Guidance for Industry
"Lookback" for Hepatitis C Virus (HCV):Product Quarantine, Consignee Notification, Further Testing, Product Disposition, and Notification of Transfusion Recipients Based on Donor Test Results Indicating Infection with HCV
[PDF printable version - 279 KB]
Additional copies of this guidance are available from Office of Communication, Training and Manufacturers Assistance (HFM-40), Suite 200N, 1401 Rockville Pike, Rockville, MD 20852-1448, or by calling 1-800-835-4709 or 301-827-1800, or from the Internet at http://www.fda.gov/cber/guidelines.htm.
For questions on the content of this guidance, contact The Division of Emerging and Transfusion Transmitted Diseases, Office of Blood Research and Review (OBRR), Center for Biologics Evaluation and Research (CBER) at 301-827-3008.
U.S. Department of Health and Human Services
Food and Drug Administration
Center for Biologics Evaluation and Research (CBER)
August 2007
- INTRODUCTION
- BACKGROUND
- DEFINITIONS
- LOOKBACK REQUIREMENTS
- BLOOD AND BLOOD COMPONENTS INTENDED FOR MANUFACTURE INTO NON-INJECTABLE PRODUCTS
- REFERENCES
Contains Nonbinding Recommendations
Guidance for Industry
"Lookback" for Hepatitis C Virus (HCV): Product Quarantine, Consignee Notification, Further Testing, Product Disposition, and Notification of Transfusion Recipients Based on Donor Test Results Indicating Infection with HCV
| This guidance represents the Food and Drug Administration's (FDA's) current thinking on this topic. It does not create or confer any rights for or on any person and does not operate to bind FDA or the public. You can use an alternative approach if the approach satisfies the requirements of the applicable statutes and regulations. If you want to discuss an alternative approach, contact the appropriate FDA staff. If you cannot identify the appropriate FDA staff, call the appropriate number listed on the title page of this guidance. |
- The identification of blood and blood components from prior collections from a donor with:
- a repeatedly reactive antibody screening test for anti-HCV, or
- a reactive NAT for HCV or other reliable test results or information indicating evidence of HCV infection (provided the testing was performed using a test approved by FDA by a laboratory compliant with the Clinical Laboratory Improvement Amendments of 1988, or has met equivalent requirements as determined by the Centers for Medicare and Medicaid Services in accordance with the provisions of 42 CFR part 493); and
- subsequent actions, such as:
- quarantine of such blood and blood components that are not expired,
- notification of consignees to quarantine such in-date blood products,
- further testing of the donor,
- destruction or relabeling of potentially infectious prior collections,
- release from quarantine of blood and blood components that do not present a greater risk of infection, and (if appropriate)
- notification of recipients of identified blood and blood components.
- quarantine prior collections that remain in inventory;
- notify consignees to quarantine prior collections;
- further test the donor and notify consignee of test results; and
- destroy or relabel potentially infectious prior collections.
- quarantine prior collections that remain in inventory;
- destroy or relabel potentially infectious prior collections; and
- notify transfusion recipients who received blood from a donor who is later determined to be infected with HCV, if appropriate.
- BLOOD AND BLOOD COMPONENTS INTENDED FOR MANUFACTURE INTO NON-INJECTABLE PRODUCTS
Multiple layers of safeguards, including donor screening and testing, are used to reduce the risk of transmitting infection through blood transfusion. However, a person may donate blood early in infection, during the period when the viral marker is not detectable by a screening test, but the infectious agent is present in the donor's blood (the "window period"). For example, if an individual donates blood on a number of occasions and each donation tests nonreactive for antibody to HCV, but the donor returns and tests repeatedly reactive for antibody to HCV at a later date, prior collections from such a donor could be at increased risk for transmitting HCV. In addition, a recipient of a transfusion of blood or blood components collected during the "window period" from a donor who is now repeatedly reactive would not know that he or she might be at increased risk of infection with HCV through the transfusion, unless he or she is notified. Furthermore, untested collections from donors who later were found to be repeatedly reactive when tested for antibodies to HCV since 1990 (when antibody tests for HCV became available) might have been at increased risk for transmitting HCV due to a chronic infection in the donor.
Chronic hepatitis due to HCV is a major health problem in the U.S. The infection is usually clinically silent until the liver is seriously damaged. As a result, infected people usually are unaware of their disease. Although transfusion-transmitted infections account for only a very small proportion of HCV infections, it is possible to identify and "lookback" at prior donations that might have been collected during the "window period."
This guidance document provides recommendations for complying with 21 CFR 610.47 and 21 CFR 610.48 to (1) blood establishments that collect blood or blood components, including Source Plasma and Source Leukocytes, (2) hospitals, and (3) other consignees. This guidance does not apply to autologous donations, all Lookback donations (see p.4 for definition) that are identified as at increased risk of transmitting HCV, pooled blood components intended solely for further manufacturing into products that are manufactured using validated viral clearance procedures, and blood and blood components that were intended for manufacture into non-injectable products subject to the labeling described in Section V.
