Guidance for Industry
Nucleic Acid Testing (NAT) for Human Immunodeficiency Virus Type 1 (HIV-1)
and Hepatitis C Virus (HCV):Testing, Product Disposition, and Donor Deferral and Reentry
[PDF version]

Draft Guidance
This guidance document is being distributed for comment purposes only.
Submit comments on this draft guidance by the date provided in the Federal Register notice announcing the availability of the draft guidance. Submit comments to the Division of Dockets Management (HFA-305), Food and Drug Administration, 5630 Fishers Lane, rm. 1061, Rockville, MD 20852. You should identify all comments by the docket number listed in the notice of availability that publishes in the Federal Register.
Additional copies of this draft guidance are available from the Office of Communication, Training and Manufacturers Assistance (HFM-40), Suite 200N, 1401 Rockville Pike, Rockville, MD 20852-1448, or by calling 1-800-835-4709 or 301-827-1800, or from the Internet at http://www.fda.gov/cber/guidelines.htm.
For questions on the content of this draft guidance, contact Paul A. Mied, Ph.D., at 301-827-3008.
U.S. Department of Health and Human Services
Food and Drug Administration
Center for Biologics Evaluation and Research
July 2005

TABLE OF CONTENTS
- INTRODUCTION
- DEFINITIONS
- BACKGROUND AND DISCUSSION
- RECOMMENDATIONS
- IMPLEMENTATION
- REFERENCES
FIGURE 1. Testing, Product Disposition, and Donor Management for an Individual Donor Sample that is Reactive on a Multiplex NAT After a Negative Antibody Screening Test
FIGURE 2. Testing, Product Disposition, and Donor Management for an Individual Donor Sample that is Reactive on an Individual NAT After a Negative Antibody Screening Test
FIGURE 3. Testing, Product Disposition, and Donor Management for a Master Pool that is Reactive on a Multiplex NAT: Resolution by Testing Individual Donor Samples
FIGURE 4. Testing, Product Disposition, and Donor Management for a Master Pool that is Reactive on an Individual NAT: Resolution by Testing Individual Donor Samples
FIGURE 5. Testing, Product Disposition, and Donor Management for a Master Pool that is Reactive on a Multiplex NAT: Resolution by Testing Subpools
FIGURE 6. Testing, Product Disposition, and Donor Management for a Master Pool that is Reactive on an Individual NAT: Resolution by Testing Subpools
FIGURE 7. Reentry for Donors Deferred Because of HIV-1 Test Results
FIGURE 8. Reentry for Donors Deferred Because of HCV Test Results
TABLE 1. Testing, Product Disposition, and Donor Management for an Individual Donor Sample that is Reactive on a Multiplex NAT After a Negative Antibody Screening Test
TABLE 2. Testing, Product Disposition, and Donor Management for an Individual Donor Sample that is Reactive on an Individual NAT After a Negative Antibody Screening Test
TABLE 3.Testing, Product Disposition, and Donor Management for a Master Pool that is Reactive on a Multiplex NAT: Resolution by Testing Individual Donor Samples
TABLE 4.Testing, Product Disposition, and Donor Management for a Master Pool that is Reactive on an Individual NAT: Resolution by Testing Individual Donor Samples
TABLE 5.Testing, Product Disposition, and Donor Management for a Master Pool that is Reactive on a Multiplex NAT: Resolution by Testing Subpools
TABLE 6. Testing, Product Disposition, and Donor Management for a Master Pool that is Reactive on an Individual NAT: Resolution by Testing Subpools
TABLE 7. Reentry for Donors Deferred Because of HIV-1 Test Results
TABLE 8. Reentry for Donors Deferred Because of HCV Test Results

Guidance for Industry
Nucleic Acid Testing (NAT) for Human Immunodeficiency Virus Type 1 (HIV-1)
and Hepatitis C Virus (HCV): Testing, Product Disposition, and Donor Deferral and Reentry
Contains Nonbinding Recommendations
Draft - Not for Implementation
| This draft guidance, when finalized, will represent the Food and Drug Administration's (FDA's) current thinking on this topic. It does not create or confer any rights for or on any person and does not operate to bind FDA or the public. You can use an alternate approach if the approach satisfies the requirements of the applicable statutes or regulations. If you want to discuss an alternate approach, contact the appropriate FDA staff. If you cannot identify the appropriate FDA staff, call the appropriate number listed on the title page of this guidance. |

- INTRODUCTION
During the past decade there has been a dramatic reduction in the transmission of Human Immunodeficiency Virus Type 1 (HIV-1) and Hepatitis C Virus (HCV) by human blood and blood components. Primarily, this is due to the implementation of sensitive tests for viral antibody, antigen (for HIV-1), and nucleic acids, and in the case of plasma derivatives, the use of effective virus removal and inactivation methods. The sources of remaining risk of HIV-1 and HCV transmission are marker-negative "window period" donations (made during the period that the donor is infected with a virus, but neither the virus nor antibodies to the virus are detectable by current tests), donors infected with immunovariant viral strains, persistent antibody-negative (immunosilent) carriers, and laboratory test procedure errors. According to a recent report, donations during the window period constitute most of the risk of HIV-1 and HCV transmission (Ref. 1). Therefore, measures to reduce the window period could further reduce significantly the low residual risk of HIV-1 and HCV transmission by human blood and blood components.
Studies performed using seroconversion panels indicate the value of Nucleic Acid Testing (NAT) in reducing the window period for HIV-1 and HCV. The estimated mean window-period reduction for HIV-1 ribonucleic acid (RNA) by pooled sample NAT is approximately 11 to 15 days relative to antibody and 5 to 9 days relative to HIV-1 p24 antigen testing (Refs. 2-4). NAT for detection of HCV has been estimated to reduce the window period by 50-60 days relative to that for HCV antibody. In large-scale studies performed nationwide, NAT for HIV-1 detected 4 antigen-negative/antibody-negative window period donations and in the case of NAT for HCV, detected 42 additional antibody-negative window period donations. As a result, subsequent to implementation of NAT, the residual risk of HIV-1 and HCV in screened human blood and blood component donations is currently estimated to be approximately 1 in 2,135,000 donations for HIV-1 and 1 in 1,935,000 donations for HCV (Ref. 3).
We, the Food and Drug Administration (FDA), have previously issued recommendations on serologic testing for HIV-1 and HCV and use of NAT to establishments that collect blood and blood components including Source Plasma and Source Leukocytes in "Use of Nucleic Acid Tests on Pooled and Individual Samples from Donors of Whole Blood and Blood Components (including Source Plasma and Source Leukocytes) to Adequately and Appropriately Reduce the Risk of Transmission of HIV-1 and HCV." In this guidance document we are providing recommendations to you, blood and plasma establishments, manufacturers, and testing laboratories that are implementing a licensed method for HIV-1/HCV NAT, on testing individual samples or pooled samples from donors of human blood and blood components for HIV-1 RNA and HCV RNA. This document contains recommendations regarding product disposition (§ 610.40(h)), and donor management (§ 610.41 and § 630.6) based on the results of NAT and serologic testing for markers of HIV-1 and HCV infection on samples, collected at the time of donation, from donors of human blood and blood component donations.
FDA's guidance documents, including this guidance, do not establish legally enforceable responsibilities. Instead, guidances describe the FDA's current thinking on a topic and should be viewed only as recommendations, unless specific regulatory or statutory requirements are cited. The use of the word should in FDA guidances means that something is suggested or recommended, but not required.
This guidance, when final, is intended to supersede the recommendations in the FDA Memorandum to Blood Establishments dated April 23, 1992, August 5, 1993, and August 8, 1995, for reentry of donors deferred because of anti-HIV-1 test results, HIV-1 p24 antigen test results, and anti-HCV test results (Refs. 5-7).
Table of Contents

- DEFINITIONS
Master Pool: A pool of donor samples on which NAT is performed as a screening test. A Master Pool is formed by pooling of samples from subpools or by directly pooling samples from individual donors.
Subpool: A pool of donor samples that was used with other (sub)pools to form the Master Pool or that was formed during "deconstruction" of the Master Pool.
Deconstruction: Resolution of the reactivity of a Master Pool by testing subpools (original or freshly made) or samples from individual donors that formed the Master Pool. Deconstruction of a Reactive Master Pool to individual units is a required step for all approved tests.
Multiplex NAT: A NAT that simultaneously detects HIV-1 RNA and HCV RNA.
Discriminatory NAT: A NAT that uses specific primers for HIV-1 or HCV to identify the RNA in the Reactive multiplex NAT sample as HIV-1 RNA or HCV RNA. Performing a Discriminatory NAT is a required step for those establishments using a multiplex test such as the Procleix HIV-1/HCV NAT.
Additional NAT: A NAT that uses an amplification technology and/or primers that are different from those that were used for the original NAT screening test, and that has been validated for use with samples from individual donors. This test is not used to make the initial determination of donor suitability, but is used for donor counseling and to determine whether lookback should include notification of transfusion recipients.
Lookback: A series of actions taken by a blood establishment based on donor test results indicating infection with HIV-1 or HCV. These actions relate to prior donations from that donor that possibly were donated during the window period when HIV-1 or HCV RNA and antibody were not detectable by screening tests but the infectious agent might be present in the donor's blood. These actions include: quarantining of prior collections from that donor that remain in inventory, notifying consignees to quarantine prior collections, further testing of the donor, destroying or relabeling potentially infectious prior collections, and notifying transfusion recipients who received human blood or blood components from that donor, when appropriate.
In the proposed HCV lookback rule published in November 2000 (Ref. 8) we proposed changes to § 610.46 that would require lookback to be performed on the basis of a reactive NAT result, even when serological testing is non-reactive. When that rule becomes final, lookback for HIV-1 and for HCV will be required. In the meantime, we recommend that you perform lookback for HIV-1 and for HCV when donor samples test Reactive using HIV-1 NAT or HCV NAT.
Donor Reentry: A procedure that qualifies a donor who was deferred as eligible to donate again. Donor reentry procedures may be used following a false positive test result and typically require the passage of time to allow for possible seroconversion prior to the performance of additional serologic testing and NAT (See sections IV.7. and IV.8.).
Table of Contents

