 |
 |
 |
 |
FDA Home Page | Search FDA Site | FDA A-Z Index | Contact FDA
Printer-friendly version (60 KB PDF)
This guidance document is for comment purposes only.
Submit comments on this draft guidance by the date provided in the Federal Register notice announcing the availability of the draft guidance. Submit written comments to the Division of Dockets Management (HFA-305), Food and Drug Administration, 5630 Fishers Lane, Rm. 1061, Rockville, MD 20852. Submit electronic comments to http://www.fda.gov/dockets/ecomments. You should identify all comments with the docket number listed in the notice of availability that publishes in the Federal Register.
For questions on the content of this guidance, contact Office of Policy (Office of the Commissioner) at 301-827-3360.
U.S. Department of Health and Human Services
Food and Drug Administration
March 2007
Contains Nonbinding Recommendations
Draft – Not for Implementation
III. WHAT ARE THE GOALS AND PRINCIPLES OF THIS GUIDANCE?
IV. HOW DOES THIS ALGORITHM OPERATE?
A. Introduction
B. Step 1
C. Step 2
D. Step 3
E. Step 4a
F. Step 4b
G. Step 5
H. Step 6
APPENDIX 1: Algorithm for Considering Advisory Committee Member Participation
Contains Nonbinding Recommendations
Draft – Not for Implementation
This draft guidance, when finalized, will represent the Food and Drug Administration's (FDA's) current thinking on this topic. It does not create or confer any rights for or on any person and does not operate to bind FDA or the public. You can use an alternative approach if the approach satisfies the requirements of the applicable statutes and regulations. If you want to discuss an alternative approach, contact the appropriate FDA staff. If you cannot identify the appropriate FDA staff, call the appropriate number listed on the title page of this guidance.
This guidance document is intended for use by FDA staff ("you") involved with advisory committee matters. This guidance document describes the factors and analyses that you should consider in determining whether an advisory committee member has a potential conflict of interest and whether participation is appropriate. This guidance describes FDA's policy in applying the statutory and regulatory requirements found in 18 U.S.C 208(b)(1), 18 U.S.C. 208(b)(3), 21 U.S.C. 355(n)(4), 5 CFR 2640. This guidance applies to special government employees (SGEs) and regular government employees invited to participate in FDA advisory committees subject to the Federal Advisory Committee Act (FACA) (5 U.S.C. App. 2). For purposes of the guidance, we refer to such SGEs and regular government employees as advisory committee "members."
This guidance document replaces the "FDA Waiver Criteria 2000" guidance document.
FDA's advisory committees play an essential role in FDA's activities to protect and promote public health through the regulation of human and animal drugs, biological products, medical devices, and foods. FDA's advisory committees provide independent expert advice to the agency on scientific, technical, and policy matters related to the development and evaluation of FDA-regulated products. Advisory committees enhance FDA's ability to protect and promote public health by ensuring it has access to such advice in a manner as public as permitted by existing laws and regulations. Although advisory committees provide recommendations to FDA, FDA makes the final decisions.
FDA is committed to strictly adhering to the laws and regulations governing the process for selecting advisory committee members. FDA for many years has screened, prior to each meeting, all potential members who are SGEs or regular government employees, to determine whether the potential for a financial conflict of interest exists. The agency may grant a waiver allowing participation in an advisory committee meeting when statutory criteria are met; for example, the need for the individual's services outweighs the potential for a conflict of interest created by the financial interest involved (18 U.S.C. 208(b)(3)). However, because FDA's conflict of interest screening process is complex and has been poorly understood, the agency has been criticized in its application of the legal framework. Moreover, while many conflict of interest laws and regulations apply to advisory committees across the federal government, the public has a particular interest in and high expectations for FDA's process.
