


   |
| FDA Home Page | CDRH Home Page | Search | A-Z Index |  |
|
The information collection provisions in this guidance have been approved under OMB control number 0910-0553. This approval expires 10/31/2007. An agency may not conduct or sponsor, and a person is not required to respond to, a collection of information unless it displays a currently valid OMB number.
For questions regarding this document contact Paula Silberberg, Office of Communication, Education and Radiation Programs, Center for Devices and Radiological Health, 240-276-3234; Terri Garvin, Office of In Vitro Diagnostic Devices Evaluation and Safety, Center for Devices and Radiological Health, 240-276-1326; or Sheryl Kochman, Office of Blood Research and Review, Center for Biologics Evaluation and Research, 301-827-6123.
 |
 |
U.S. Department of Health and Human Services Center for Devices and Radiological Health |
Written comments and suggestions may be submitted at any time for Agency consideration to the Division of Dockets Management, Food and Drug Administration, 5630 Fishers Lane, Room 1061, (HFA-305), Rockville, MD, 20852.
Alternatively, electronic comments may be submitted to http://www.fda.gov/dockets/ecomments. When submitting comments, please refer to Docket No. 2003D-0383. Comments may not be acted upon by the Agency until the document is next revised or updated.
Additional copies are available from the Internet at: http://www.fda.gov/cdrh/ocd/guidance/4444.pdf, or http://www.fda.gov/cber/guidelines.htm.
To receive this document by fax, call the CDRH Facts-On-Demand system at 800-899-0381 or 301-827-0111 from a touch-tone telephone. Press 1 to enter the system. At the second voice prompt, press 1 to order a document. Enter the document number (4444) followed by the pound sign (#). Follow the remaining voice prompts to complete your request.
Or, you may contact:
Office of Communication, Training, and
Manufacturers Assistance, HFM-40
Center for Biologics Evaluation and Research
Food and Drug Administration
1401 Rockville Pike, Rockville, MD 20852-1448
Phone: 800-835-4709 or 301-827-1800
Introduction
Background
Legal Considerations
FDA Recognizes Selected ISO 15223 and EN 980 Medical Device Symbols
For In Vitro Diagnostic Devices
For Professional Labels and Labeling
Glossary of Terms
Educational Outreach
Application of Guidance to Other Symbols
Implementation
Contains Nonbinding Recommendations
| This guidance represents the Food and Drug Administration's (FDA's) current thinking on this topic. It does not create or confer any rights for or on any person and does not operate to bind FDA or the public. You can use an alternative approach if the approach satisfies the requirements of the applicable statutes and regulations. If you want to discuss an alternative approach, contact the FDA staff responsible for implementing this guidance. If you cannot identify the appropriate FDA staff, call the appropriate number listed on the title page of this guidance. |
This document provides guidance on the use of selected symbols in place of text to convey some of the information required for in vitro diagnostic devices (IVDs) intended for professional use by 21 CFR 809.10, FDA’s labeling requirements for in vitro diagnostic devices, and 21 CFR parts 610 and 660, FDA’s labeling requirements for biologics (including IVDs) that are licensed under the Public Health Service (PHS) Act. These recommendations apply to the use of symbols on the labels and in labeling only of IVDs intended for professional use, and not for over-the-counter or prescription home-use IVDs. This guidance does not address the use of “unique and generally recognized” symbols to identify the manufacturer of a device, as described in Section 502(u) of the Food, Drug and Cosmetic Act.
Note: The term “symbols” used throughout this guidance refers to the use of graphical symbols without equivalent accompanying text.
This guidance is intended for both industry and FDA.
FDA's guidance documents, including this guidance, do not establish legally enforceable responsibilities. Instead, guidances describe the Agency's current thinking on a topic and should be viewed only as recommendations, unless specific regulatory or statutory requirements are cited. The use of the word should in Agency guidances means that something is suggested or recommended, but not required.