- BACKGROUND AND DISCUSSION
In September 1994 we held a workshop to discuss the potential application of nucleic acid based methods to donor screening for HIV-1. We concluded at the time that these methods clearly were sensitive, but they were not ready for implementation on a large scale.
The industry actively pursued the development of NAT for screening donors of human blood and blood components. Because of the cost and labor intensiveness of NAT, there was much interest in testing pools of plasma donor samples (minipools) by NAT, and by 1997, some manufacturers in Europe had voluntarily instituted NAT on minipools. At about that time, the European Union issued a directive that, by July 1, 1999, HCV RNA testing would be required in Europe for all plasma for fractionation, and that the requirement for HIV-1 RNA testing would follow at a later date.
Large-scale clinical studies were needed to demonstrate the efficacy of NAT because of the low frequency of window period donations. Small-scale studies would not identify adequate numbers of window period donations. Test kit manufacturers and testing laboratories submitted Investigational New Drug (IND) applications describing their test method and in-house validation of that method. Blood organizations and establishments intending to use the assay for donor screening also filed INDs to describe their clinical trial protocol for validation of pooled-donor sample NAT and individual donor sample NAT.
In December 1999 we issued guidance for industry on the validation of NAT methods to screen plasma donors (Ref. 9). This document provided guidance on test standards, manufacturing requirements, and clinical trial requirements for licensure of the test method for use in donor screening for transfusion transmitted viruses.
In September 2001 we licensed the first NAT system, the National Genetics Institute (NGI) UltraQual HIV-1 and HCV Reverse Transcription Polymerase Chain Reaction (RT-PCR) assays. Under that license, NGI performs these assays on pooled samples from donors of Source Plasma.
In February 2002 we licensed the Procleix HIV-1/HCV Assay, a qualitative NAT for detection of HIV-1 RNA and/or HCV RNA in plasma from donors of human blood and blood components for transfusion. This assay was approved for use with individual donor samples or pooled donor samples.
In December 2002 we licensed the COBAS AmpliScreen HCV Test, v 2.0 and the COBAS AmpliScreen HIV-1 Test, v 1.5. These tests are qualitative in vitro tests for the direct detection of HCV RNA and HIV-1 RNA in plasma samples from individual human donors, including donors of Whole Blood and blood components, Source Plasma, and other living donors. They are also intended for use in screening organ donors when specimens are obtained while the donor's heart is still beating. These assays were approved for use with individual donor samples or pooled donor samples.
In October 2004 we issued a final guidance, "Use of Nucleic Acid Tests on Pooled and Individual Samples from Donors of Whole Blood and Blood Components (including Source Plasma and Source Leukocytes) to Adequately and Appropriately Reduce the Risk of Transmission of HIV-1 and HCV." That guidance combined and finalized the draft guidance "Use of Nucleic Acid Tests on Pooled Samples from Source Plasma Donors to Adequately and Appropriately Reduce the Risk of Transmission of HIV-1 and HCV" dated December 2001 (January 31, 2002, 67 FR 4719) and the draft guidance "Use of Nucleic Acid Tests on Pooled and Individual Samples from Donors of Whole Blood and Blood Components for Transfusion to Adequately and Appropriately Reduce the Risk of Transmission of HIV-1 and HCV" dated March 2002 (April 9, 2002, 67 FR 17077).
That guidance informed establishments that collect blood and blood components that we have licensed NAT as tests to screen blood donors for HIV-1 RNA and HCV RNA, that these licensed tests can detect evidence of infection at a significantly earlier stage than is possible under previously approved tests using antibody or antigen detection technology, including the HIV-1 p24 antigen test, and that we believe that these newly licensed tests are now widely available and meet the criteria in 21 CFR 610.40(b) for screening tests that are necessary to reduce adequately and appropriately the risk of transmission of communicable disease through blood products.
In that guidance we recommend the use of HIV-1 NAT and HCV NAT on units that are not reactive on a donor-screening test for the detection of antibodies to HIV or HCV, respectively. However, for donations that are reactive on a test for the detection of antibodies to HIV-1 and are to be discarded or used in the manufacture of non-injectable products, we do not believe that HIV-1 NAT and HCV NAT are necessary as part of the adequate and appropriate testing required under § 610.40(b). Nevertheless, you may decide to perform HIV-1 and HCV NAT for these donations in order to obtain useful information regarding the donor's infection status. This information may be useful as part of donor notification.
This guidance is intended to assist you with testing, product disposition, donor deferral, donor notification, donor reentry, and lookback. We have written this document in general form because additional NAT may be approved in the future. However, where appropriate, we will identify sections that apply to NAT that are already approved. You must follow manufacturers' instructions regarding testing (§ 610.40(b)). Note that screening of donors of human blood and blood components for HIV-1 p24 antigen may be replaced by a NAT that has been validated by the manufacturer as a replacement for the HIV-1 p24 antigen EIA.
- NAT Algorithms
Under § 610.40(b), you must use approved screening tests "in accordance with the manufacturer's instructions." If you perform NAT on pooled samples and obtain a Reactive NAT result on a Master Pool, the manufacturer's instructions instruct you to perform subsequent testing to identify the individual unit(s) that contains the RNA identified in the Master Pool test. Once you have identified a positive unit, either by subsequent testing of a Master Pool, or by initial individual test, you must not use the donation for transfusion or for manufacturing into injectable products (§ 610.41(h)(1)) unless an exception applies (§ 610.40(h)(2)). You must defer the donor (§610.41(a)), and you must inform the donor of the deferral and the basis for the deferral including test results (§ 630.6). A Reactive NAT result may indicate ongoing infection of the donor, and thus prior donations from that donor, although NAT-Non-Reactive, may pose a risk to transfusion recipients. We recommend that you perform lookback when donor samples test Reactive for HIV-1 NAT or HCV NAT.
At the meeting of the Blood Products Advisory Committee (BPAC) in March 2001, we requested advice on appropriate algorithms for management of donations of human blood and blood components tested by pooled donor sample NAT for both HIV-1 RNA and HCV RNA. In particular, FDA sought comment on actions to be taken in the event of discrepant testing results, such as when the Master Pool is Reactive but individual donor samples test Non-Reactive. Data generated using NAT under IND that was presented in the BPAC meeting showed that in each discrepant case it was the Master Pool that was falsely Reactive, due to contamination either during specimen handling or during the assay run. In response to FDA questions, the BPAC voted to consider the NAT result on samples from individual donors as the definitive test result, and recommended release from quarantine for donations from those donors, on the basis of Non-Reactive test results.
This draft guidance document contains six recommended algorithms for use when NAT-Reactive results are obtained on individual samples or pooled samples from donors of human blood and blood components. This draft guidance also contains recommendations on product disposition, donor deferral criteria, follow-up testing of the donor, donor notification and donor reentry criteria that combine NAT and serologic test results, and lookback. This guidance is not intended to replace manufacturers' instructions for testing using approved tests.
The first and second algorithms (See Recommendations IV.1. and IV.2, Figures 1 and 2, and Tables 1 and 2) recommend actions to be taken when a NAT-Reactive result is obtained on an individual sample from a donor of human blood or blood components. The third and fourth algorithms (See Recommendations IV.3. and IV.4, Figures 3 and 4, and Tables 3 and 4) recommend actions to deconstruct a comparatively small Reactive Master Pool by testing individual donors. The fifth and sixth algorithms (See Recommendations
IV.5 and IV.6, Figures 5 and 6, and Tables 5 and 6) recommend actions to deconstruct a larger Reactive Master Pool by testing archived or freshly pooled subpools to identify the Reactive individual donor(s).
- Donor Reentry
Each year, thousands of donors are deferred from donating blood for an indefinite period, because of a false positive test result on a serological test, followed by a Negative or Indeterminate supplemental test for antibodies to HIV-1 or HCV. In addition to these deferrals, the implementation of individual donor sample and pooled donor sample NAT for HIV-1 RNA and HCV RNA has resulted in deferrals of several hundred donors each year due to potentially false Reactive NAT results.
These deferred donors are eligible to be considered for reentry to donate blood or blood components. Under § 610.41(b), a deferred donor subsequently may be found to be suitable as a donor by a re-qualification method or process found acceptable for such purposes by FDA. However, some establishments are not attempting to reenter donors because of the complexity of the current reentry algorithms and concerns about inappropriately reentering a donor. Although we do not require reentry of donors deferred because of false positive test results, we issued guidance in April of 1992 and August of 1993 on reentry of donors deferred because of HIV or HCV test results (Refs. 5, 7).
For those establishments that choose to perform donor reentry, this guidance recommends criteria for reentry of donors deferred because of Reactive HIV-1 or HCV NAT or certain other test results in accordance with § 610.41. We find these criteria to be acceptable within the meaning of § 610.41(b).
These recommendations include two new reentry algorithms based on the combined use of NAT and serologic testing: one for donors deferred because of HIV-1 test results, and a second for donors deferred because of HCV test results. In this draft guidance we recommend that you consider for reentry three classes of donors deferred because of HIV-1 test results (See Recommendation IV.7, Figure 7, and Table 7):
- Donors who had HIV NAT-Reactive results but were seronegative. This includes donors previously deferred because of Reactive test results on an investigational HIV-1 NAT.
- Donors with Non-Reactive NAT who have a Repeatedly Reactive screening test for HIV-1 antibody, and Negative or Indeterminate HIV-1 Western Blot or immunofluorescence assay (IFA) results or an HIV-1 Western Blot or IFA was not performed. This includes donors previously deferred because of Repeatedly Reactive HIV serologic test results prior to the initiation of testing by NAT. This class actually includes three subsets of donors, those with a Western Blot that was: (1) Indeterminate with viral bands present, (2) Indeterminate with non-viral bands only, and (3) Negative or not performed.
- Donors with a Repeatedly Reactive result on an HIV-1 p24 antigen test and with an Indeterminate (an invalid or a non-neutralized) result on the HIV-1 p24 Neutralization test (Ref. 6). In addition, donors with a Positive result on the HIV-1 p24 antigen Neutralization test also may be eligible for reentry because there are many donors who had (false) positive Neutralization test results who are currently Non-Reactive by HIV-1 NAT and Negative by anti-HIV-1/2 EIA. FDA has advised that it no longer recommends that blood and plasma establishments using certain approved NAT methods perform screening for HIV-1 p24 antigen. If antigen testing continues to be performed concurrent with NAT and antibody testing, donors deferred because of HIV-1 p24 test results would continue to be eligible for reentry.
Data presented at the June 2001 BPAC meeting demonstrated that an 8-week waiting period encompasses the pre-seroconversion window period for HIV-1 with sufficient confidence that Negative tests after at least 8 weeks have passed rule out HIV-1 infection (Ref. 10). Absent evidence for seroconversion, the Negative NAT on follow-up testing would be evidence that any prior Reactive (but unconfirmed) NAT result was an error.
Accordingly, for all three classes of donors, after a minimum time period of 8 weeks, we recommend that you take a follow-up sample from the donor for testing by both HIV-1 NAT and HIV-1 antibody enzyme immunoassay (EIA). Performing follow-up testing first on a sample from the donor before they donate again may prevent a potentially contaminated unit from being collected and placed in inventory at the blood establishment. If the NAT is Non-Reactive and the EIA is Negative on the follow-up sample, the donor may be reentered. The donor would then be tested again at the time of his/her next donation using the battery of screening tests required under § 610.40(b). Thus, two HIV-1 NAT tests would be performed and must be Non-Reactive and two HIV-1 EIA tests would be performed and must be Negative before a unit from that donor could be used. For purposes of donor counseling, you may choose to test the deferred donor with an HIV-1 NAT and an anti-HIV-1/2 EIA test at any time prior to the end of this 8-week waiting period after the original donation. However, if an HIV-1 NAT is Reactive or an anti-HIV-1/2 EIA is Repeatedly Reactive prior to the end of this 8-week waiting period, the donor would not be eligible for reentry and we recommend that you defer the donor permanently.
In this guidance we recommend that you consider for reentry two classes of donors deferred because of HCV test results. (See Recommendation IV.8., Figure 8, and Table 8):
- Donors who had HCV NAT-Reactive results but were seronegative on the HCV antibody test. This includes donors previously deferred because of Reactive test results on an investigational HCV NAT.
- Donors with Non-Reactive NAT who have a Repeatedly Reactive screening test for HCV antibody, and radioimmunoblot assay (RIBA) results that were Indeterminate or Negative or a RIBA was not performed. This includes donors previously deferred because of Repeatedly Reactive HCV serologic test results prior to the initiation of testing by NAT.
Data presented at the June 2001 BPAC meeting demonstrated that a 6-month follow-up period encompasses the pre-seroconversion window period with sufficient confidence that Negative serologic tests after at least 6 months have passed rule out HCV infection (Ref. 10).
For purposes of reentering both of these classes of deferred donors, we recommend that a sample be taken from the donor, after a minimum time period of 6 months, for follow-up testing by both HCV NAT and anti-HCV EIA. Current research indicates that detectable viremia may be intermittent or may also be resolved in about 15-25% of cases of HCV infection (Refs. 11, 12). If the NAT is Non-Reactive and the EIA is Negative on the follow-up sample, the donor may be reentered. For purposes of donor counseling and to detect possible HCV viremia, you may also choose to test the deferred donor with an HCV NAT and an anti-HCV EIA test at any time prior to the completion of this 6-month period after the original donation. However, if an HCV NAT is Reactive or an anti-HCV EIA is Repeatedly Reactive prior to the end of this 6-month period, the donor would not be eligible for reentry and we recommend that you defer the donor permanently.
Table of Contents