FDA administers several laws and regulations that govern conflict of interest determinations; these laws are not entirely consistent and set out different standards. For advisory committees that involve clinical investigation of, or marketing approval for, human drugs (including biological products), FDA is required to exclude from voting any member who could gain financially from the advice given by the advisory committee, or whose immediate family could gain financially, unless a waiver is necessary to afford the panel essential expertise (21 U.S.C. 355(n)). A waiver may not be granted when the member's own scientific work is involved. FDA must also apply the provisions of 18 U.S.C. 208(b)(1) or 208(b)(3) to these same committees, as well as to committees not covered by 21 U.S.C. 355(n). The test for a regular government employee who seeks to participate in an advisory committee meeting is whether the financial interest is "not so substantial as to be deemed likely to affect the integrity of the services which the Government may expect" from the employee (18 U.S.C. 208(b)(1)). However, in the case of an SGE, the test is whether the "need for the individual's services outweighs the potential for a conflict of interest created by the financial interest involved" (18 U.S.C. 208(b)(3)). The scope of the inquiry regarding financial interests is also broader under 18 U.S.C. 208(b). FDA must determine not only the financial interests of the employee and his immediate family members, but also those of the employee's business partner, organizations for which he serves as officer, director, trustee, general partner, or employee, and any prospective employer of the member. Finally, several regulations promulgated pursuant to 18 U.S.C. 208(b) further explain and delineate the parameters that FDA must apply in applying these statutes, as well as detailing certain exemptions to the conflict of interest prohibitions (see 5 CFR Part 2640).
FDA's Waiver Criteria 2000 guidance attempted to address this complex set of variables by setting out a series of tables indicating involvement levels and expected action that FDA advisory committee staff would take. The tables varied depending on the type of interest (e.g., stocks and investments, primary employment, consulting work, contracts and grants, patents/royalties/trademarks, expert witness work, teaching/speaking/writing, contracts/grants for department heads, and institutional directors), level of involvement (low, medium, or high), type of meeting (involving specific parties or matters of general applicability), as well as a number of other factors. In applying the tables, FDA staff also considered enumerated circumstances favoring the use of the member and additional criteria that would exclude a member.
The Waiver Criteria 2000 guidance was an attempt to address comprehensively the multiple variables that can be applied in reaching a determination about an individual advisory committee member. However, because of its complexity and discretionary elements, Centers and offices found it difficult to achieve consistent results that the public could readily understand.
As part of FDA's recent internal assessment of its advisory committee process, the agency has targeted its assessment of potential conflicts of interest and granting of waivers as an area that needs improvement. This guidance greatly simplifies and streamlines the process by which we determine meeting participation. FDA intends that this guidance increase the transparency, clarity, and consistency of the advisory committee process and enhance public trust in this important function.
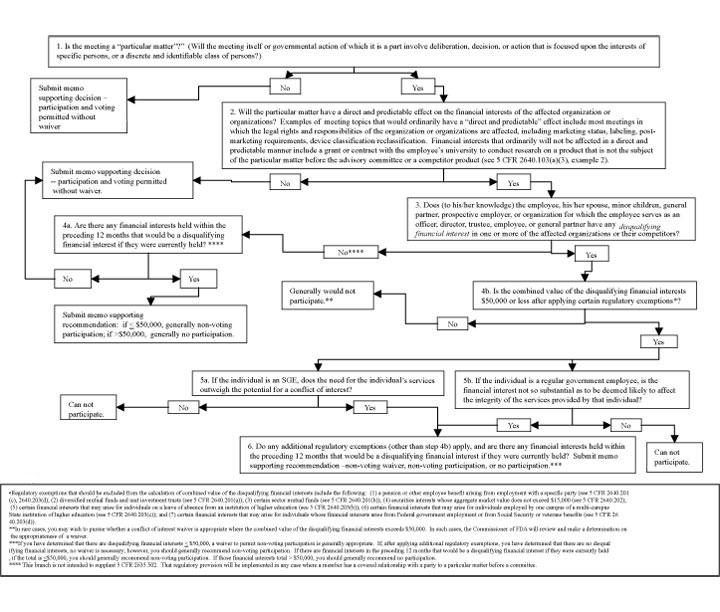
This guidance sets out a simple, streamlined approach for considering who may participate in an advisory committee meeting. As a policy matter, FDA is choosing to implement a more stringent policy for considering eligibility for participation than would be permitted under the current legal framework. Under this approach, participation of members with potential conflicts of interest generally would occur under narrow circumstances where the potential conflict is minimal and the member's expertise is needed for the committee's deliberations. The principal tool in considering advisory committee participation is a flowchart, or algorithm, that sets out the questions and considerations to address in a step-wise manner. This algorithm is discussed in detail in Part IV of this guidance, and is attached as Appendix 1.