This document supersedes the HCV sections of the Food and Drug Administration (FDA) memorandum of July 19, 1996, entitled "Recommendations for the Quarantine and Disposition of Units from Prior Collections from Donors with Repeatedly Reactive Screening Tests for Hepatitis B Virus (HBV), Hepatitis C Virus (HCV), and Human T-Lymphotropic Virus Type I (HTLV-I)." This guidance document also supersedes the September 1998 guidance entitled "Guidance for Industry: Current Good Manufacturing Practice for Blood and Blood Components: (1) Quarantine and Disposition of Units from Prior Collections from Donors with Repeatedly Reactive Screening Tests for Antibody to Hepatitis C Virus (Anti-HCV); (2) Supplemental Testing, and the Notification of Consignees and Blood Recipients of Donor Test Results for Anti-HCV." Additionally, this guidance finalizes FDA's draft guidance dated June 1999 entitled "Guidance for Industry: Current Good Manufacturing Practice for Blood and Blood Components: (1) Quarantine and Disposition of Prior Collections from Donors with Repeatedly Reactive Screening Tests for Hepatitis C Virus (HCV); (2) Supplemental Testing, and the Notification of Consignees and Transfusion Recipients of Donor Test Results for Antibody to HCV (Anti-HCV)."
FDA's guidance documents, including this guidance, do not establish legally enforceable responsibilities. Instead, guidances describe FDA's current thinking on a topic and should be viewed only as recommendations, unless specific regulatory or statutory requirements are cited. The use of the word should in FDA's guidances means that something is suggested or recommended, but not required.
"Lookback" related to HCV testing has been discussed at open public meetings, including meetings of FDA's Blood Products Advisory Committee (BPAC), on multiple occasions since October 1989. As a response to these discussions, FDA provided detailed guidance in the July 19, 1996, memorandum on the quarantine and disposition of certain prior collections of blood and blood components from donors who subsequently test repeatedly reactive for anti-HCV. The 1996 memorandum recommended that blood establishments notify consignees (such as the transfusion service, physician, or fractionator) for the purpose of quarantine and eventual disposition of products made from prior collections. Prior to recent advances in medical diagnosis and therapy, FDA did not recommend notification of recipients of blood from donors who subsequently test positive for anti-HCV because no clear consensus on the public health benefit (i.e., disease prevention and treatment) of such action had emerged. Improvements in the management and treatment of chronic hepatitis C have occurred over time, and it is now evident that there is a strong correlation between a positive test for anti-HCV in a supplemental assay (the Chiron Recombinant Immuno Blot Assay (RIBA) HCV 2.0 Strip Immunoblot Assay (SIA) or the Chiron RIBA HCV 3.0 SIA1) and HCV infection. More specifically, in studies of blood donors tested by RIBA 2.0, 73-95% of test-positive and 14-21% of test-indeterminate blood samples had detectable HCV ribonucleic acid (RNA) by polymerase chain reaction (PCR) (Refs. 1-3). In addition, a reactive result on a nucleic acid test (NAT) is presumptive evidence of ongoing infection with HCV. Prior collections from donors later found to be reactive for anti-HCV might also be at increased risk of transmitting HCV.
At public meetings on April 24 and 25, 1997, and August 11 and 12, 1997, the Public Health Service (PHS) Advisory Committee on Blood Safety and Availability recommended notification of recipients of transfused blood and blood components that are at increased risk of transmitting HCV infection based on donor screening with a licensed multiantigen screening test (Enzyme Immuno Assay (EIA) 2.0 or EIA 3.0) since 1992. Consistent with these recommendations, in March 1998, FDA issued guidance for implementation and public comment regarding recipient notification (63 FR 13675, March 20, 1998). In response to comments received, FDA issued a revised guidance for implementation in September 1998 (63 FR 56198, October 21, 1998).
At public meetings on November 24, 1998, and January 28, 1999, the PHS Advisory Committee on Blood Safety and Availability reconsidered the issue of recipient notification related to repeatedly reactive results on the single antigen (EIA 1.0) screening test that was licensed in 1990. The PHS Advisory Committee recommended the expansion of the targeted HCV lookback program to include recipients of blood and blood components from donors subsequently identified as repeatedly reactive by the EIA 1.0 screening test. In addition, the PHS Advisory Committee considered that, in cases where no supplemental test result is available, it is reasonable to perform lookback for EIA 1.0 based on a Signal to Cutoff ratio of the screening test of greater than 2.5 to capture the vast majority of the true positives and minimize unnecessary recipient notifications based on false reactive screening test results.