- RECOMMENDATIONS
Currently approved tests on individual donor samples for HIV-1 RNA and HCV RNA may be either Multiplex NAT for the simultaneous detection of HIV-1 RNA and/or HCV RNA or separate tests for the RNA of the two viruses.
- Testing, Product Disposition, and Donor Management for an Individual Donor Sample that is Reactive on a Multiplex NAT after a Negative Antibody Screening Test
If you obtain a Reactive Multiplex HIV-1 RNA/HCV RNA NAT result on an individual donor sample, you must do the following (See Figure 1 and Table 1):
- Follow the manufacturer's instructions, which instruct you to test the Reactive donation using Discriminatory NAT(s) (§ 610.40(b)) (Ref. 13).
- If the Discriminatory NAT is Reactive for HIV-1 RNA and/or HCV RNA, you must quarantine the unit (§ 610.40(h)). You must not ship or use the unit unless one of the exceptions described in 610.40(h)(2) applies. If you choose not to destroy the unit, you may release it for research or further manufacture with written approval from FDA. If released for one of these uses, you must re-label the unit consistent with the labeling requirements in § 606.121(f) and § 610.40(h). The unit must be labeled as "Biohazard" and with one of the following cautionary statements as applicable:
"Reactive for HIV-1 RNA"
OR
"Reactive for HCV RNA"
OR
"Reactive for HIV-1 RNA and HCV RNA"
AND EITHER
"Caution: For Further Manufacturing Into In Vitro Diagnostic Reagents For Which There Are No Alternative Sources"
OR
"Caution: For Laboratory Research Use Only."
Further, we recommend that you include on the label after the appropriate Reactive test results one of the following statements:
"Increased risk of transmission of HIV"
OR
"Increased risk of transmission of HCV"
OR
"Increased risk of transmission of HIV and HCV."
You must defer the donor (§ 610.41). The donor may be eligible for reentry (See sections IV.7 and IV.8). You must notify the donor of his/her deferral, providing information about the test results (§ 630.6).
We recommend that you perform lookback (product quarantine/retrieval and notification of recipients of prior collections for HIV-1 and/or HCV), as appropriate.
- If the Discriminatory NAT is Non-Reactive for both HIV-1 RNA and HCV RNA, you must quarantine the unit and destroy or relabel the unit as described in section IV.1.a.i above. You must defer the donor (§ 610.41). The donor may be eligible for reentry (See sections IV.7 and IV.8). You must notify the donor of his/her deferral, providing information about the test results (§ 630.6). We recommend that you perform lookback for HIV-1 and HCV.
Alternatively, for purposes of donor notification, you may choose to perform another NAT (the original NAT, or Discriminatory NAT(s), or an Additional NAT)) on a new sample or the same sample from the original donation. If an Additional NAT is performed, we recommend that the test be one that has been validated for use with individual donor samples.
(a) If you perform another test on a sample from the original donation and it is Reactive, you must quarantine the unit and destroy or relabel the unit as described in section IV.1.a.i above. You must defer the donor (§ 610.41). The donor may be eligible for reentry (See sections IV.7 and IV.8). You must notify the donor of his/her deferral, providing information about the test results (§ 630.6). We recommend that you perform lookback for HIV-1 and/or HCV, as appropriate.
(b) If you perform another test on a sample from the donation and it is Non-Reactive, you must quarantine the unit and destroy or relabel the unit as described in section IV.1.a.i above. You must defer the donor (§ 610.41). The donor may be eligible for reentry (See sections IV.7 and IV.8). You must notify the donor of his/her deferral, providing information about the test results (§ 630.6). In this case you may explain to the donor that the test result, while initially Reactive, is not conclusive. There is a slight risk that the initial test result was a Positive result that cannot be excluded without follow-up testing of the donor. We recommend that you quarantine/retrieve prior collections; however, due to the low probability that any of the prior collections was infectious, we do not recommend that you notify transfusion recipients.
- Testing, Product Disposition, and Donor Management for an Individual Donor Sample that is Reactive on an Individual NAT after a Negative Antibody Screening Test
If you obtain a Reactive HIV-1 RNA/HCV RNA NAT result for an individual donor sample (not by Multiplex NAT), you must do the following (See Figure 2 and Table 2):
- Quarantine the unit (§ 610.40(h)).
You must not ship or use the unit unless one of the exceptions described in § 610.40(h)(2) applies. If you choose not to destroy the unit, you may release it for research or further manufacture with written approval from FDA. If released for one of these uses, you must re-label the unit as described in section IV.1.a.i.
- Defer the donor (§ 610.41). The donor may be eligible for reentry if serologic test results are negative. (See sections IV.7. and IV.8).
- Notify the donor of his/her deferral including information about the test results (§ 630.6).
- We recommend that you perform lookback (product quarantine/retrieval and notification of recipients of prior collections for HIV-1 and/or HCV), as appropriate.
Currently approved tests on Master Pools of donor samples for HIV-1 RNA and HCV RNA may be either Multiplex NAT for the simultaneous detection of HIV-1 RNA and/or HCV RNA or separate tests for the RNA of the two viruses.
In general, there are two approaches to resolving a Master Pool that is Reactive on a Multiplex NAT or a Master Pool that is Reactive using separate tests for the RNA of the two viruses. If you would like to directly test all individual donor samples in a Reactive Master Pool, we recommend that you follow the test algorithms described in sections IV.3 and IV.4. These test algorithms are illustrated in Figures 3 and 4 and described in Tables 3 and 4. If you would like to test subpools that are used to construct a NAT-Reactive Master Pool, we recommend that you follow the test algorithms described in sections IV.5 and IV.6. These test algorithms are illustrated in Figures 5 and 6 and described in Tables 5 and 6.
- Testing, Product Disposition, and Donor Management for a Master Pool that is Reactive on a Multiplex NAT: Resolution by Testing Individual Donor Samples
If you obtain a Reactive Multiplex HIV-1 RNA/HCV RNA NAT result for a Master Pool, the test instructions for use instruct you to perform subsequent testing to identify the donor sample(s) that is (are) NAT-Reactive as the basis for the NAT-Reactive result on the pool. For comparatively small Master Pools, you may wish to directly test individual donor samples (See Figure 3 and Table 3). You must follow the instructions in the package insert for a licensed NAT test that provides a specific testing algorithm. (§ 610.40(b).)
- If you directly test the samples from individual donors that constituted the Multiplex NAT-Reactive Master Pool, consistent with the manufacturer's instructions you must test the individual donor samples using the same Multiplex NAT method that was used in the original NAT on the Master Pool (§ 610.40(b)) (Ref.13).
NOTE: In some cases the manufacturer's instructions provide for a different sample preparation procedure. However, the primers and probes would be the same as those used in the original NAT on the Master Pool.
- If all individual donor samples are Non-Reactive, you may release from quarantine all individual donations (if serologic tests on those donor samples are Negative and the donations are otherwise suitable for release). However, you must investigate the unexplained discrepancy in testing (§ 211.192). Laboratory control procedures must make adequate provisions for monitoring the reliability, accuracy, precision, and performance of laboratory test procedures and instruments, and must include adequate identification and handling of all test samples (§ 606.140(b), (c)). Use of supplies and reagents must be in a manner consistent with the instructions provided by the manufacturer (§§ 606.65(e), 610.40(b)). In addition, as part of an overall Quality Assurance program, we recommend that you conduct additional investigation to determine the cause of the initial reactivity of the Master Pool.
- If one (or more) individual donor sample(s) is (are) Reactive, perform the steps in section IV.1.a. above.
You may release from quarantine all Non-Reactive individual donations (if serologic tests on those donor samples are Negative and the donations are otherwise suitable for release).
- Testing, Product Disposition, and Donor Management for a Master Pool that is Reactive on an Individual NAT: Resolution by Testing Individual Donor Samples
If you obtain a Reactive result for a NAT for HIV-1 RNA and/or HCV RNA performed separately on a Master Pool, the test instructions for use instruct you to perform subsequent testing to identify the donor sample(s) that is (are) NAT-Reactive as the basis for the NAT-Reactive result on the pool. For comparatively small Master Pools, you may wish to directly test individual donor samples (See Figure 4 and Table 4). You must follow the instructions in the package insert for a licensed NAT that provides a specific testing algorithm (§ 610.40(b)).
- If you directly test the samples from individual donors that constituted the NAT-Reactive Master Pool, consistent with the manufacturer's instructions you must test the individual donor samples using the same NAT method that was used in the original NAT on the Master Pool (§ 610.40(b)) (Ref. 13).
NOTE: In some cases the manufacturer's instructions provide for a different sample preparation procedure. However, the primers and probes would be the same as those used in the original NAT on the Master Pool.
- If all individual donor samples are Non-Reactive, you may release from quarantine all individual donations (if serologic tests on those donor samples are Negative and the donations are otherwise suitable for release). However, you must investigate the unexplained discrepancy in testing (§ 211.192). Laboratory control procedures must make adequate provisions for monitoring the reliability, accuracy, precision, and performance of laboratory test procedures and instruments, and must include adequate identification and handling of all test samples (§ 606.140(b), (c)). Use of supplies and reagents must be in a manner consistent with the instructions provided by the manufacturer (§§ 606.65(e), 610.40(b)). In addition, as part of an overall Quality Assurance program, we recommend that you conduct additional investigation to determine the cause of the initial reactivity of the Master Pool.
- If one (or more) individual donor sample(s) is (are) Reactive, perform steps a-d in section IV.2. above.
You may release from quarantine all Non-Reactive individual donations (if serologic tests on those donor samples are Negative and the donations are otherwise suitable for release).
- Testing, Product Disposition, and Donor Management for a Master Pool that is Reactive on a Multiplex NAT: Resolution by Testing Subpools
If you obtain a Reactive Multiplex HIV-1 RNA/HCV RNA NAT result for a Master Pool, the test instructions for use instruct you to perform subsequent testing to identify the donor sample(s) that is (are) NAT-Reactive as the basis for the NAT-Reactive result on the pool. Deconstruction of the NAT-Reactive Master Pool may be performed by testing the subpools, (original or freshly made), that formed the Master Pool. This deconstruction of the Master Pool to determine the basis for the reactivity may involve several layers of testing using original or freshly pooled subpools, followed by testing of individual donor samples in the Reactive subpool(s) (See Figure 5 and Table 5). You must follow the instructions in the package insert for a licensed NAT that provides a specific testing algorithm (§ 610.40(b)).
- If you test subpools that were used to construct a Multiplex NAT-Reactive Master Pool, consistent with the manufacturer's instructions you must test the original subpools or freshly pooled subpools using the same Multiplex NAT method that was used in the original NAT on the Master Pool (§ 610.40(b)) (Ref. 13).
NOTE: In some cases the manufacturer's instructions provide for a different sample preparation procedure. However, the primers and probes would be the same as those used in the original NAT on the Master Pool.
- If all subpools are Non-Reactive, you may release from quarantine all individual donations that comprise the Non-Reactive subpools (if serologic tests on those donor samples are Negative and the donations are otherwise suitable for release). However, you must investigate the unexplained discrepancy in testing (§ 211.192). Laboratory control procedures must make adequate provisions for monitoring the reliability, accuracy, precision, and performance of laboratory test procedures and instruments, and must include adequate identification and handling of all test samples (§ 606.140(b), (c)). Use of supplies and reagents must be in a manner consistent with the instructions provided by the manufacturer (§§ 606.65(e), 610.40(b)). In addition, as part of an overall Quality Assurance program, we recommend that you conduct additional investigation to determine the cause of the initial reactivity of the Master Pool.
- If one (or more) of the subpools is (are) Reactive, you may release from quarantine the individual donations that comprise the Non-Reactive subpools (if serologic tests on those donor samples are Negative and the donations are otherwise suitable for release). Consistent with the manufacturer's instructions, you must test the individual donor samples that comprise the Reactive subpool using the same Multiplex NAT method that was used in the original NAT on the Master Pool (§ 610.40(b)) (Ref. 13).
(1) If all individual donor samples are Non-Reactive, you may release from quarantine all individual donations (if serologic tests on those donor samples are Negative and the donations are otherwise suitable for release). However, you must investigate the unexplained discrepancy in testing (§ 211.192). Laboratory control procedures must make adequate provisions for monitoring the reliability, accuracy, precision, and performance of laboratory test procedures and instruments, and must include adequate identification and handling of all test samples (§ 606.140(b), (c)). Use of supplies and reagents must be in a manner consistent with the instructions provided by the manufacturer (§§ 606.65(e), 610.40(b)). In addition, as part of an overall Quality Assurance program, we recommend that you conduct additional investigation to determine the cause of the initial reactivity of the Master Pool.