The algorithm consolidates the various standards and tests found in the applicable statutes into a series of straightforward steps that generally apply to all meetings, regardless of the subject matter or type of meeting and irrespective of the type of financial interest(s) held by the member. This unified, simpler approach will improve consistency within the agency in considering advisory committee participation and will provide greater clarity to the public regarding how FDA selects members.
Advisory committee members will be considered under a more stringent policy regarding the level of financial interests in organizations that potentially could be affected by the meeting deliberations. First, if an individual has disqualifying financial interests whose combined value exceeds $50,000 , she generally would not participate in the meeting, regardless of the need for her expertise. Second, if the disqualifying financial interests are $50,000 or less, the individual would be eligible to participate only if she met the applicable statutory standard for participation; e.g., the need for her services outweighs the potential conflict. Third, even where the standard for participation is met, the individual's participation would be limited to non-voting. Fourth, FDA intends to generally limit participation in certain cases where there may be a perception of a conflict of interest, even though full participation would be permitted under the applicable statutes.
This guidance reduces variability among meetings in determining who may participate in FDA advisory committee meetings. The consolidated, simplified approach will enhance the public's understanding of and trust in the selection process, and will promote consistency in FDA's advisory committee process.
This part of the guidance discusses each step in the algorithm. The algorithm consists of six steps, and we will discuss each step sequentially.
B. Step 1 – Is the Meeting a "Particular Matter?"
The first step is to ask, "Will the meeting itself or governmental actions of which it is a part involve a ‘particular matter'?" The term "particular matter" includes only matters that involve deliberation, decision, or action that is focused upon the interests of specific persons, or a discrete and identifiable class of persons. It does not cover consideration or adoption of broad policy options directed to the interests of a large and diverse group of persons such as actions that will affect all companies or the economy in general (5 CFR 2640.103(a)(1)). While most FDA advisory committee meeting topics will involve "particular matters," some topics are so wide-ranging in nature and could potentially affect such a large number of organizations, that they would not be considered a "particular matter."
If your answer to this question is "no," then you do not have to proceed further to determine whether there is a conflict of interest. The member may fully participate 1 in the meeting, and you should prepare a memorandum for the record to support your categorization of the meeting. You should obtain concurrence from the Director of Advisory Committee Oversight and Management (DACOM) on your memorandum.
If your answer to the question is "yes," then proceed to step 2.
C. Step 2 – Is There a Direct and Predictable Effect on the Financial Interests?
Under step 2, the question is, "Will the meeting have a direct and predictable effect on financial interests of the affected organization or organizations?" Under 5 CFR 2640.103(a)(3)(i), a particular matter will have a "direct" effect on a financial interest if there is a close causal link between any decision or action to be taken in the matter and any expected effect of the matter on the financial interest. An effect may be direct even though it does not occur immediately. A particular matter will not have a direct effect on a financial interest, however, if the chain of causation is attenuated or is contingent upon the occurrence of events that are speculative or that are independent of, and unrelated to, the matter. A particular matter will have a "predictable" effect if there is a real, as opposed to a speculative, possibility that the matter will affect the financial interest. It is not necessary, however, that the magnitude of the gain or loss be known, and the dollar amount of the gain or loss is immaterial (5 CFR 2640.103(a)(3)(ii)).
For example, a meeting that will affect the legal rights or responsibilities of a known organization or organizations, such as most potential advisory committee recommendations pertaining to marketing status, labeling, post-marketing requirements, and device classification or reclassification, would ordinarily have a "direct and predictable effect" on financial interests. Financial interests that ordinarily will not be affected in a direct and predictable manner include a grant or contract with the employee's university to conduct research on a product that is not the subject of the particular matter before the advisory committee or a competitor product (see 5 CFR 2640.103(a)(3), example 2).
