); or sponsor code name with short description of product, Dosage Form, Strengths if applicable >
- Drug Trade Name (if applicable)
If requesting an NDA or ANDA, please include the following:
- Indicate whether or not the application for which a pre-assigned number is being requested is an “old antibiotic”. An “old antibiotic” is an application for a drug that contains an antibiotic that was the subject of any marketing application received by the Secretary on or before November 20, 1997. See http://www.fda.gov/cder/Guidance/2468fnl.pdf for additional information.
If requesting for an IND, NDA, or BLA please include the following:
- Indication
- Review Division, if known
If requesting for a Master File please include the following:
- Manufacturing Site Name
- Manufacturing Site Address
If requesting for an ANDA, please include the following:
Note: ANDA pre-assigned numbers expire after 60 days
- Reference Listed Drug Name and RLD Number
- Is there a NCE exclusivity? YES or NO
- If yes, when does it expire?
- Have you filed any applications for this active ingredient before? If YES, answer question below
- Please specify the ANDA Number, Drug, Dosage Form, Strength
- A pre-assigned eCTD number will be issued within 3 business days.
Sample e-mail
The fastest and most direct route to your reviewers is through the Electronic Submission Gateway. Please consider using it for all your IND, NDA, ANDA, or BLA regulatory submissions. See www.fda.gov/esg for more information.
 PDF requires the free Adobe Acrobat Reader PDF requires the free Adobe Acrobat Reader
 Back to Top Back to Top  Back to eCTD
Back to eCTD
Date created: April 29, 2008 |
 |