(2) If one (or more) individual donor sample(s) is (are) Reactive, perform the steps in section IV.1.a. above.
You may release from quarantine all Non-Reactive individual donations (if serologic tests on those donor samples are Negative and the donations are otherwise suitable for release).
- Testing, Product Disposition, and Donor Management for a Master Pool that is Reactive on an Individual NAT: Resolution by Testing Subpools
If you obtain a Reactive result for a NAT for HIV-1 RNA and/or HCV RNA performed separately on a Master Pool, the test instructions for use instruct you to perform subsequent testing to identify the donor sample(s) that is (are) NAT-Reactive as the basis for the NAT-Reactive result on the pool. Deconstruction of the NAT-Reactive Master Pool may be performed by testing the subpools (original or freshly made), that formed the Master Pool. This deconstruction of the Master Pool to determine the basis for the reactivity may involve several layers of testing using original or freshly pooled subpools, followed by testing of individual donor samples in the Reactive subpool(s) (See Figure 6 and Table 6). You must follow the instructions in the package insert for a licensed NAT that provides a specific testing algorithm. (§ 610.40(b).)
- If you test subpools that were used to construct a NAT-Reactive Master Pool, consistent with the manufacturer's instructions you must test the original subpools or freshly pooled subpools using the same NAT method that was used in the original NAT on the Master Pool (§ 610.40(b)) (Ref. 13).
NOTE: In some cases the manufacturer's instructions provide for a different sample preparation procedure. However, the primers and probes would be the same as those used in the original NAT on the Master Pool.
- If all subpools are Non-Reactive, you may release from quarantine all individual donations that comprise the Non-Reactive subpools (if serologic tests on those donor samples are Negative and the donations are otherwise suitable for release). However, you must investigate the unexplained discrepancy in testing (§ 211.192) . Laboratory control procedures must make adequate provisions for monitoring the reliability, accuracy, precision, and performance of laboratory test procedures and instruments, and must include adequate identification and handling of all test samples (§ 606.140(b), (c)). Use of supplies and reagents must be in a manner consistent with the instructions provided by the manufacturer (§§ 606.65(e), 610.40(b)). In addition, as part of an overall Quality Assurance program, we recommend that you conduct additional investigation to determine the cause of the initial reactivity of the Master Pool.
- If one (or more) of the subpools is (are) Reactive, you may release from quarantine the individual donations that comprise the Non-Reactive subpools (if serologic tests on those donor samples are Negative and the donations are otherwise suitable for release). Consistent with the manufacturer's instructions, you must test the individual donations that comprise the Reactive subpool using the same NAT method that was used in the original NAT on the Master Pool (§ 610.40(b)) (Ref. 13).
(1) If all individual donor samples are Non-Reactive, you may release from quarantine all individual donations (if serologic tests on those donor samples are Negative and the donations are otherwise suitable for release). However, you must investigate the unexplained discrepancy in testing (§ 211.192). Laboratory control procedures must make adequate provisions for monitoring the reliability, accuracy, precision, and performance of laboratory test procedures and instruments, and must include adequate identification and handling of all test samples (§ 606.140(b), (c)). Use of supplies and reagents must be in a manner consistent with the instructions provided by the manufacturer (§§ 606.65(e), 610.40(b)). In addition, as part of an overall Quality Assurance program, we recommend that you to conduct additional investigation to determine the cause of the initial reactivity of the Master Pool.
(2) If one (or more) individual donor sample(s) is (are) Reactive, perform steps a-d in section IV.2. above.
You may release from quarantine all Non-Reactive individual donations (if serologic tests on those donor samples are Negative and the donations are otherwise suitable for release).
- Reentry for Donors Deferred Because of HIV-1 Test Results
Currently, FDA has not approved a process for reentry of donors with the following HIV-1 test results:
- NAT-Reactive for HIV-1 (either by a Discriminatory NAT after a Reactive Multiplex NAT or by a separate NAT for HIV-1 RNA) and anti-HIV-1/2 EIA Repeatedly Reactive (regardless of HIV-1 Western Blot or IFA or HIV-1 p24 EIA test result);
OR
- NAT-Reactive for HIV-1 (either by a Discriminatory NAT after a Reactive Multiplex NAT or by a separate NAT for HIV-1 RNA) and HIV-1 p24 EIA Repeatedly Reactive (regardless of anti-HIV-1/2 EIA test result);
OR
- NAT-Non-Reactive for HIV-1 (or HIV-1 NAT not performed) and anti-HIV-1/2 EIA Repeatedly Reactive, HIV-1 Western Blot Positive (regardless of HIV-1 p24 EIA test result).
FDA has approved a method or process for reentry of deferred donors in the following classes:
- Donors who were NAT-Reactive and seronegative. This includes donors previously deferred because of Reactive test results on an investigational HIV-1 NAT. The HIV-1 p24 antigen EIA may not have been performed if it was replaced by an approved NAT that was validated to replace the HIV-1 p24 antigen test. The HIV-1 Discriminatory NAT may have been either Positive or Negative. If an Additional NAT for HIV-1 (validated for use with individual donor samples) was performed, it must have been Non-Reactive.
NOTE: If the original donation that was NAT-Reactive was Negative on the Discriminatory NAT for HIV-1 but was Positive on the Discriminatory NAT for HCV, you may attempt to reenter the donor according to the recommendations in section IV.8. (See Figure 8 and Table 8). If the original donor sample that was NAT-Reactive was Positive or Negative on both the Discriminatory NAT for HIV-1 and on the Discriminatory NAT for HCV, you may attempt to reenter the donor according to the recommendations in both sections IV.7 and IV.8 (See Figures 7 and 8, and Tables 7 and 8).
- Donors who were NAT-Non-Reactive (or NAT was not performed) and who were Repeatedly Reactive on a screening test for HIV-1 antibody, with an HIV-1 Western blot or IFA that was Negative (or was not performed), or an HIV-1 Western blot result that was Indeterminate (viral bands may be present). This includes donors previously deferred because of Repeatedly Reactive HIV serologic test results prior to the initiation of testing by NAT.
These donors may be eligible for reentry only if the HIV-1 p24 antigen EIA (if done) was Negative and if a second, different, licensed HIV-2 EIA was Negative, or, if the second HIV-2 EIA was Repeatedly Reactive, an investigational HIV-2 supplemental test was not Positive. Currently, we have not approved a supplemental (additional, more specific) test for HIV-2.
- Donors who were NAT Non-Reactive and who were Negative on a screening test for HIV-1 antibody, but who were Repeatedly Reactive on an HIV-1 p24 antigen EIA with a Positive or an Indeterminate (that is, an Invalid or a Non-Neutralized) result on the Neutralization test.
- To reenter a donor who meets FDA eligibility criteria (i.e., the donor is otherwise eligible to donate again), we recommend that you do the following (See Figure 7 and Table 7):
- At least 8 weeks after the original donation obtain a new sample from the donor (no donation is made at this time) and perform follow-up testing using:
(1) a licensed HIV-1 NAT that is the same as the NAT (i.e., the Discriminatory NAT for HIV-1) that was run on the original donor sample or a licensed HIV-1 NAT that is labeled as sensitive for HIV-1 group O and HIV-1 group M variants;
AND
(2) a licensed anti-HIV-1/2 EIA. If the original donor sample was Repeatedly Reactive on the anti-HIV-1/2 EIA, we recommend that you use that same EIA to test this follow-up sample. If the original donor sample was Negative on the anti-HIV-1/2 EIA, we recommend that you use an Alternate EIA that is labeled as sensitive for HIV-1 Group O.
NOTE: If you wish to perform follow-up testing on a donor who is deferred because of HIV-1 test results, you may do so prior to the end of this 8-week waiting period for donor notification purposes or for medical reasons. Negative results on a follow-up HIV-1 test conducted before the 8 week period ends may be useful in donor counseling. However, only a Negative screening test result obtained at least 8 weeks after the NAT-Reactive or Repeatedly Reactive anti-HIV-1/2 or HIV-1 p24 EIA test result would qualify the donor for reentry. If you again obtain a Reactive NAT or a Repeatedly Reactive anti-HIV-1/2 EIA result during this 8-week waiting period, the donor would not be eligible for reentry and we recommend that you defer the donor permanently.
- Evaluate the results of the follow-up testing on the donor's new sample as follows:
(1) If the NAT is Reactive and the anti-HIV-1/2 EIA is Repeatedly Reactive, we recommend that you defer the donor permanently.
(2) If the NAT is Reactive and the anti-HIV-1/2 EIA is Negative, we recommend that you defer the donor permanently.
(3) If the NAT is Non-Reactive and the anti-HIV-1/2 EIA is Repeatedly Reactive, you may reconsider the donor for reentry by additional follow-up testing after a second waiting period of 8 weeks.
When there is a persistent anti-HIV-1/2 EIA Repeatedly Reactive result, you may wish to further test the donor's new sample using an HIV-1 Western Blot. If the Western Blot test result is Negative, or an Indeterminate blot pattern has not progressed, you may reconsider the donor for reentry by additional follow-up testing after a second waiting period of 8 weeks. If the Western blot result is Positive, we recommend that you defer the donor permanently.
(4) If the NAT is Non-Reactive and the anti-HIV-1/2 EIA is Negative, you may reenter the donor (i.e., the donor is eligible to donate in the future, provided the donor meets all donor eligibility criteria).
- Reentry for Donors Deferred Because of HCV Test Results
Currently, FDA has not approved a process for reentry of donors with the following HCV test results:
- NAT-Reactive for HCV (either by a Discriminatory NAT after a Reactive Multiplex NAT or by a separate NAT for HCV RNA) and anti-HCV EIA Repeatedly Reactive (regardless of HCV RIBA test result).
OR
- NAT-Non-Reactive for HCV (or HCV NAT not performed) and anti-HCV EIA Repeatedly Reactive, HCV RIBA Positive.
FDA has approved a method or a process for reentry of deferred donors in the following classes:
- Donors who were NAT-Reactive and seronegative. This includes donors previously deferred because of Reactive test results on an investigational HCV NAT. The HCV Discriminatory NAT may have been either Positive or Negative. If an Additional NAT for HCV (validated for use with individual donor samples) was performed, it must have been Non-Reactive.
NOTE: If the original donor sample that was NAT-Reactive was Negative on the Discriminatory NAT for HCV but was Positive on the Discriminatory NAT for HIV-1, you may attempt to reenter the donor according to the recommendations in section IV.7. (See Figure 7 and Table 7). If the original donor sample that was NAT-Reactive was Positive or Negative on both the Discriminatory NAT for HCV and on the Discriminatory NAT for HIV-1, you may attempt to reenter the donor according to the recommendations in both sections IV.7 and IV.8 (See Figures 7 and 8 and Tables 7 and 8).
- Donors who were NAT-Non-Reactive (or NAT was not performed) and who were Repeatedly Reactive on a screening test for HCV antibody, with an HCV RIBA that was Indeterminate or Negative (or was not performed). This includes donors previously deferred because of Repeatedly Reactive HCV serologic test results prior to the initiation of testing by NAT.
- To reenter a donor who meets FDA eligibility criteria (i.e., the donor is otherwise eligible to donate again), we recommend that you do the following (See Figure 8 and Table 8):
- At least 6 months after the original donation obtain a new sample from the donor (no donation is made at this time) and perform follow-up testing using:
(1) A licensed HCV NAT
AND
(2) A licensed anti-HCV EIA.
NOTE: If you wish to perform follow-up testing on a donor who is deferred because of HCV test results, you may do so prior to the end of this 6-month waiting period for donor notification purposes or for medical reasons. Negative results on a follow-up HCV test conducted before the 6-month period ends may be useful in donor counseling. However, only a Negative screening test result obtained at least 6 months after the NAT-Reactive or Repeatedly Reactive anti-HCV test result would qualify the donor for reentry. If you again obtain a Reactive NAT or a Repeatedly Reactive anti-HCV EIA result during this 6-month waiting period, the donor would not be eligible for reentry and we recommend that you defer the donor permanently.
- Evaluate the results of the follow-up testing on the donor's new sample as follows:
(1) If the NAT is Reactive and the anti-HCV EIA is Repeatedly Reactive, we recommend that you defer the donor permanently.
(2) If the NAT is Reactive and the anti-HCV EIA is Negative, we recommend that you defer the donor permanently.
(3) If the NAT is Non-Reactive and the anti-HCV EIA is Repeatedly Reactive, you may reconsider the donor for reentry by additional follow-up testing after a second waiting period of 6 months.
When there is a persistent anti-HCV EIA Repeatedly Reactive result, you may wish to further test the donor's new sample using an HCV RIBA. If the RIBA test result is Negative, you may reconsider the donor for reentry by additional follow-up testing after a second waiting period of 6 months. If the RIBA test result is Positive or Indeterminate, we recommend that you defer the donor permanently.
(4) If the NAT is Non-Reactive and the anti-HCV EIA is Negative, you may reenter the donor (i.e., the donor is eligible to donate in the future, provided the donor meets all donor eligibility criteria).
Table of Contents

