 |
 |
 |
 |
FDA Home Page | Search FDA Site | FDA A-Z Index | Contact FDA
This guidance document is being distributed for comment purposes only.
Comments and suggestions regarding this draft document should be submitted within 90 days of publication in the Federal Register of the notice announcing the availability of the draft guidance. Submit comments to the Division of Dockets Management (HFA-305), Food and Drug Administration, 5630 Fishers Lane, rm. 1061, Rockville, MD 20852. All comments should be identified with the docket number listed in the notice of availability that publishes in the Federal Register.
For questions regarding this draft document contact (OC) Office of Policy, Ilisa Bernstein at 301-796-4830; (CDER) Office of Compliance, Jennifer Devine at 301-796-344, or (CBER) Stephen Ripley at 301-827-6210.
U.S. Department
of Health and Human Services
Food and Drug Administration
Office of the Commissioner (OC)
Center for Drug Evaluation and Research (CDER)
Center for Biologics Evaluation and Research (CBER)
Office of Regulatory Affairs (ORA)
January 2009
Procedural
Guidance for Industry
Standards
for Securing the Drug Supply Chain - Standardized Numerical Identification
for Prescription Drug Packages
Additional
copies are available from:
Office of Training and Communications
Division of Drug Information, WO51, Room 2201
10903 New Hampshire Ave.
Silver Spring, MD 20993
Phone: 301-796-3400; Fax: 301-847-8714
druginfo@fda.hhs.gov
http://www.fda.gov/cder/guidance/index.htm
and/or
Office
of Communication, Training and
Manufacturers Assistance, HFM-40
Center for Biologics Evaluation and Research
Food and Drug Administration
1401 Rockville Pike, Rockville, MD 20852-1448
http://www.fda.gov/cber/guidelines.htm.
(Tel) Voice Information System at 800-835-4709 or 301-827-1800
and/or
Office of Policy
Office of the Commissioner
Food and Drug Administration
10903 New Hampshire Ave.
Silver Spring, MD 20993
Phone: 301-796-4830
U.S. Department
of Health and Human Services
Food and Drug Administration
Office of the Commissioner (OC)
Center for Drug Evaluation and Research
Center for Biologics Evaluation and Research
Office of Regulatory Affairs
January 2009
Procedural
![]()
TABLE OF CONTENTS
I. INTRODUCTION
II. BACKGROUND
A. Food and Drug Administration Amendments
Act of 2007
B. Scope of this Guidance
III. STANDARDIZED NUMERICAL IDENTIFIERS
A. What should be designated as a package-level SNI?
B. Does the SNI include expiration date and/or lot or batch number?
C. Why did FDA select the serialized NDC for package-level SNI?
![]()
Guidance
for Industry1
Standards for Securing the Drug Supply Chain - Standardized Numerical Identification
for Prescription Drug Packages
This draft guidance, when finalized, will represent the Food and Drug Administration's (FDA's) current thinking on this topic. It does not create or confer any rights for or on any person and does not operate to bind FDA or the public. You can use an alternative approach if the approach satisfies the requirements of the applicable statutes and regulations. If you want to discuss an alternative approach, contact the FDA staff responsible for implementing this guidance. If you cannot identify the appropriate FDA staff, call the appropriate number listed on the title page of this guidance.
This guidance is intended to address provisions set forth in Section 505D of the Federal Food, Drug, and Cosmetic Act (the act) regarding development of standardized numerical identifiers (SNIs) for prescription drug packages. In this guidance, FDA is identifying package-level SNIs, as an initial step to facilitating other measures for securing the drug supply chain.
FDA's guidance documents, including this guidance, do not establish legally enforceable responsibilities. Instead, guidances describe the Agency's current thinking on a topic and should be viewed only as recommendations, unless specific regulatory or statutory requirements are cited. The use of the word should in Agency guidances means that something is suggested or recommended, but not required.