In accordance with these PHS Advisory Committee recommendations, FDA issued draft guidance dated June 1999 for public comment (64 FR 33309, June 22, 1999). Additionally, FDA published a Proposed Rule on HCV Lookback (65 FR 69378, November 16, 2000). In response to public comments received on the draft guidance and the Proposed Rule, FDA is now issuing this guidance on HCV lookback for implementation. This guidance is consistent with the requirements in the HCV Lookback regulations under 21 CFR 610.47 and 21 CFR 610.48.
Alternative Test: A licensed test for anti-HCV that is of equal or greater sensitivity in comparison to the licensed HCV EIA 3.0.
Blood and blood components: Whole Blood and blood components, including Source Plasma and Source Leukocytes.
Lookback:
Lookback Donations: Collectively, the blood and blood components from prior collections from the same donor.
Reactive NAT: A result for an HCV RNA test that uses only one set of primers specific for HCV that is performed initially on an individual donation, or is performed as a discriminatory NAT subsequent to a reactive multiplex NAT, which tests for multiple viruses, including HCV RNA.
Repeatedly Reactive Screening Test: The result when an initially reactive screening test is repeated in duplicate samples on the same test run and one or both samples are reactive.
Transfusion Recipient Notification: The actions taken by a hospital, transfusion service, or patient's physician of record to notify patients that they received a transfusion of a Lookback Donation that is at increased risk of transmitting HCV infection.
In this guidance, FDA addresses "lookback" related to donor screening by the EIA 1.0, EIA 2.0, and EIA 3.0 screening tests and NAT. Under 21 CFR 610.47 and 21 CFR 610.48, blood establishments must search records of prior collections from donors whose test results on current or historical review donations show evidence of infection with HCV (either repeatedly reactive on screening tests for anti-HCV or reactive on NAT for HCV RNA). Blood establishments must search their records to identify prior collections dating back 10 years (for current donations) or dating back to January 1, 1988 (for historical review donations). If an establishment maintains computerized electronic records relating to collections made before January 1, 1988, the blood establishment must perform an historical review search of those records dating back indefinitely, as far back as they exist in computerized, electronic form (21 CFR 610.48(b)(1)(i)). The blood establishment must search records of prior collections, if available, back to the date 12 months prior to the donor's most recent nonreactive licensed multiantigen screening test for anti-HCV (if applicable), if that is a shorter period, since prior infection in the donor is highly unlikely based on the known duration of the window period (21 CFR 610.48(b)(1)(iii)(A)(B)). FDA believes that this date (12 months prior to the last nonreactive multiantigen screening test for anti-HCV) will antedate the window period for infection in that donor.
This guidance also addresses appropriate "lookback" actions based on results obtained using RIBA 1.0 and the Abbott HCV Neutralization EIA and Peptide-1 and Peptide-2 assays ("Neutralization-Peptide EIA"),2 unapproved supplemental tests for EIA 1.0 repeatedly reactive specimens. Donors found to be repeatedly reactive on EIA 1.0 and positive on the unlicensed RIBA 1.0 may be as likely to be infected as donors detected and confirmed positive by approved multiantigen tests (Ref. 4). (Direct comparison of the unapproved Neutralization-Peptide EIA and RIBA 2.0 found few differences in overall test performance (Ref. 5)).
FDA believes that results for NAT are of value in (1) lookback related to current collections when a NAT result exists and (2) in historical lookback, when a NAT result, including NAT performed under an Investigational New Drug Application (IND), exists in the record of the previous donation(s). Blood establishments should consider a reactive NAT result to be presumptive evidence of ongoing HCV infection. A reactive NAT must serve as a basis for initiating lookback (i.e., both product retrieval and recipient notification). However, a nonreactive NAT should not be a reason for not performing lookback for a repeatedly reactive donation. This recommendation is based on current research that indicates that in about 15-25% of cases of HCV infection, viremia may be intermittently detectable (Ref. 6) or resolved (Ref. 7).
Blood establishments that collect blood or blood components, including Source Plasma and Source Leukocytes, must perform the following within 3 days after a donor tests reactive for evidence of HCV infection (21 CFR 610.47(a)); or during an historical record review, after identifying donors who tested reactive for evidence of HCV infection (21 CFR 610.48(b)):
Under 21 CFR 610.47(b) and 21 CFR 610.48(c), transfusion services, as consignees, must perform the following:
Blood establishments must initiate lookback based on a reactive NAT although a screening test or supplemental test for antibodies to HCV performed on the same collection is nonreactive. In this situation, blood establishments must search records of prior collections for the 12 months prior to the date of the NAT-reactive donation, since this configuration of test results is consistent with recent HCV infection in the donor (Ref. 8).