- IMPLEMENTATION
This guidance is being distributed for comment purposes only.
Table of Contents

- REFERENCES
- Busch MP. Closing the windows on viral transmission by blood transfusion. In Stramer SL ed. Blood Safety in the New Millenium. Bethesda, MD: American Association of Blood Banks, 2001:Chapter 2, p.36.
- Glynn SA, Kleinman SH, Wright DJ, Busch MP. International application of the incidence rate/window period model. Transfusion 42:966-972 (2002).
- Dodd RY, Notari EP, Stramer SL. Current prevalence and incidence of infectious disease markers and estimated window period risk in the American Red Cross blood donor population. Transfusion 42:975-979 (2002).
- Fiebig EW, Wright DJ, Rawal BD, et. al. Dynamics of HIV-1 viremia and antibody seroconversion in plasma donors: Implications for diagnosis and staging of primary HIV-1 infection. AIDS 17:1871-1879 (2003).
- FDA Memorandum to All Registered Blood Establishments: "Revised Recommendations for the Prevention of Human Immunodeficiency Virus (HIV-1) Transmission by Blood and Blood Products," April 23, 1992.
- FDA Memorandum to All Registered Blood and Plasma Establishments: "Recommendations for Donor Screening with a Licensed Test for HIV-1 Antigen," August 8, 1995.
- FDA Memorandum to All Registered Blood Establishments: "Revised Recommendations for Testing Whole Blood, Blood Components, Source Plasma and Source Leukocytes for Antibody to Hepatitis C Virus Encoded Antigen (Anti-HCV)," August 5, 1993.
- Federal Register, 11/16/00 (65 FR 69378), Proposed Rule: Current Good Manufacturing Practice for Blood and Blood Components; Notification of Consignees and Transfusion Recipients Receiving Blood and Blood Components at Increased Risk of Transmitting HCV Infection ("Lookback")
- Federal Register, 12/14/99 (64 FR 71147), Guidance for Industry: In the Manufacture and Clinical Evaluation of In Vitro Tests to Detect Nucleic Acid Sequences of Human Immunodeficiency Viruses Types 1 and 2, December 1999.
- Blood Products Advisory Committee, 69th Meeting, June 14, 2001, http://www.fda.gov/ohrms/dockets/ac/cber01.htm-Blood Products Advisory Committee.
- Alter HJ. To C or not to C: These are the questions. Blood 85:1681-1695 (1995).
- CDC, Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR 47, (RR-19) (1998).
- See 21 CFR 610.40(b) for licensed test kits or 21 CFR 601.20(a) for licensed in-house assays.
Table of Contents