On September 27, 2007, the Food and Drug
Administration Amendments Act of 2007 (FDAAA) (Public Law 110-85) was
signed into law. Section 913 of this legislation created section 505D
of the Federal Food, Drug, and Cosmetic Act (the act), which requires the
Secretary of Health and Human Services (the Secretary) to develop standards
and identify and validate effective technologies for the purpose of securing
the drug supply chain against counterfeit, diverted, subpotent, substandard,
adulterated, misbranded, or expired drugs. Section 505D directs the
Secretary to consult with specific entities to prioritize and develop standards
for identification, validation, authentication, and tracking and tracing
of prescription drugs. No later than 30 months after the date of enactment
of FDAAA, the statute also directs the Secretary to develop an SNI to be
applied to a prescription drug at the point of manufacturing and repackaging
at the package or pallet level, sufficient to facilitate the identification,
validation, authentication, and tracking and tracing of the prescription
drug. An SNI applied at the point of repackaging is to be linked to
the SNI applied at the point of manufacturing, and to the extent practicable,
the SNI should be harmonized with international consensus standards for such
an identifier. (See Section 505D(b)(2).) The provisions in section
505D(b) of the act complement and build on FDA’s longstanding efforts
to further secure the U.S. drug supply.
FDA sought public comment on specific questions related to development of an
SNI by opening a docket to receive information. 73 FR14988 (March 20,
2008). We also shared this request with State governments, other Federal
agencies, and with foreign governments. We received 59 comments from
a range of stakeholders, including manufacturers, wholesalers, pharmacies,
trade and health professional organizations, technology vendors, health professionals,
consumers, and state governments. The standards included in this guidance
are based on information received in response to our request for comment and
the agency's familiarity with identification standards already in use for certain
prescription biologics.
This guidance addresses only package-level SNI. For this purpose, FDA considers the package to be the smallest saleable unit placed into interstate commerce by the manufacturer or the repackager for sale to the pharmacy or other dispenser of the drug product. Standards for prescription drug SNI for the pallet level or other intermediate levels, such as cases, are not included in this guidance. Linking of a repackager SNI to a manufacturer SNI is also not addressed in this guidance. Additionally, standards for track and trace, authentication, and validation are not included in this guidance. This guidance is intended to be the first of several guidances and regulations that FDA may issue to implement section 505D of the act; issuance of this guidance is intended to assist with the development of standards and systems for identification, authentication, and tracking and tracing of prescription drugs.
A. What should be designated as a package-level SNI?
Although manufacuters and repackagers are not required to use an SNI, for
those manufactures and repackagers who do, the SNI for most prescription drug
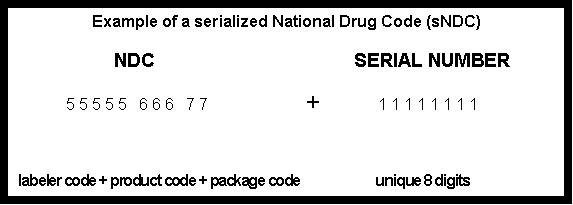
packages should be a serialized National Drug Code (sNDC). The sNDC is composed
of the National Drug Code (NDC) (as set forth in 21 CFR Part 207)2 that reflects
each corresponding manufacturer or repackager, combined with a unique
8-digit numerical serial number generated by the manufacturer or repackager
for each individual package. An example is shown below with a 10-digit
NDC.
FDA recognizes that some prescription drugs approved under Section 351 of the Public Health Service Act, such as blood and blood components, do not use NDC numbers. Instead, such products currently use other recognized consensus standards for identification and labeling, e.g. ISBT 128 (see http://iccbba.org/about_gettoknowisbt128.html, and Guidance for Industry: Recognition and Use of a Standard for Uniform Blood and Blood Component Container Labels (http://www.fda.gov/cber/gdlns/unilabbld.htm) or Codabar. In addition, hematopoietic stem cells derived from peripheral and cord blood use the ISBT 128 standard for product package identification. Using these standards, a unique identification number is created for each individual product package. Therefore, for such products that do not use NDC numbers, FDA is considering use of ISBT 128 or Codabar as the SNI.