The issues identified in this guidance document represent those that we believe should be addressed before your device can be marketed. In developing the guidance, we carefully considered the relevant statutory criteria for Agency decision-making. We also considered the burden that may be incurred in your attempt to follow the guidance and address the issues we have identified. We believe that we have considered the least burdensome approach to resolving the issues presented in the guidance document. If, however, you believe that there is a less burdensome way to address the issues, you should follow the procedures outlined in the “A Suggested Approach to Resolving Least Burdensome Issues” document. It is available on our Center web page at http://www.fda.gov/cdrh/modact/leastburdensome.html.
The market for in vitro diagnostic devices is international. European Union (EU) member countries have attempted to harmonize their national legislation governing IVDs through the European Union’s Directive on In Vitro Diagnostic Medical Devices, (Directive 98/79/EC) (“IVD Directive”). The EU’s IVD Directive went into full effect on December 8, 2003. As of that date, IVD products marketed in the EU must comply with the IVD Directive and bear the CE mark (mark showing that the product is certified for sale in the European community) to indicate compliance.
The EU’s IVD Directive and FDA regulations in 21 CFR 809.10, 21 CFR part 610, and 21 CFR part 660 all require substantial information to appear on the IVD itself and/or in its labeling. The IVD Directive specifically allows each EU member state to require that such information appear in its national language, so that a single IVD could be required to bear labeling in multiple languages in order to be sold in the EU. As an alternative, the IVD Directive encourages that, in place of text, IVDs use symbols from harmonized standards to convey the required information. Given that the use of national languages may be required by individual member states and that most IVDs and their packaging are quite small, the IVD Directive’s symbols provision represents an avenue through which manufacturers can achieve compliance in an international marketplace.
Similarly, the use of symbols helps IVD manufacturers to create uniform labels and labeling for the United States and European Union (and any other countries that may permit use of symbols from these international standards), instead of needing designated labels for each marketplace. Because symbols take up less space than the text for which they may substitute, the use of symbols promotes less crowded and more legible IVD labels. An additional advantage is that there are likely to be fewer labeling errors when using a single label, rather than having one set of labels for use in the United States and another set for use in the European Union. Of course, it is essential that the symbol convey the substance of the deleted text and be widely understood.
FDA regulations in 21 CFR 809.10 and 21 CFR parts 610 and 660 define information required to appear on the label and in labeling for IVDs marketed in the United States. These regulations specify the content of labels and labeling and the order in which this information should be presented. With a few exceptions, these regulations do not specify wording that manufacturers must use to meet these requirements.
Under Section 502(c) of the Food, Drug, and Cosmetic Act (the Act), a drug or device is misbranded “If any word, statement, or other information required by or under authority of this Act to appear on the label or labeling is not prominently placed thereon with such conspicuousness (as compared with other words, statements, designs, or devices, in the labeling) and in such terms as to render it likely to be read and understood by the ordinary individual under customary conditions of purchase and use." The information required by 21 CFR 809.10 and 21 CFR parts 610 and 660 thus must also meet the requirements of Section 502(c) of the Act.
For a symbol to be used to convey information required by the regulations, it must be a “term” that is “. . . likely to be read and understood by the ordinary individual under customary conditions of purchase and use.” This guidance addresses specific symbols from two harmonized international standards that FDA believes can satisfy this requirement. Thus, in accordance with section 514(c) of the Act, FDA recognizes these specific symbols for use on the label and in the labeling of certain IVDs. (See Federal Register of April 28, 2003 (68 FR 22391); corrected with respect to the extent of recognition by October 28, 2003 (68 FR 61448). In this context, we narrowly define the “ordinary user” as the professional user of IVDs. The “customary conditions of purchase and use” are within the laboratory environment. Through the process for recognizing international consensus standards, under section 514(c) of the Act, FDA received acceptable evidence in the form of a user comprehension study conducted in the United States that indicates that this target audience can understand labels and labeling that use the 25 symbols identified here in place of equivalent text.
Use of these symbols can also help to satisfy the “conspicuousness” requirement of section 502(c). As explained above, most IVD devices are small and have limited label space. By using symbols in place of some textual statements, manufacturers may enhance the legibility of labeling and thus improve the “conspicuousness” of required information. In the study submitted to FDA in support of recognition of these standard symbols, participants overwhelmingly favored the use of the tested symbols in place of the text equivalents.