FIGURE 1. TESTING, PRODUCT DISPOSITION, AND DONOR MANAGEMENT FOR AN INDIVIDUAL DONOR SAMPLE THAT IS REACTIVE ON A MULTIPLEX NAT AFTER A NEGATIVE ANTIBODY SCREENING TEST
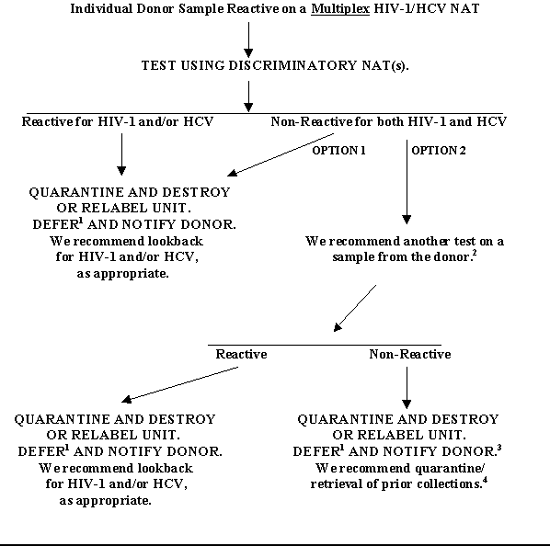
1 The donor may be eligible for reentry (See Figures 7 and 8).
2 If you test a new sample from the original donation, you may use the original NAT or Discriminatory NAT(s) or an Additional NAT. Alternatively, you may test the same sample as in the previous NAT tests (e.g., using an Additional NAT).
3 You may explain to the donor that the test result, while initially Reactive, is not conclusive. There is a slight risk that the initial test result was a Positive result that cannot be excluded without follow-up testing of the donor.
4 We do not recommend that you notify transfusion recipients.
Table of Contents

FIGURE 2. TESTING, PRODUCT DISPOSITION, AND DONOR MANAGEMENT FOR AN INDIVIDUAL DONOR SAMPLE THAT IS REACTIVE ON AN INDIVIDUAL NAT AFTER A NEGATIVE ANTIBODY SCREENING TEST

We recommend lookback for HIV-1 and/or HCV, as appropriate.
1 The donor may be eligible for reentry (See Figures 7 and 8.)
Table of Contents

FIGURE 3. TESTING, PRODUCT DISPOSITION, AND DONOR MANAGEMENT FOR A MASTER POOL THAT IS REACTIVE ON A MULTIPLEX NAT: RESOLUTION BY TESTING INDIVIDUAL DONOR SAMPLES
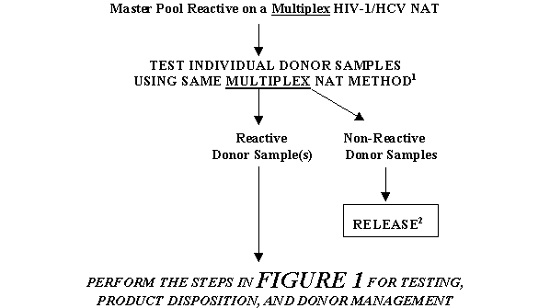
1 In some cases a different sample preparation procedure may be used per manufacturer's instructions. However, primers and probes should be same as those used in the NAT on Master Pool.
2 Units may be released only if serologic tests for HIV-1 and HCV are Negative and the units are otherwise suitable for release.
Table of Contents

FIGURE 4. TESTING, PRODUCT DISPOSITION, AND DONOR MANAGEMENT FOR A MASTER POOL THAT IS REACTIVE ON AN INDIVIDUAL NAT: RESOLUTION BY TESTING INDIVIDUAL DONOR SAMPLES
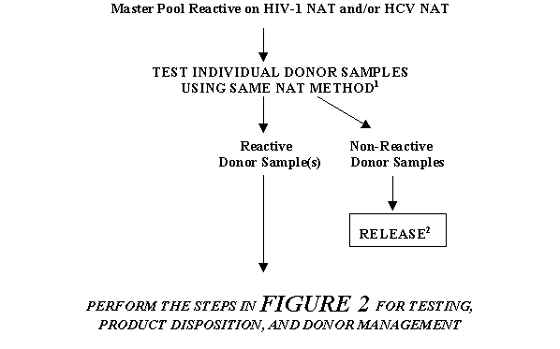
1 In some cases a different sample preparation procedure may be used per manufacturer's instructions. However, primers and probes should be same as those used in the NAT on Master Pool.
2 Units may be released only if serologic tests for HIV-1 and HCV are Negative and the units are otherwise suitable for release.
Table of Contents