"Affected organization or organizations" generally means a company or entity that could be affected by the outcome of the advisory committee proceedings and any FDA decision based on the committee's recommendations. For example, the sponsor of a new drug application that is being presented to an advisory committee and sponsors of drugs that closely compete with the subject drug would all be "affected organizations" for which the financial interest of the SGE or regular government employee in the organization would need to be considered for potential conflict of interest.
If your answer to this question is "no," then you do not have to proceed further to determine whether there is a conflict of interest, and the individual may fully participate in the meeting. You should prepare a memorandum for the record supporting your categorization, and you should obtain concurrence from the DACOM on your memorandum.
If your answer to this question is "yes," proceed to step 3.
D. Step 3 – Is There a Disqualifying Financial Interest?
Once you have determined that the meeting will have a direct and predictable effect on the financial interests of an organization or organizations, you need to determine whether the SGE or regular government employee or persons or organizations whose interests are imputed to him have financial interests that may be affected by the deliberations at the meeting or the resolution of the governmental action of which the meeting is a part. The term "financial interest" means the potential for gain or loss to the employee (or persons/organizations whose interests are imputed to him) as a result of governmental action on the particular matter (5 CFR 2640.103(b)). In general, you should consider the financial interests (if any) of:
If you determine that the member and persons or organizations whose interests are imputed to him do not have any disqualifying financial interests, then you may recommend that the individuals may participate in the meeting unless the member is disqualified for reasons unrelated to 18 U.S.C. 208. In this case you should proceed to step 4a. Alternatively, if you find that the member or persons or organizations whose interests are imputed to him has disqualifying financial interests, you should proceed to step 4b.
E. Step 4a – Are There any Financial Interests Held Within the Preceding Twelve Months that Would Be a Disqualifying Financial Interest if They Were Currently Held?
If you have reached this step, there are no disqualifying financial interests that would constitute a conflict of interest under 18 U.S.C. 208. However, we believe that the public may perceive some financial interests in organizations potentially affected by advisory committee recommendations as problematic, even though those interests are not currently held. For example, we may wish to limit the participation of a member who has had a previous consulting arrangement within the last few months with an organization who is a party in the particular matter that is the subject of the advisory committee meeting. Accordingly, we intend to implement a policy of generally limiting participation when a member has a financial interest within the preceding twelve months that would be a disqualifying financial interest if it were currently held, even though full participation would be permitted under 18 U.S.C. 208. See 21 U.S.C. 393, 5 U.S.C. App. 2, 41 CFR 102-3.130.
If you determine that the member has a financial interest held within the preceding twelve months that would be a disqualifying financial interest if it were currently held, you should generally consider whether the member should not participate or whether he should participate but not vote. If the sum of those financial interests is greater than $50,000, the member generally would not participate 3. If the sum of those financial interests is less than or equal to $50.000, the member generally would participate but not vote. You should prepare a memorandum for the record supporting your recommendation and obtain concurrence from the DACOM on your memorandum.
If your answer to the question at Step 4a is "no," then you may recommend that the individual may fully participate in the meeting. You should prepare a memorandum for the record supporting your recommendation, and you should obtain concurrence from the DACOM on your memorandum. However, prior to making such recommendation, you should also consider the appropriateness of the member's participation under any other applicable regulatory provisions such as 5 CFR 2635.502.
F. Step 4b – How Large Are the Disqualifying Financial Interests, and Do I Apply Exemptions?
At this step, you have determined that there are disqualifying financial interests, and you need to determine the aggregate amount and apply applicable exemptions listed below. If the combined value of the disqualifying financial interests is greater than $50,000, after applying the exemptions listed in Step 4 below, you should not, in a large majority of cases, continue to seek the participation of the member. In limited cases, FDA may determine that a conflict of interest waiver is appropriate, provided that the relevant statutory and regulatory standards are met, even if the combined value of the disqualifying financial interests exceeds $50,000 (after applying the exemptions enumerated below). In such cases, the Commissioner of FDA will review the request and make a determination on the appropriateness of a waiver.