This guidance document recognizes that the symbols for which comprehension data have been presented to, and accepted by, the FDA through the consensus standards recognition process may be used as terms to communicate to professional users of IVDs information required under 21 CFR 809.10 and 21 CFR parts 610 and 660.1 Manufacturers remain responsible for adhering to the substantive requirements of these provisions, and to all other labeling requirements under the Act and regulations.
As stated above, some provisions of 21 CFR 809.10 and 21 CFR parts 610 and 660 do specify particular labeling language. As a matter of enforcement discretion, FDA does not intend to object to the use of:
The symbol that represents “Authorized representative in the European Community” is not needed to fulfill the labeling requirements of 21 CFR 809.10 or 21 CFR parts 610 or 660, as U.S. regulations do not require this information to be provided. IVD manufacturers who wish to use this symbol on the label or labeling to be used in both the U.S. and European Union in order to fulfill European labeling requirements may do so as long as the use of this symbol does not violate other U.S. labeling requirements. For example, if the “Authorized representative in the European Community” symbol is used in a manner that interferes with the communication of information required by U.S. law, the device could be misbranded under section 502(c) of the Act.
__________________
1 21
CFR 801.15(c) says that a device is misbranded under section 502(c) of the
Act unless:
“All words, statements, and other information required by or under authority
of the act to appear on the label or labeling shall appear thereon in the English
language: Provided, however, That in the case of articles distributed
solely in the Commonwealth of Puerto Rico or in a Territory where the predominant
language is one other than English, the predominant language may be substituted
for English.” Because, as explained above, FDA concludes that the symbols
from international consensus standards recognized in this guidance document
can be understood by relevant users in the United States and thus satisfy the
underlying requirements of section 502(c), the Agency does not intend to interpret
their use to violate 21
CFR 801.15(c). This interpretation is consistent with international harmonization.
In accordance with the consensus standards recognition process, established by section 514(c) of the Act, in the Federal Register of April 28, 2003 (68 FR 22391), FDA published a notice recognizing certain standards for use in premarket reviews including the two standards listed below concerning the use of symbols in labeling. In the Federal Register of October 28, 2003 (68 FR 61448), FDA published a notice correcting the April 28th notice with respect to the extent of the recognition of the two standards. FDA recognizes 25 symbols for IVD devices for professional use from the following two international consensus standards:
The following chart displays the symbols with their definitions.
| Symbol | Used for |
Symbol | Used for |
|---|---|---|---|
 |
Do not reuse |  |
Use by YYYY-MM-DD or YYYY-MM |
 |
Batch code |  |
Serial number |
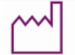 |
Date of manufacture |  |
Sterile |
 |
Sterilized using ethylene oxide |  |
Sterilized using irradiation |
 |
Sterilized using steam or dry heat |  |
Catalog number |
 |
Caution, consult accompanying documents |  |
Sterilized using aseptic processing technique |
 |
Manufacturer |  |
Authorized representative in the European Community |
 |
Contains sufficient for < n > tests | 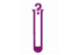 |
For IVD Performance Evaluation only |
 |
In vitro diagnostic medical device | 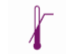 |
Upper limit of temperature |
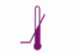 |
Lower limit of temperature | 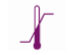 |
Temperature limitation |
 |
Consult instructions for use |  |
Biological risks |
 |
Control |  |
Negative control |
 |
Positive control | Graphic
symbols for use in labeling |
|
Validation data introduced in FDA’s consensus standards recognition process satisfies FDA that professional users of IVDs, the intended audience for this labeling, will understand the 25 symbols identified above. Specifically, industry-sponsored studies conducted in the United States with professional laboratory test users from a variety of educational backgrounds demonstrated end-user comprehension of the symbols in context, with an accompanying glossary and other educational outreach. Sections V and VI of this guidance explain the limitations of this recognition, which flow from the validation data.