FIGURE 5. TESTING, PRODUCT DISPOSITION, AND DONOR MANAGEMENT FOR A MASTER POOL THAT IS REACTIVE ON A MULTIPLEX NAT: RESOLUTION BY TESTING SUBPOOLS
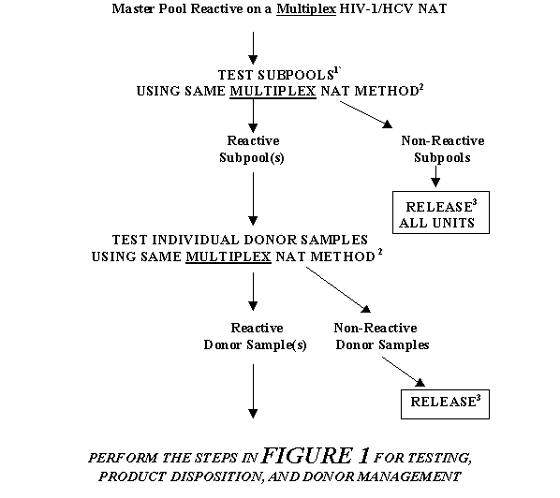
1Can be several layers of deconstruction using original or freshly pooled Subpools.
2 In some cases a different sample preparation procedure may be used per manufacturer's instructions. However, primers and probes should be same as those used in the NAT on Master Pool.
3 Units may be released only if serologic tests for HIV-1 and HCV are Negative and the units are otherwise suitable for release.
Table of Contents

FIGURE 6. TESTING, PRODUCT DISPOSITION, AND DONOR MANAGEMENT FOR A MASTER POOL THAT IS REACTIVE ON AN INDIVIDUAL NAT: RESOLUTION BY TESTING SUBPOOLS
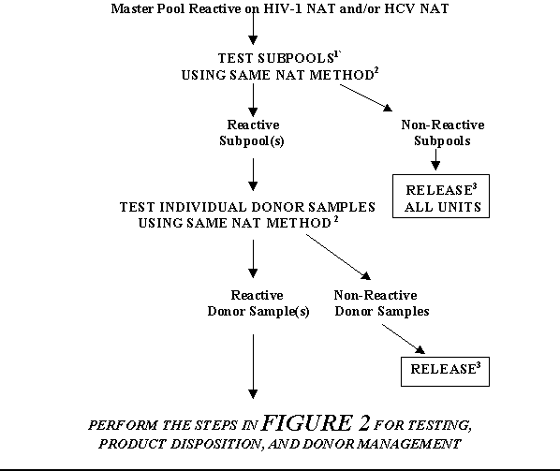
1Can be several layers of deconstruction using original or freshly pooled Subpools.
2 In some cases a different sample preparation procedure may be used per manufacturer's instructions. However, primers and probes should be same as those used in the NAT on Master Pool.
3 Units may be released only if serologic tests for HIV-1 and HCV are Negative and the units are otherwise suitable for release.
Table of Contents

FIGURE 7. REENTRY FOR DONORS DEFERRED BECAUSE OF HIV-1 TEST RESULTS
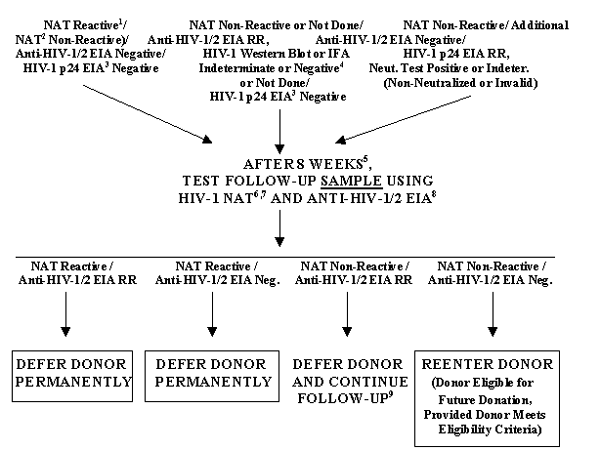
1 HIV-1 Discriminatory NAT may be Positive or Negative; however, if Negative and if HCV Discriminatory NAT is Positive, use HCV Reentry Algorithm only (See Figure 8).
2 An Additional NAT that has been validated for use with individual donor samples.
3 May not have been performed, depending upon conditions of specific NAT approval.
4 If a second, different, licensed HIV-2 EIA was Negative or, if Repeatedly Reactive, an investigational HIV-2 Supplemental Test was not Positive.
5 HIV-1 NAT and/or anti-HIV-1/2 EIA, if performed prior to 8 weeks, must be Negative.
6 If the original donor sample was Non-Discriminated using Discriminatory NAT for HIV-1 and HCV or was Positive on both of the Discriminatory NAT tests, test a follow-up sample using HCV NAT and Anti-HCV EIA also, as in HCV Reentry Algorithm (See Figure 8).
7 Using the same NAT (i.e., the Discriminatory NAT for HIV-1) or a NAT labeled as sensitive for HIV-1 Group O and HIV-1 Group M variants.
8 If the original donor sample was Repeatedly Reactive on the anti-HIV-1/2 EIA, we recommend that you use that same EIA to test this follow-up sample. If the original donor sample was Negative on the anti-HIV-1/2 EIA, we recommend that you use an Alternate EIA that is labeled as sensitive for HIV-1 Group O.
9 At your option you may further test the donor's sample using HIV-1 Western Blot. If Western Blot is Negative, or if an Indeterminate blot pattern has not progressed, you may reconsider the donor for reentry by additional follow-up testing after a second waiting period of 8 weeks. If Western Blot is Positive, defer the donor permanently.
Table of Contents

FIGURE 8. REENTRY FOR DONORS DEFERRED BECAUSE OF HCV TEST RESULTS
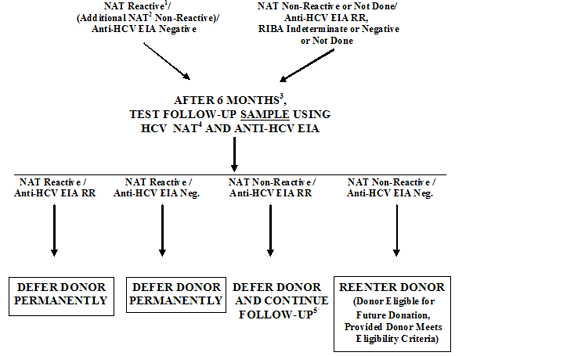
1HCV Discriminatory NAT may be Positive or Negative; however, if Negative and if HIV-1 Discriminatory NAT is Positive, use HIV-1 Reentry Algorithm only (See Figure 7).
2 An Additional NAT that has been validated for use with individual donor samples.
3 HCV NAT and/or anti-HCV EIA, if performed prior to 6 months, must be Negative.
4 If the original donor sample was Non-Discriminated using Discriminatory NAT for HIV-1 and HCV or was Positive on both of the Discriminatory NAT tests, test a follow-up sample using HIV-1 NAT and Anti-HIV-1/2 EIA also, as in HIV-1 Reentry Algorithm (See Figure 7).
5 At your option you may further test the donor's sample using HCV RIBA. If RIBA is Negative, you may reconsider the donor for reentry by additional follow-up testing after a second waiting period of 6 months. If RIBA is Positive or Indeterminate, defer the donor permanently.
Table of Contents

TABLE 1. TESTING, PRODUCT DISPOSITION, AND DONOR MANAGEMENT FOR AN INDIVIDUAL DONOR SAMPLE THAT IS REACTIVE ON A MULTIPLEX NAT AFTER A NEGATIVE ANTIBODY SCREENING TEST
| If: |
Then: |
After that if: |
Then: |
After that if: |
Then: |
Individual Donor Sample Reactive on a Multiplex
HIV-1/HCV NAT |
Test the sample using Discriminatory NAT(s) |
Reactive for HIV-1 and/or HCV |
Quarantine and destroy or relabel unit; defer1 and notify donor; we recommend lookback for HIV-1 and/or HCV as appropriate |
|
|
| Non-Reactive for both HIV-1 and HCV |
Quarantine and destroy or relabel unit; defer1 and notify donor; we recommend lookback for HIV-1 and/or HCV as appropriate |
|
|
OR:
We recommend another test2 on a sample from the donor. |
Another test is Reactive |
Quarantine and destroy or relabel unit; defer1 and notify donor; we recommend lookback for HIV-1 and/or HCV as appropriate |
| Another test is Non-Reactive |
Quarantine and destroy or relabel unit; defer1 and notify donor3; We recommend quarantine/
retrieval of prior collections4 |
1 The donor may be eligible for reentry (See Figures 7 and 8).
2 If you test a new sample from the original donation, you may use the original NAT or Discriminatory NAT(s) or an Additional NAT. Alternatively, you may test the same sample as in the previous NAT tests (e.g., using an Additional NAT).
3 You may explain to the donor that the test result, while initially Reactive, is not conclusive. There is a slight risk that the initial test result was a Positive result that cannot be excluded without follow-up testing of the donor.
4 We do not recommend that you notify transfusion recipients.
Table of Contents

