U.S. Food and Drug Administration
Performance Plan
2002
Ensure the safety of food and feed, and safety and effectiveness of drug, device, and biological products that are derived from biotechnology.
| Publish a final rule to require premarket notification for bioengineered foods. |
Biotechnology refers to the techniques that allow scientists to modify DNA, the genetic material of living things. From bioengineered corn, to drugs such as insulin, to gene therapy research, to diagnostic test kits, biotechnology is incorporated into almost all the product areas that FDA regulates.
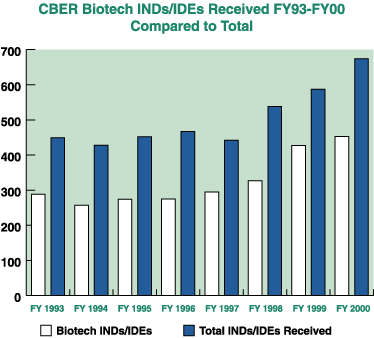
The following chart shows the increase in biologic biotech IND's/IDE's received by FDA from 1993 - 2000.
While drugs and biologics produced using biotechnology have been widely accepted by the public, that has not been entirely true for foods from bioengineered plants. There are also safety and ethical concerns regarding the use of cell and gene therapies. Without rigorous FDA oversight, the promises of gene therapy and biotech medicines will not be realized. Safety and effectiveness concerns may not be adequately addressed, and public confidence in bioengineered foods will not be assured.
Foods: Currently, FDA has a voluntary process through which companies marketing bioengineered foods and feeds consult with the Agency on safety and other regulatory issues. However, FDA has recently proposed a regulation that will make this process mandatory. Companies will be required to notify the Agency at least 120 days before marketing a new bioengineered food or feed, and to provide the Agency with sufficient data and other information to establish that the food or feed is as safe as its conventionally-derived counterparts. The proposed rule, if finalized, will ensure that FDA has the appropriate amount of information about bioengineered foods to help to ensure that all market entry decisions by the industry are made consistently and in full compliance with the law.
FDA also recently issued draft guidance on the voluntary labeling of foods indicating whether they have or have not been developed through bioengineering. The guidance will aid manufacturers in ensuring that their labeling is truthful and not misleading.
Gene Therapy: From 1989 to 1993, FDA received 48 gene therapy investigational new drug applications (INDs). In contrast, FDA received 265 gene therapy INDs from 1994 through 2000. Additionally, there have been over 800 amendments (changes to the product or new protocols, etc.) to these INDs submitted since FY 97. The Agency has yet to receive the first application to license a gene therapy product.
FDA is continually evaluating its review and oversight processes and has taken numerous steps to ensure better patient protection, such as issuing a Dear Gene Therapy IND Sponsor/Principal Investigator Letter and conducting workshops for gene therapy sponsors, investigators, and monitors to make them aware of reporting requirements. The Agency also has provided additional substantive guidance and standards to facilitate preparation of INDs through educational outreach conferences, meetings, and policy development.
Also, FDA and NIH together are committed to establish a gene therapy database that will support collection of short-and long-term effects of gene-transfer products that can be analyzed for safety trends.
Devices: Industry is researching and developing many types of biotechnology devices, and FDA is responsible for reviewing these devices for safety and efficacy. Some exciting new technologies include:
Animal Drugs: Genetically engineered animals fall into two product categories: Bio-Pharm, used to produce products such as tissues for harvest and use as medical products, and Ag-Biotech, improving animal health or productivity. FDA has taken the position that these products and the animals producing them are subject to pre-market approval as new animal drugs. FDA expects to prepare guidance on this issue this year. In addition, the Agency is contracting with the National Academy of Sciences/National Research Council to examine risks and risk assessment methods for animal biotechnology products.
Biotechnology offers many benefits and for these to be realized, FDA must continue to keep pace with the explosive growth of new science. State-of-the-art scientific expertise is essential for FDA to determine the safety and efficacy of these biotechnological products. Without this expertise, public health could be compromised and public confidence in these products will erode.
Foods: FDA believes no safety problem exists with any genetically engineered food or feed that is currently on the market. However, FDA has proposed requiring companies to provide the Agency with information prior to marketing foods and feeds because it expects that biotechnology methods likely will be used to an increasingly greater extent by plant breeders and that the products of this technology are likely in some cases to present more complex safety and regulatory issues than have been seen to date.
Approvals: FDA has approved about 130 drug and biologic biotechnology products since 1987, and has approved over 500 device biotechnology products in the last 10 years.
Vaccines: FDA conducts research to ensure the safety and efficacy of new technology-based products such as DNA plasmid vaccines. DNA vaccines have successfully prevented infection in a number of animal models, including flu, malaria, TB, herpes, anthrax, and others.
Gene Therapy: FDA has not yet approved any human gene therapy product for sale. However, FDA has received many requests from medical researchers and manufacturers to study gene therapy and to develop gene therapy products. Such research could lead to gene-based treatments for cancer, cystic fibrosis, heart disease, hemophilia, wounds, infectious diseases such as AIDS, and graft-versus-host disease.