In addition, blood establishments must initiate lookback based on a reactive NAT when a supplemental test for antibodies to HCV performed on the same collection is indeterminate. In this situation, in order to notify consignees and quarantine components, blood establishments must search records of prior collections dating back 10 years (for current donations) or dating back to January 1, 1988 (for historical review donations), and, if the establishment maintains computerized electronic records relating to collections made before January 1, 1988, the blood establishment must also search those records dating back indefinitely, as far back as they exist in computerized, electronic form. The blood establishment must notify consignees to destroy or label all prior collections identified in that search. The blood establishment must search records of prior collections back to the date 12 months prior to the donor's most recent nonreactive licensed multiantigen screening test for anti-HCV (if applicable), if that is a shorter period. However, if the donation is indeterminate using a RIBA 3.0, blood establishments must notify transfusion recipients of prior collections from the same donor only if the collection took place in the 12 months before the date of the NAT-reactive donation, since recent infection of the donor is likely based on this test configuration (Centers for Disease Control and Prevention, unpublished data).
To assist blood establishments in complying with the regulations in 21 CFR 610.47 and 21 CFR 610.48, this guidance provides applicable figures and tables that will assist in interpreting testing scenarios and the appropriate steps to take with each scenario.
There may be some limited uses for quarantined prior collections that are not suitable for release from quarantine for the component's original intended use. Such prior collections must not be used for transfusion or routinely used for manufacturing into injectable products 21 CFR 610.40(h). These prior collections should be destroyed as a general practice; however, in limited situations, release for research or for further manufacture is acceptable. If released for research or for further manufacture into in vitro diagnostic reagents, the prior collections must be relabeled consistent with general labeling requirements in 21 CFR 606.121 and 21 CFR 640.70. Additionally, the blood and blood components must be labeled as "Biohazard" (see 21 CFR 610.40(h)(2)(ii)(B)) and with two of the following statements:
- "Collected from a donor who subsequently tested reactive for anti-HCV or HCV RNA. An increased risk of transmission of HCV is present."
and either
- "Caution: For Further Manufacturing Into In Vitro Diagnostic Reagents For Which There Are No Alternative Sources" (see 21 CFR 610.40(h)(2)(ii)(D)).
or
- "For Laboratory Research Use Only."
If the units are to be released for further manufacture into injectable products, the units must include a statement on the container label indicating the exempted use specifically approved by FDA (21 CFR 610.40(h)(2)(ii)(D)).
1. Sayers, M.H., and Gretch, D.R. Recombinant immunoblot and polymerase chain reaction testing in volunteer whole blood donors screened by a multi-antigen assay for hepatitis C virus antibodies. Transfusion 33:809-813 (1993).
2. Kleinman, S. et al. Increased detection of hepatitis C virus (HCV)-infected blood donors by a multiple-antigen HCV enzyme immunoassay. Transfusion 32:805-813 (1992).
3. Yun, Z. et al. Detection of Hepatitis C Virus (HCV) RNA by PCR Related to HCV Antibodies in Serum and Liver Histology in Swedish Blood Donors. J Med Virol 39:57-61 (1993).
4. Menozzi, D. et al. HCV lookback in the United States: effectiveness of an extended lookback program. Transfusion 40:1393-1398 (2000).
5. Evans, C.S. et al. Comparative evaluation of supplemental hepatitis C virus antibody test systems. Transfusion 32:408-414 (1992).
6. Alter, H.J. To C or Not to C: These Are the Questions. Blood 85:1681-1695 (1995).
7. CDC. Recommendations for Prevention and Control of Hepatitis C Virus (HCV) Infection and HCV-Related Chronic Disease. MMWR Morb Mortal Wkly Rep 47 (RR-19) (1998).
8. Schreiber, G.B. et al. The Risk of Transfusion-Transmitted Viral Infections. N Engl J Med 334:1685-1690 (1996).
Figure 1 - "Lookback" for Hepatitis C Virus (HCV) Based on Current Donor Test Results Using a Licensed EIA 2.0 or EIA 3.0 Test for HCV Antibody or Nucleic Acid Testing (NAT) for HCV RNA
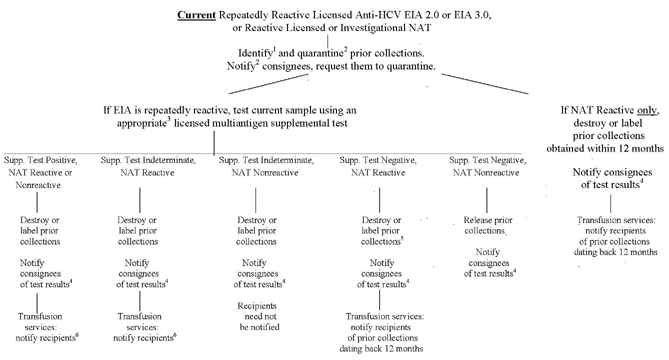
1 If repeatedly reactive for anti-HCV, prior collections should be identified from the same donor dating back to the date 12 months prior to the donor's most recent nonreactive licensed multiantigen screening test. If NAT-reactive only, prior collections should be identified from the same donor dating back 12 months prior to the reactive NAT.
2 Within 3 calendar days after the day of obtaining the reactive NAT or repeatedly reactive anti-HCV screening test result.
3 An appropriate supplemental test is one that includes all the antigens contained in the screening test that was performed.