In calculating the combined value of the disqualifying financial interests, you need to consider whether exemptions apply. Certain financial interests have been determined by the Director of the Office of Government Ethics to be too remote or too inconsequential to affect the integrity of the services of the Government officers or employees (see 18 U.S.C. 208(b)(2)). The regulations issued by the Office of Government Ethics (OGE) expressly exempt these financial interests from consideration (see 5 CFR 2640. 201-206). To streamline the conflicts of interest screening process, increase its transparency, and add stringency, we have decided, as a policy matter, generally to apply only a simplified subset of these exemptions at this stage of the algorithm. Although we will consider the applicability of additional exemptions at step 6, we intend, in most cases, to recommend that participation is not warranted even where a step 6 exemption may apply, if the combined value of the financial interests (before taking into consideration the step 6 exemptions) exceeds $50,000. If you determine at this stage that the combined value of the financial interests is $50,000 or less, and you proceed to step 5, you will later need to evaluate whether additional regulatory exemptions apply in determining whether a waiver is appropriate to permit non-voting participation.
In brief, you do not need to include in your calculation of the combined value of the disqualifying financial interests the following:
If the combined value of the disqualifying financial interests in the preceding 12 months is less than or equal to $50,000 (after applying the selected exemptions), then you should proceed to step 5. (Since the $50,000 will become smaller in real terms as prices rise with inflation, we intend each year to adjust this figure according to reported increases in the Consumer Price Index above its level for 2006. We plan to revise this guidance to reflect the first such adjustment in early 2008.)
G. Step 5 – Does the Need for the Individual's Services Outweigh the Potential for a Conflict of Interest, or Is the Member's Financial Interest Not So Substantial as to be Deemed Likely to Affect the Integrity of the Services Provided by that Individual?
The conflict of interest waiver provisions establish similar, but not identical, standards and criteria for deciding whether to grant a waiver for an individual's participation. Under 18 U.S.C. 208(b)(3), which applies to advisory committee members who are SGEs, the standard is whether the need for the individual's services outweighs the potential for a conflict of interest created by the individual's financial interests. Under 18 U.S.C. 208(b)(1), a provision that applies to advisory committee members who are regular government employees, the standard is whether the member's financial interest is not so substantial as to be deemed likely to affect the integrity of the services provided by that individual. For members serving on drug or biologic advisory committees that provide scientific advice and recommendations regarding a clinical investigation or marketing approval, the standard for a waiver to permit voting, under section 505(n)(4) of the Act, is whether a waiver is "necessary" to afford the committee "essential expertise."
If you have reached this stage in the algorithm, you should determine whether the member may participate as a non-voting member, or, alternatively, that he may not participate in the advisory committee meeting. Because 21 U.S.C. 505(n)(4) applies only to voting waivers, and we have decided, as a policy matter, to consider only non-voting participation when the individual has financial conflicts of interest, 4 your evaluation of whether a waiver is appropriate will first focus on whether the applicable standard for granting a waiver pursuant to 18 U.S.C. 208 is met.
Accordingly, you first need to identify whether the individual is an SGE or a regular government employee. If the individual is an SGE, you need to determine whether the need for the individual's services outweighs the potential for a conflict of interest.
In determining whether the need for the individual's services outweighs the potential for a conflict of interest, we may consider a number of factors, including the type of interest that is creating the disqualification, the relationship of the person whose financial interest is involved to the member, the uniqueness of the individual's qualifications, the difficulty of locating a similarly qualified individual without a disqualifying financial interest, the dollar value of the disqualifying financial interest, and the extent to which the disqualifying financial interest could be affected by the actions of the advisory committee (see 5 CFR 2640.302(b)).
If the individual is a regular government employee, you should determine whether the member's financial interest is not so substantial as to be deemed likely to affect the integrity of the services provided by that individual. In determining whether the member's financial interest is not so substantial as to be deemed likely to affect the integrity of the services provided by that individual, we may consider a number of factors, including the type of financial interest that is creating the disqualification, the relationship of the person whose financial interest is involved to the member or member, the dollar value of the disqualifying financial interest, the nature and importance of the employee's role in the matter, and the need for the employee's services in the particular matter (see 5 CFR 2640.301(b)).