TABLE 2. TESTING, PRODUCT DISPOSITION, AND DONOR MANAGEMENT FOR AN INDIVIDUAL DONOR SAMPLE THAT IS REACTIVE ON AN INDIVIDUAL NAT AFTER A NEGATIVE ANTIBODY SCREENING TEST
| If: |
Then: |
| Individual Donor Sample Reactive on HIV-1 NAT and/or HCV NAT |
Quarantine the unit |
| Destroy or relabel the unit |
| Defer the donor1 |
| Notify the donor |
| We recommend lookback for HIV-1 and/or HCV, as appropriate |
1The donor may be eligible for reentry (See Figures 7 and 8).
Table of Contents

TABLE 3. TESTING, PRODUCT DISPOSITION, AND DONOR MANAGEMENT FOR A MASTER POOL THAT IS REACTIVE ON A MULTIPLEX NAT: RESOLUTION BY TESTING INDIVIDUAL DONOR SAMPLES
| If: |
Then: |
After that if: |
Then: |
| Master Pool Reactive on a Multiplex HIV-1/HCV NAT |
Test the individual donor samples using same Multiplex NAT method1 |
Reactive donor sample(s) |
Perform the steps in Table 1 for Testing, Product Disposition, and Donor Management |
| Non-Reactive donor samples |
Release2
|
1In some cases a different sample preparation procedure may be used per manufacturer's instructions. However, primers and probes should be same as those used in NAT on Master Pool.
2Units may be released only if serologic tests for HIV-1 and HCV are Negative and the units are otherwise suitable for release.
Table of Contents

TABLE 4. TESTING, PRODUCT DISPOSITION, AND DONOR MANAGEMENT FOR A MASTER POOL THAT IS REACTIVE ON AN INDIVIDUAL NAT: RESOLUTION BY TESTING INDIVIDUAL DONOR SAMPLES
| If: |
Then: |
After that if: |
Then: |
| Master Pool Reactive on HIV-1 NAT and/or HCV NAT |
Test the individual donor samples using same NAT method1 |
Reactive donor sample(s) |
Perform the steps in Table 2 for Testing, Product Disposition, and Donor Management |
| Non-Reactive donor samples |
Release2 |
1 In some cases a different sample preparation procedure may be used per manufacturer's instructions. However, primers and probes should be same as those used in NAT on Master Pool.
2Units may be released only if serologic tests for HIV-1 and HCV are Negative and the units are otherwise suitable for release.
Table of Contents

TABLE 5. TESTING, PRODUCT DISPOSITION, AND DONOR MANAGEMENT FOR A MASTER POOL THAT IS REACTIVE ON A MULTIPLEX NAT: RESOLUTION BY TESTING SUBPOOLS
| If: |
Then: |
After that if: |
Then: |
After that if: |
Then: |
Master Pool Reactive on a Multiplex
HIV-1/HCV NAT |
Test subpools1 using same Multiplex NAT method2 |
Reactive subpool(s) |
Test the individual donor samples using same Multiplex NAT method2 |
Reactive donor sample(s) |
Perform the steps in Table 1 for Testing, Product Disposition, and Donor Management |
| Non-Reactive Donor samples |
Release3 |
| Non-Reactive subpool(s) |
Release all units3 |
|
|
1 Can be several layers of deconstruction using original or freshly pooled Subpools.
2 In some cases a different sample preparation procedure may be used per manufacturer's instructions. However, primers and probes should be same as those used in the NAT on Master Pool.
3 Units may be released only if serologic tests for HIV-1 and HCV are Negative and the units are otherwise suitable for release.
Table of Contents

TABLE 6. TESTING, PRODUCT DISPOSITION, AND DONOR MANAGEMENT FOR A MASTER POOL THAT IS REACTIVE ON AN INDIVIDUAL NAT: RESOLUTION BY TESTING SUBPOOLS
| If: |
Then: |
After that if: |
Then: |
After that if: |
Then: |
| Master Pool Reactive on HIV-1 NAT and/or HCV NAT |
Test subpools1 using same NAT method2 |
Reactive Subpool(s) |
Test the individual donor samples using same NAT Method2 |
Reactive donor sample(s) |
Perform the steps in Table 2 for Testing, Product Disposition, and Donor Management |
| Non-Reactive donor samples |
Release3 |
| Non-Reactive Subpools |
Release all units3 |
|
|
1 Can be several layers of deconstruction using original or freshly pooled Subpools.
2 In some cases a different sample preparation procedure may be used per manufacturer's instructions. However, primers and probes should be same as those used in the NAT on Master Pool.
3 Units may be released only if serologic tests for HIV-1 and HCV are Negative and the units are otherwise suitable for release.
Table of Contents

TABLE 7. REENTRY FOR DONORS DEFERRED BECAUSE OF HIV-1 TEST RESULTS
| If: |
Then: |
After that if: |
Then: |
NAT Reactive1 /
(Additional NAT2 Non-Reactive)/
Anti-HIV-1/2 EIA Negative/
HIV-1 p24 EIA3 Negative
OR
NAT Non-Reactive or Not Done/
Anti-HIV-1/2 EIA RR, HIV-1 WB or IFA Indeterminate or Negative4 or Not Done/HIV-1
p24 EIA3 Negative
OR
NAT Non-Reactive/
Anti-HIV-1/2 EIA Negative/
HIV-1 p24 EIA RR, Neut.Test Positive or Indeterminate (Non-Neutralized or Invalid)
|
After 8 weeks5 test follow-up sample using HIV-1 NAT6,7 and Anti-HIV-1/2 EIA8 |
NAT Reactive/Anti-HIV-1/2 EIA RR |
Defer donor permanently |
NAT Reactive/Anti-
HIV-1/2 EIA Negative |
Defer donor permanently |
NAT Non-Reactive/Anti-
HIV-1/2 EIA RR |
Defer donor and continue follow-up9 |
| NAT Non-Reactive/Anti-HIV-1/2 Negative |
REENTER DONOR (Donor eligible for future donation, provided donor meets eligibility criteria)
|
1HIV-1 Discriminatory NAT may be Positive or Negative; however, if Negative and if HCV Discriminatory NAT is Positive, use HCV Reentry Algorithm only (See Table 8).
2An Additional NAT that has been validated for use with individual donations.
3 May not have been performed, depending upon conditions of specific NAT approval.
4If a second, different, licensed HIV-2 EIA was Negative or, if Repeatedly Reactive, an investigational HIV-2 Supplemental Test was not Positive.
5HIV-1 NAT and/or anti-HIV-1/2 EIA, if performed prior to 8 weeks, must be Negative.
6If the original donor sample was Non-Discriminated using Discriminatory NAT for HIV-1 and HCV or was Positive on both of the Discriminatory NAT tests, test a follow-up sample using HCV NAT and Anti-HCV EIA also, as in HCV Reentry Algorithm (See Table 8).
7Using the same NAT (i.e., the Discriminatory NAT for HIV-1) or a NAT labeled as sensitive for HIV-1 Group O and HIV-1 Group M variants.
8If the original donor sample was Repeatedly Reactive on the anti-HIV-1/2 EIA, we recommend that you use that same EIA to test this follow-up sample. If the original donor sample was Negative on the anti-HIV-1/2 EIA, we recommend that you use an Alternate EIA that is labeled as sensitive for HIV-1 Group O.
9At your option you may further test the donor's sample using HIV-1 Western Blot. If Western Blot is Negative, or if an Indeterminate blot pattern has not progressed, you may reconsider the donor for reentry by additional follow-up testing after a second waiting period of 8 weeks. If Western Blot is Positive, defer the donor permanently.
Table of Contents

TABLE 8. REENTRY FOR DONORS DEFERRED BECAUSE OF HCV TEST RESULTS
| If: |
Then: |
After that if: |
Then: |
NAT Reactive1/
(Additional NAT2 Non-Reactive)/
Anti-HCV EIA Negative
OR
NAT Non-Reactive or Not Done/
Anti-HCV EIA RR,
RIBA Indeterminate or
Negative or Not Done |
After 6 months3 test follow-up sample using HCV NAT4 and Anti-HCV EIA |
NAT Reactive/ Anti-HCV EIA RR |
Defer donor permanently |
| NAT Reactive/Anti-HCV EIA Negative |
Defer donor permanently |
| NAT Non-Reactive/Anti-HCV EIA RR |
Defer donor and continue follow-up5 |
| NAT Non Reactive/ Anti-HCV EIA Negative |
REENTER DONOR (Donor eligible for future donations, provided donor meets eligibility criteria)
|
1 HCV Discriminatory NAT may be Positive or Negative; however, if Negative and if HIV-1 Discriminatory NAT is Positive, use HIV-1 Reentry Algorithm only (See Table 7).
2 An Additional NAT that has been validated for use with individual donations.
3 HCV NAT and/or anti-HCV EIA, if performed prior to 6 months, must be Negative.
4 If the original donor sample was Non-Discriminated using Discriminatory NAT for HIV-1 and HCV or was Positive on both of the Discriminatory NAT tests, test a follow-up sample using HIV-1 NAT and Anti-HIV-1/2 EIA also, as in HIV-1 Reentry Algorithm (See Table 7).
5 At your option you may further test the donor's sample using HCV RIBA. If RIBA is Negative, you may reconsider the donor for reentry by additional follow-up testing after a second waiting period of 6 months. If RIBA is Positive or Indeterminate, defer the donor permanently.
Table of Contents
|