FDA recognizes use of these symbols only for the labels and labeling of in vitro diagnostic devices. Recognition supports FDA’s efforts to harmonize its regulatory work with international standards-making bodies. Recognition is supported by the scope and results of the studies performed and submitted to FDA. FDA has not received reports of studies supporting use of any additional symbols on IVDs, nor supporting recognition of any symbols for use on other devices, for which ordinary users and conditions of purchase and use would differ from IVDs.
FDA recognizes these symbols only for use on the professional label and in the professional labeling of in vitro diagnostic devices. FDA does not recognize the symbols for use in the labels and labeling of over-the-counter or prescription home-use IVDs. Validation data introduced through FDA’s consensus standards recognition process supported the use of symbols for IVD professional labels and labeling, not for consumer labeling.
FDA recommends that a glossary of terms accompany each IVD to define all of the symbols used on that device’s labels and/or labeling. This glossary may also contain other symbols identified by FDA in its recognition of ISO 15223 and EN 980, whether or not used in labeling for the particular device. The glossary allows users to become familiar with the meaning of the symbols and also acts as a reference for users to look up any definitions they may not recall. In these respects, the glossary helps to satisfy the requirements of Section 502(c) of the Act by ensuring that IVD users, under customary conditions of use, have access to necessary reference materials, making it more likely that they can understand the symbols. Since users can keep the package insert readily accessible, FDA encourages the inclusion of the glossary in the package insert. The glossary accompanying the IVD may be a separate labeling piece in the form of a separate sheet of paper or card while IVD labeling is being updated and revised, but FDA recommends that to ensure ease of access, the glossary of symbols ultimately appear as part of the package insert accompanying the IVD.
FDA recommends that manufacturers conduct an educational outreach effort for the intended audience to enhance the understanding of newly introduced symbols. The educational outreach should target the various professional users of IVDs (e.g., laboratory technologists, nurses, laboratory assistants, medical assistants). FDA recommends the following possible methods for education:
The educational outreach should extend to all of those involved in the IVD distribution chain, such as wholesalers and distributors, who need to be aware of symbols relating to expiration date and storage requirements.
Such educational programs also help satisfy the requirements of Section 502(c) of the Act by creating an environment in which IVD users are likely to understand the symbols used.
FDA recommends that the manufacturers evaluate the educational outreach activities to determine if these activities are effective in educating IVD users to comprehend the meaning of the symbols.
CDRH will consider recognizing other symbols for use on the labels and in the labeling of IVDs through FDA’s consensus standards recognition process. For additional information about this process, refer to the web page for FDA’s Standards Program: http://www.fda.gov/cdrh/stdsprog.html.
For IVDs intended for professional use for which FDA has approved an application for Premarket Approval (PMA), manufacturers may change the labeling to include FDA recognized symbols in place of equivalent text without approval of PMA supplements. Other changes to labeling may require new PMA submissions, in accordance with 21 CFR 814.39(b). PMA holders that implement this type of change should notify the Agency of the change in the next annual report to the PMA, in accordance with 21 CFR 814.84. Similarly, biologics license holders that implement this type of change should notify the Agency of the change, which the Agency will consider an editorial or similar minor change, in the next annual report to the Manufacturers Biologics License Application (BLA), in accordance with 21 CFR 601.12(f)(3)(A). Manufacturers may substitute recognized symbols in place of equivalent text on existing labels and labeling for IVDs intended for professional use that received Premarket Notification [510(k)] clearance without submitting a new 510(k). (For information on other labeling changes that may require submission of a new 510(k), please see 21 CFR 807.81(a)(3), and FDA’s guidance document, "Deciding When to Submit a 510(k) for a Change to an Existing Device," available at http://www.fda.gov/cdrh/ode/510kmod.pdf.)
Manufacturers and importers should report to FDA any adverse events that arise from the use of symbols in labeling of IVDs intended for professional use, as required by 21 CFR part 803. Reporting forms and instructions are available at http://www.fda.gov/medwatch/safety.htm.
Updated November 18, 2004
![]()
CDRH Home Page | CDRH A-Z Index | Contact CDRH | Accessibility | Disclaimer
FDA Home Page | Search FDA Site | FDA A-Z Index | Contact FDA | HHS Home Page
Center for Devices and Radiological Health / CDRH