4 Notify consignees of all test results within 45 calendar days of the current repeatedly reactive anti-HCV or NAT-reactive test result.
5 Prior collections obtained more than 12 months prior to the date of the NAT-reactive donation may be released.
6 Transfusion services must identify and notify recipients of identified prior collections.
Figure 2 - "Lookback" for Hepatitis C Virus (HCV) Based on Historical Donor Test Results Using a Licensed EIA 2.0 or EIA 3.0 Test for HCV Antibody or Nucleic Acid Testing (NAT) for HCV RNA
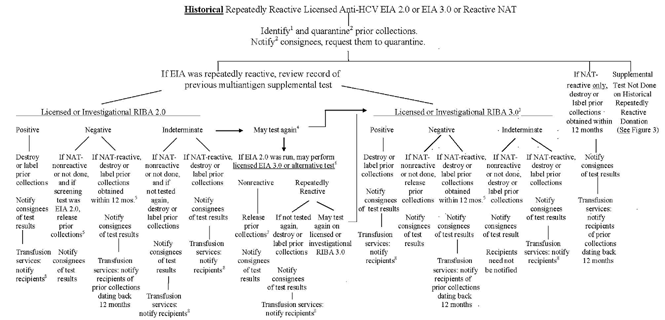
1 If repeatedly reactive, prior collections should be identified from the same donor dating back to the date 12 months prior to the donor's most recent nonreactive licensed multiantigen screening test. If NAT-reactive only, prior collections should be identified from the same donor dating back 12 months prior to the date of the NAT-reactive donation.
2 Within 3 calendar days after the day of identifying the reactive NAT or repeatedly reactive anti-HCV screening test result.
3 Testing already performed as an in-house service by Chiron Corp. using the research HCV Strip Immunoassay (SIA) Version 3, or testing already performed using an investigational RIBA 3.0 in accordance with previous guidance, is also acceptable.
4 A previously frozen serum or plasma sample from the repeatedly reactive/RIBA 2.0 indeterminate donation or a fresh sample from the donor may be tested again. Notify consignees within 45 calendar days of obtaining the additional test result.
5 If the repeatedly reactive screening test was EIA 3.0 and the negative supplemental test was RIBA 2.0, destroy or label prior collections and notify transfusion recipients. Alternatively, may test again using RIBA 3.0 (see outcomes at right). Notify consignees within 45 calendar days of obtaining the supplemental test result.
6 An alternative test for anti-HCV is a test of equal or greater sensitivity in comparison to the licensed HCV EIA 3.0.
7If a RIBA 2.0 or RIBA 3.0 is negative, prior collections obtained more than 12 months prior to the date of the NAT-reactive donation may be released.
8 Transfusion services must identify and notify recipients of identified prior collections to the date 12 months prior to the donor's most recent nonreactive licensed multiantigen screening test for anti-HCV.
Figure 3 - "Lookback" for Hepatitis C Virus (HCV) Based on Historical Donor Test Results Using a Licensed EIA 2.0 or EIA 3.0 Test for HCV Antibody or Nucleic Acid Testing (NAT) for HCV RNA (Cont.)
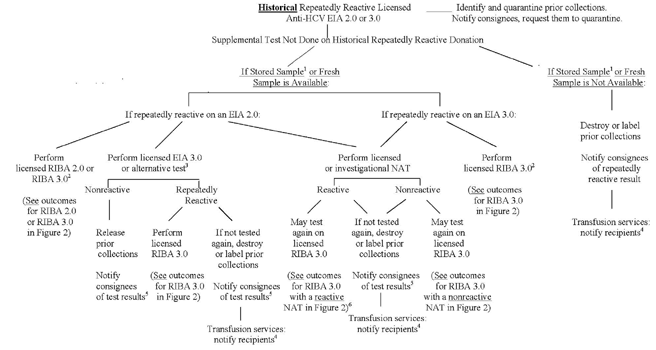
1 A previously frozen serum or plasma sample from the repeatedly reactive donation.
2 If a licensed RIBA 2.0 test or an investigational RIBA 3.0 test was performed consistent with previous guidance, refer to Figure 2 for outcomes.
3 An alternative test for anti-HCV is a test of equal or greater sensitivity in comparison to the licensed HCV EIA 3.0.
4 Transfusion services must identify and notify recipients of identified prior collections dating back to the date 12 months prior to the donor's most recent nonreactive licensed multiantigen screening test for anti-HCV.
5 Notify consignees within 45 calendar days of obtaining the additional test result.
6 If a NAT is reactive and further testing by RIBA 3.0 is negative or indeterminate, transfusion services should identify and notify recipients of identified prior collections dating back 12 months prior to the date of the NAT-reactive donation. If the RIBA 3.0 is negative, prior collections obtained more than 12 months prior to the date of the NAT-reactive donation may be released. If the RIBA 3.0 is indeterminate, prior collections should be destroyed or labeled consistent with section IV.F. Note that a nonreactive NAT does not obviate lookback for a repeatedly reactive donation.