A common factor to be considered for an SGE advisory committee member and a regular government employee is the "need" for the individual's services. In deciding whether there is a need for the member's services, you should consider:
Furthermore, simple recitation of the member's credentials would not ordinarily be sufficient to establish "need." In other words, the issue is not whether John Doe is qualified to be a member, but rather whether John Doe – as opposed to another member or another person who could be recruited to become a member – is critical for that particular meeting. Need will be most persuasively shown when a reasonably thorough search for a similarly or better qualified candidate with fewer conflicts can be documented.
If you conclude that, for an SGE, the potential for a conflict of interest is not outweighed by the need for the member's services, or, for a regular government employee, that the member's financial interest is so substantial as to be deemed likely to affect the integrity of the services provided by that individual, you should not continue to seek his participation in the advisory committee meeting..
Alternatively, if you conclude that, for an SGE, the need for the member's services outweighs the potential for a conflict of interest, or, for a regular government employee, that the member's financial interest is not so substantial as to be deemed likely to affect the integrity of the services provided by that individual, you should proceed to step 6.
H. Step 6 – Do Any Additional Regulatory Exemptions Apply, and Are There Any Financial Interests Held Within the Preceding Twelve Months that Would Be a Disqualifying Financial Interest if They Were Currently Held?
If you have reached this step in the algorithm, you will evaluate two more questions to consider whether non-voting participation in a specific advisory committee meeting or topic may be justified. As a policy matter, we generally intend to limit participation of members who reach this step of the algorithm to non-voting participation. Advisory committee members who are non-voting members may participate in consensus recommendations.
In order to determine whether a waiver is needed to consider participation by the member on a non-voting basis, you will need to examine whether any of the regulatory exemptions issued pursuant to 18 U.S.C. 208(b)(2) that have not previously been considered apply. These include exemptions for interests in securities found in 5 CFR 2640.202(b) and (c). If, after applying additional applicable exemptions, the member no longer has any conflicts of interests, there is no violation of 18 U.S.C. 208(a), and a waiver is not necessary to permit participation. However, because we believe that the public may perceive some financial interests involving securities in organizations potentially affected by advisory committee recommendations as problematic, notwithstanding the applicability of these regulatory exemptions, we intend to implement a policy of generally limiting participation to non-voting in such circumstances even where full participation would be permitted under 18 U.S.C. 208.
You also need to determine whether the member has a financial interest held within the preceding twelve months that would be a disqualifying financial interest if it were currently held. Although financial interests that are not currently held do not constitute a conflict of interest under 18 U.S.C. 208, we believe that the public may perceive some previously held financial interests in organizations potentially affected by advisory committee recommendations as problematic. Accordingly, we intend to implement a policy of generally limiting participation when a member has a financial interest within the preceding twelve months that would be a disqualifying financial interest if it were currently held, even though full participation would be permitted under 18 U.S.C. 208.
If you determine that the member has a financial interest held within the preceding twelve months that would be a disqualifying financial interest if it were currently held, you should generally consider whether the member should not participate or whether he should participate but not vote. If the sum of those financial interests is greater than $50,000, the member generally would not participate 5. If the sum of those financial interests is less than or equal to $50.000, the member generally would participate but not vote.
Based on your answers to the questions in step 6, you should recommend that (a) a non-voting waiver is appropriate, (b) non-voting participation is warranted, or (c) no participation is warranted.
You should prepare a memorandum for the record supporting your recommendation and obtain concurrence from the DACOM on your memorandum.
Algorithm for Considering Advisory Committee Member Participation
Printable version of algorithm [674 KB PDF]
1. Full participation includes voting.
2. A prospective employer would be anyone with whom the employee has any arrangement concerning future employment or with whom he/she is seeking or negotiating for employment.
3. In limited cases, FDA may determine that participation is appropriate even if the combined value of the non-current financial interests exceeds $50,000. In such cases, the Commissioner of FDA will review the request and make a determination on the appropriateness of participation.
4. Under 505(n)(4) no waivers may be granted for a member of a panel when the member's own scientific work is involved. Because we do not plan to consider voting participation for individuals with conflicts of interest, we do not specifically ask in the algorithm whether the member's own work is involved.
5. In limited cases, FDA may determine that participation is appropriate even if the combined value of the non-current financial interests exceeds $50,000. In such cases, the Commissioner of FDA will review the request and make a determination on the appropriateness of participation.
![]()