Expiration date and/or lot or batch number are not part of the SNI. Addition of this information within the SNI will increase the length of, and introduce complexity into, the SNI. Expiration date and/or lot or batch number are already readily accessible because FDA regulations require this information to be included on the label of each drug product. (See 21 CFR §§ 201.17, 201.18, 211.130, 211.137, 610.60, and 610.61.) However, if a manufacturer or repackager chooses to include expiration date and/or lot or batch number with the SNI, it should ensure that the resulting number still permits users to distinguish and make use of the SNI. For example, expiration date and lot or batch number may be incorporated in accordance with the GS1 standards for use of Global Trade Item Numbers (GTIN)3 (discussed below).
FDA chose the sNDC because we believe that it serves the needs of the drug supply chain as a means of identifying individual prescription drug packages. That identification can in turn facilitate authentication and tracking and tracing of the prescription drugs. Because the sNDC incorporates an 8-digit numerical serial number with the NDC, it should provide appropriate robustness to support billions of units of marketed products without duplication of an SNI. This approach will allow manufacturers and repackagers to assign serial numbers to combine with the NDC for unique identification of individual product packages. The SNI can also be linked to other identifiers used for manufacturing and shipping purposes. As already noted, defining the SNI is expected to be a first step to facilitate the development of other standards and systems for securing the drug supply chain. Many aspects of the implementation of package-level SNI will take shape in the future, as the standards that make use of SNI are developed.
At this time, FDA is not specifying a particular means of incorporating the SNI onto the package. The SNI identified in this guidance is compatible with, and flexible for, encoding into a variety of machine readable forms of data carriers, such as 2-dimensional bar codes and RFID,4 leaving options open as technologies useful for securing the supply chain continue to be identified, and standards making use of SNI are developed. FDA expects that SNI generally will be applied to each package in both human readable and machine readable forms. A redundant human readable SNI on the package will provide the ability to identify the package when electronic means are unavailable (e.g., in the event of hardware/software failure). FDA also is not specifying a location on the package where an SNI should be placed, although any SNI would need to be placed on the package in a manner that does not obstruct FDA required labeling information.
In addition to facilitating other actions to secure the drug supply chain, adoption of the sNDC as the SNI satisfies the requirement in 505D(b)(2) that the SNI developed by FDA be harmonized, to the extent practicable, with international standards for such an identifier.5 Specifically, use of sNDC is compatible with, and may be presented within, a serialized Global Trade Item Number (serialized GTIN or sGTIN). GTIN is a global standard for item and object identification, established by GS1, a consensus-based, not-for-profit, international standards organization that works with manufacturers, distributors, retailers, and others in the drug supply chain. FDA has been an active observer and participant in GS1 standards development related to healthcare and drug products. According to documentation from GS1, the GTIN is used worldwide by twenty-three industry sectors, including healthcare, and has been adopted by sixty-five countries to uniquely identify pharmaceutical products. A GTIN may be used to uniquely identify items at the package level throughout the supply chain; combining a serial number with the GTIN ("serializing") results in an sGTIN that is unique to the individual package.
![]()
1This guidance has been prepared by the Office of the Commissioner (OC), the Center for Drug Evaluation and Research (CDER), the Center for Biologics Evaluation and Research (CBER), and the Office of Regulatory Affairs (ORA) at the Food and Drug Administration.
2Use of the sNDC as SNI is consistent with both existing provisions of part 207 and with FDA's proposed amendments to that provision, which would affect the assignment of NDCs. See 71 FR 51276 (August 29, 2006).
3See www.GS1.org -- Healthcare GTIN Allocation Rules (http://www.gs1.org/docs/gsmp/healthcare/GS1_Healthcare_GTIN_Allocation_Rules.pdf ).
4 FDA's enforcement policy with respect to the application of current good manufacturing practices to RFID technology is provided in Compliance Policy Guide (CPG) Section 400.210. See http://www.fda.gov/oc/initiatives/counterfeit/rfid_cpg.html. This CPG would apply if an SNI were embedded into an RFID tag.
5The potential alternative SNI for blood and certain other biologics identified above also use international consensus standards (ISBT 128).
![]()