Figure 4 - "Lookback" for Hepatitis C Virus (HCV) Based on Historical Donor Test Results Using an EIA 1.0 for HCV Antibody
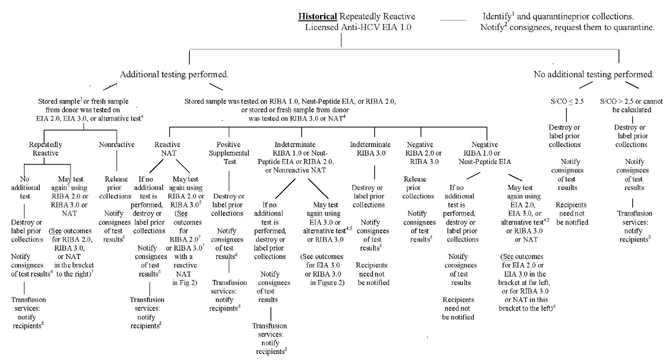
1 Previously distributed units should be identified dating back 12 months prior to the donor's most recent nonreactive licensed screening test for anti-HCV.
2 Within 3 calendar days after the day of identifying the repeatedly reactive anti-HCV screening test results.
3 A previously frozen serum or plasma sample from the repeatedly reactive donation.
4 An alternative test for anti-HCV is a test of equal or greater sensitivity in comparison to the licensed HCV EIA 3.0.
5 The supplemental test performed should include all the antigens contained in the screening test that was performed.
6 Notify consignees within 45 calendar days of obtaining the supplemental or additional test result.
7 If a NAT is reactive and further testing by RIBA 2.0 or RIBA 3.0 is negative, prior collections obtained more than 12 months prior to the date of the NAT-reactive donation may be released, and transfusion servicesmust identify and notify recipients of prior collections dating back 12 months prior to the date of the NAT-reactive donation. If the RIBA 2.0 is indeterminate, destruction or labeling of prior collections and notification of recipients are not limited to collections within 12 months prior to the NAT-reactive donation. If the RIBA 3.0 is indeterminate, destruction or labeling of prior collections is not limited to collections within 12 months prior to the NAT-reactive donation, but notification of recipients should extend back only 12 months prior to the NAT-reactive donation. Note that a nonreactive NAT does not obviate lookback for a repeatedly reactive donation.
8 Transfusion services must identify and notify recipients of identified prior collections.
Table 1 - Current Review
| You must take the following steps when donor test results for antibody to Hepatitis C Virus (anti-HCV) by Licensed HCV EIA 2.0 or EIA 3.0 are repeatedly reactive, or a licensed or investigational HCV RNA Nucleic Acid Test (NAT) is reactive. You must identify and quarantine prior collections, notify the consignees and request them to quarantine prior collections. Then. | ||||||||
| If the initial test is a licensed . | And the additional testing1 is a licensed or investigational. | Then . | ||||||
| Referenced Row in Guidance Document | Notify Consignee of test result | Destroy/Label prior collection | Transfusion service notify recipient | Release prior collection | ||||
| EIA | NR | NAT | Reactive | |||||
| EIA 2.0 or 3.0 | RR | Appropriate Multiantigen Supplemental Test | Positive | |||||
| NAT | Nonreactive | |||||||
| EIA 2.0 or 3.0 | RR | Appropriate Multiantigen Supplemental Test | Positive | |||||
| NAT | Reactive | |||||||
| EIA 2.0 or 3.0 | RR | Appropriate Multiantigen Supplemental Test | Indeterminate | |||||
| NAT | Nonreactive | |||||||
| EIA 2.0 or 3.0 | RR | Appropriate Multiantigen Supplemental Test | Indeterminate | |||||
| NAT | Reactive | |||||||
| EIA 2.0 or 3.0 | RR | Appropriate Multiantigen Supplemental Test | Negative | |||||
| NAT | Nonreactive | |||||||
| EIA 2.0 or 3.0 | RR | Appropriate Multiantigen Supplemental Test | Negative | |||||
| NAT | Reactive | |||||||
1 Appropriate additional testing includes all the antigens contained in the screening test that was performed
2 Collections during the 12 months prior to the date of the donation testing NAT reactive
3Release collections from more than 12 months prior to the date of the donation testing NAT reactive
NR means nonreactive
RR means repeatedly reactive
Table 2 - Historical Review
| You must take the following steps when donor test results for antibody to Hepatitis C Virus (anti-HCV) by Licensed HCV EIA 2.0 or EIA 3.0 are repeatedly reactive, or a licensed or investigational HCV RNA Nucleic Acid Test (NAT) is reactive. You must identify and quarantine prior collections, notify the consignees and request them to quarantine prior collections. Then . | ||||||||
| If the historical initial test is a licensed . | And the historical additional testing1 is a licensed or investigational. | Then . | ||||||
| Referenced Row in Guidance Document | Notify Consignee of test result | Destroy/Label prior collection | Transfusion service notify recipient | Release prior collection | ||||
| EIA 2.0 or 3.0 | RR | NFT | Sample available | |||||
| EIA 2.0 or 3.0 | RR | RIBA 2.0 | Positive | |||||
| NAT | Reactive, Nonreactive or NP | |||||||
| EIA 2.0 | RR | RIBA 2.0 | Negative | |||||
| NAT | Nonreactive or NP | |||||||
| EIA 3.0 | RR | RIBA 2.0 | Negative | |||||
| NAT | Nonreactive or NP | |||||||
| EIA 2.0 | RR | RIBA 2.0 | Negative | |||||
| NAT | Reactive | |||||||
| EIA 3.0 | RR | RIBA 2.0 | Negative | |||||
| NAT | Reactive | |||||||
| EIA 2.0 or 3.0 | RR | RIBA 2.0 | Indeterminate | |||||
| NAT | Nonreactive or NP | |||||||
| EIA 2.0 or 3.0 | RR | RIBA 2.0 | Indeterminate | |||||
| NAT | Reactive | |||||||
| EIA 2.0 | RR | RIBA 2.0 | Indeterminate | |||||
| (MTA) EIA 3.0 or alternative | Nonreactive | |||||||
| NAT | Nonreactive or NP | |||||||
| EIA 2.0 | RR | RIBA 2.0 | Indeterminate | |||||
| (MTA) EIA 3.0 or alternative | Reactive | |||||||
| NAT | Nonreactive or NP | |||||||
| EIA 2.0 or 3.0 | RR | RIBA 2.0 | Indeterminate | |||||
| NAT | Nonreactive or NP | |||||||
| RIBA 3.0 | ||||||||
| EIA 2.0 or 3.0 | NR | NAT only | Reactive | |||||
1 Appropriate additional testing includes all the antigens contained in the screening test that was performed
2 Collections during the 12 months prior to the date of the donation testing NAT reactive
3 Release collections more than 12 months prior to the date of the donation testing NAT reactive
NR means non-reactive
RR means repeatedly reactive
MTA means may test again
NFT means not further tested
NP means not performed
Table 3 - Historical Review
| You must take the following steps when donor test results for antibody to Hepatitis C Virus (anti-HCV) by Licensed HCV EIA 2.0 or EIA 3.0 are repeatedly reactive, or a licensed or investigational HCV RNA Nucleic Acid Test (NAT) is reactive. You must identify and quarantine prior collections, notify the consignees and request them to quarantine prior collections. Then . | ||||||||
| If the historical initial test is a licensed . | And the historical additional testing1 is a licensed or investigational . | Then . | ||||||
| Referenced Row in Guidance Document | Notify Consignee of test result | Destroy/Label prior collection | Transfusion service notify recipient | Release prior collection | ||||
| EIA 2.0 or 3.0 | RR | RIBA 3.0 | Positive | |||||
| NAT | Reactive, Nonreactive or NP | |||||||
| EIA 2.0 or 3.0 | RR | RIBA 3.0 | Negative | |||||
| NAT | Nonreactive or NP | |||||||
| EIA 2.0 or 3.0 | RR | RIBA 3.0 | Negative | |||||
| NAT | Reactive | |||||||
| EIA 2.0 or 3.0 | RR | RIBA 3.0 | Indeterminate | |||||
| NAT | Nonreactive or NP | |||||||
| EIA 2.0 or 3.0 | RR | RIBA 3.0 | Indeterminate | |||||
| NAT | Reactive | |||||||
1 Appropriate additional testing includes all the antigens contained in the screening test that was performed
2 Collections during the 12 months prior to the date of the donation testing NAT reactive
3 Release collections more than 12 months prior to the date of the donation testing NAT reactive
RR means repeatedly reactive
NFT means not further tested
NP means not performed
Table 4 - Historical Review
| You must take the following steps when donor test results for antibody to Hepatitis C Virus (anti-HCV) by Licensed HCV EIA 2.0 or EIA 3.0 are repeatedly reactive, or a licensed or investigational HCV RNA Nucleic Acid Test (NAT) is reactive. You must identify and quarantine prior collections, notify the consignees and request them to quarantine prior collections. Then . | ||||||||
| If the historical initial test is a licensed . | And the historical additional testing1 is a licensed or investigational . | Then . | ||||||
| Referenced Row in Guidance Document | Notify Consignee of test result | Destroy/Label prior collection | Transfusion service notify recipient | Release prior collection | ||||
| EIA 2.0 | RR | RIBA 2.0 | ||||||
| RIBA 3.0 | ||||||||
| EIA 2.0 | RR | EIA 3.0 or alternative | Nonreactive | |||||
| EIA 2.0 | RR | EIA 3.0 or alternative | Reactive | |||||
| (NFT) | ||||||||
| EIA 2.0 | RR | EIA 3.0 or alternative | Reactive | |||||
| RIBA 3.0 | ||||||||
| EIA 3.0 | RR | RIBA 3.0 | ||||||
| EIA 2.0 or 3.0 | RR | NAT | Nonreactive | |||||
| (MTA) RIBA 3.0 | ||||||||
| EIA 2.0 or 3.0 | RR | NAT | Nonreactive or Reactive | |||||
| (NFT) | ||||||||
| EIA 2.0 or 3.0 | RR | NAT | Reactive | |||||
| (MTA) RIBA 3.0 | ||||||||
| EIA 2.0 or 3.0 | RR | (NFT) | No Sample Available | |||||
1 Appropriate additional testing includes all the antigens contained in the screening test that was performed
2 Collections during the 12 months prior to the date of the donation testing NAT reactive
3Release collections more than 12 months prior to the date of the donation testing NAT reactive
RR means repeatedly reactive
MTA means may test again NFT means not further tested
Table 5 - Historical Review
| You must take the following steps when donor test results for antibody to Hepatitis C Virus (anti-HCV) by Licensed HCV EIA 1.0 are repeatedly reactive. You must identify and quarantine prior collections, notify the consignees and request them to quarantine prior collections. Then . | ||||||||
| If the historical initial test is a licensed . | And the additional testing1 is a licensed or investigational . | Then . | ||||||
| Referenced Row in Guidance Document | Notify Consignee of test result | Destroy/Label prior collection | Transfusion service notify recipient | Release prior collection | ||||
| EIA 1.0 | S/CO< 2.5 | (NFT) | ||||||
| EIA 1.0 | S/CO >2.5 or cannot be calculated | (NFT) | ||||||
| EIA 1.0 | EIA 2.0 or 3.0 or alternative | Reactive | ||||||
| (NFT) | ||||||||
| EIA 1.0 | EIA 2.0 or 3.0 or alternative | Reactive | ||||||
| (MTA)RIBA 2.0 or 3.0 or NAT | ||||||||
| EIA 1.0 | EIA 2.0 or 3.0 or alternative | Nonreactive | ||||||
| EIA 1.0 | NAT | Reactive | ||||||
| (NFT) | ||||||||
| EIA 1.0 | NAT | Reactive | ||||||
| (MTA) RIBA 2.0 | Negative | |||||||
| Indeterminate | ||||||||
| (MTA) RIBA 3.0 | Negative | |||||||
| Indeterminate | ||||||||
| EIA 1.0 | RIBA 1.0, 2.0, or 3.0; or Neut-Peptide EIA | Positive | ||||||
| EIA 1.0 | RIBA 1.0 or 2.0; or Neut-Peptide EIA | Indeterminate | ||||||
| (NFT) | ||||||||
| EIA 1.0 | NAT | Nonreactive | ||||||
| (NFT) | ||||||||
| EIA 1.0 | RIBA 1.0 or 2.0; or Neut-Peptide EIA | Indeterminate | ||||||
| NAT | Nonreactive | |||||||
| (MTA) EIA 3.0 or alternative; or RIBA 3.0 | ||||||||
| EIA 1.0 | RIBA 1.0 or 2.0; or Neut Peptide EIA | Indeterminate | ||||||
| NAT | Nonreactive or NP | |||||||
| (MTA) EIA 3.0 or alternative; or | Nonreactive | |||||||
| RIBA 3.0 | Negative | |||||||
| EIA 1.0 | RIBA 3.0 | Indeterminate | ||||||
| EIA 1.0 | RIBA 2.0 or 3.0 | Negative | ||||||
| NAT | Nonreactive or NP | |||||||
| EIA 1.0 | RIBA 2.0 or 3.0 | Negative | ||||||
| NAT | Reactive | |||||||
| EIA 1.0 | RIBA 1.0 or Neut-Peptide EIA | Negative | ||||||
| (NFT) | ||||||||
| EIA 1.0 | RIBA 1.0 or Neut-Peptide EIA | Negative | ||||||
| (MTA) EIA 2.0 or 3.0 or alternative; or RIBA 3.0; or NAT | ||||||||
1 Appropriate additional testing includes all the antigens contained in the screening test that was performed
2 Collections during the 12 months prior to the date of the donation testing NAT reactive
3Release collections more than 12 months prior to the date of the donation testing NAT reactive
NR means non-reactive
RR means repeatedly reactive
MTA means may test again
NFT means not further tested
Footnotes
1The Chiron Corporation (Emeryville, CA) RIBA 2.0 and RIBA 3.0 are immunoblot assays based on recombinant antigens of HCV.
2 The RIBA 1.0 and the Abbott HCV neutralization EIA and peptide-1 and peptide-2 supplemental tests were not approved by FDA. Data from these supplemental tests are available for only a limited number of donations. However, FDA believes that data gathered from these tests, when available, are useful in defining the scope of HCV lookback.


