Guidance for Industry
Providing Regulatory Submissions to the Center for Biologics Evaluation and Research (CBER) in Electronic Format - Lot Release Protocols
[PDF printable version - 77 KB]
This guidance is for immediate implementation.
FDA is issuing this guidance for immediate implementation in accordance with 21 CFR 10.115(g)(4)(i). Submit written comments on this guidance at anytime to the Division of Dockets Management (HFA-305), Food and Drug Administration, 5630 Fishers Lane, Rm. 1061, Rockville, MD 20852. Submit electronic comments to either http://www.fda.gov/dockets/ecomments or http://www.regulations.gov. You should identify all comments with Docket No. 1998D-0315.
Additional copies of this guidance are available from the Office of Communication, Training and Manufacturers Assistance (HFM-40), 1401 Rockville Pike, Suite 200N, Rockville, MD 20852-1448, or by calling 1-800-835-4709 or 301-827-1800, or from the Internet at http://www.fda.gov/cber/guidelines.htm.
For questions on the content of this guidance, contact the CBER Product Release Branch at 301-594-6517.
U.S. Department of Health and Human Services
Food and Drug Administration
Center for Biologics Evaluation and Research
November 2007
Contains Nonbinding Recommendations
Table of Contents
- INTRODUCTION
- DISCUSSION
- GENERAL FILE AND FOLDER FORMAT
- File and Folder Organization
- Hypertext Links and Bookmarks
- SUBMITTING LOT RELEASE PROTOCOLS AND TEST RESULTS IN ELECTRONIC FORMAT
- Media Labeling
- Packaging and Shipping
- Delivery Address
- APPENDIX
- Example of PDF Bookmarks From an Electronic Lot Release Protocol
- Sample CD-ROM Label
Guidance for Industry
Providing Regulatory Submissions to the Center for Biologics Evaluation and Research (CBER) in Electronic Format - Lot Release Protocols
| This guidance represents the Food and Drug Administration's (FDA's) current thinking on this topic. It does not create or confer any rights for or on any person and does not operate to bind FDA or the public. You can use an alternate approach if the approach satisfies the requirements of the applicable statute and regulations. If you want to discuss an alternative approach, contact the appropriate FDA staff. If you cannot identify the appropriate FDA staff, call the appropriate number listed on the title page of this guidance. |
File and Folder Organization
- Description of the submission
- Identification of each lot release protocol as a separate PDF file with its corresponding filename
- Statement that the submission is virus free with a description of the software (name, version, and company) used to check the files for viruses
- Regulatory and technical point of contact for the submission
Hypertext Links and Bookmarks
Lot release protocols are typically 8-10 pages in length. We recommend that you use functional bookmarks to facilitate navigating the protocols. We provide an example of PDF bookmarks in Appendix 1 at the end of this guidance.
SUBMITTING LOT RELEASE PROTOCOLS AND TEST RESULTS IN ELECTRONIC FORMAT
Media Labeling
Packaging and Shipping
Delivery Address
-
APPENDIX
Example of PDF Bookmarks From an Electronic Lot Release Protocol
- ELECTRONIC PROTOCOL - 20040001.PO
- License No./ Product Code /Type of Lot[-B, -FC, -C]
- Lot Number
- Proper Name of Product
- Firm Name and Address
- Reason for Submission
- Test Results
- Potency
- Specific Activity
- pH
- Moisture
- Total Protein
- Solubility
- Sterility
- Sterility Bulk
- Sterility Final Container
- General Safety
- Purity
- LAL (limulus amebocyte lysate)
- Pyrogen
- Laser Densitometer Scan
- LD Scan
- LD Scan Reference
- Pass Statement
- Signature Block
- Electronic Protocol: 20040001.P0
Sample CD-ROM Label
We, the Center for Biologics Evaluation and Research (CBER), are issuing this guidance under 21 CFR 601.14(a) to assist you, manufacturers of biological products regulated by CBER, in submitting lot release protocols in electronic format to CBER's Product Release Branch. This guidance supersedes the guidance of the same title dated July 2006. We are updating this guidance to delete references to 3.5 inch diskettes due to changes in technology that are phasing out the use of this type of electronic format.
FDA's guidance documents, including this guidance, do not establish legally enforceable responsibilities. Instead, guidances describe the FDA's current thinking on a topic and should be viewed only as recommendations, unless specific regulatory or statutory requirements are cited. The use of the word should in FDA guidances means that something is suggested or recommended, but not required.
In accordance with section 610.2 (a) in Title 21 of the Code of Federal Regulations (CFR), CBER may require you to submit, for CBER review and confirmatory testing, samples of any lot of any licensed product, together with the protocols showing results of applicable tests. Regulatory submissions in electronic formats, consistent with lot release requirements applicable to your product, will facilitate our review of your submission, provided that you submit your data to us in an electronic format that we can readily access. Pursuant to 21 CFR 11.2(b)(2), FDA has identified such submissions in public Docket No. 92S-0251 as being the type of submission the agency accepts in electronic form (e.g., compact disk-read only memory (CD-ROM) or other formats that may become available in the future). This guidance is intended to provide you with recommendations for submitting lot release protocols showing results of applicable tests in an electronic format, as provided in 21 CFR Part 11.1 By following these recommendations for preparation and submission of electronic lot release documents, you might prevent a delay in the product release processing.
In the Federal Register of December 8, 1995 (60 FR 63048), we announced that we no longer require routine lot-by-lot release for specified categories of biological products subject to licensure (21 CFR 601.2 (c)) previously referred to as well-characterized therapeutic recombinant DNA-derived and monoclonal antibody biotechnology products. This guidance is not intended to modify that document.
We are not providing specific instructions for the construction of portable document format (PDF) files in this guidance. We expect that the draft guidance titled, "Providing Regulatory Submissions in Electronic Format - General Considerations, October 2003," when finalized, will do so.
We recommend that you submit lot release submissions to CBER's Product Release Branch in an electronic format. Currently, our preferred format is CD-ROM, as formats such as 3.5 diskettes are becoming obsolete. Each CD-ROM should include a Cover Letter (cover.pdf) file with the following information:
We recommend that you submit lot release information for each lot under a separate and unique filename constructed as follows. We recommend that you use the old Disk Operating System (DOS) standard of 8.3 characters because of the simplicity of the naming system, and that you avoid the use of special characters. We do not recommend that you use standard file extensions such as .pdf. We describe our recommendations below. In order to use different extensions, we recommend that you use any conversion program commercially available to change the word-processing document to PDF, and select the File "Save As" command. This should allow you to change the .pdf extension to one of the extensions described below. We recommend that you do not use the Security option and passwords on the submission, as these will make it difficult for us to access your data.
You should divide the filename into three sections: (1) the first four digits represent the year of the submission (e.g., 2004), (2) the next four digits represent the sequential submission number of that year (e.g., 0003), (3) the two to three alphanumeric extension (the three allowable characters, numbers or letters following the period) represents the type of submission (e.g., .P0 (zero) designates a submission under the original protocol). Thus, 20040003.P0 represents the third lot release submission of 2004 under the original protocol.
A corrected lot release protocol is a submission to correct minor clerical or transcription errors, or to clarify lot release information in response to questions by FDA. For submissions under a corrected lot release protocol, designate each corrected protocol using ".PC" followed by the correction number (i.e., .PC1 for first corrected protocol, .PC2 for second corrected protocol, etc.). Thus, the first correction of the third original protocol submission of 2004 should be 20040003.PC1.
We recommend that you attach physical labels constructed as follows on CD-ROMs, and CD-ROM jewel cases to provide visible identification of your submission. You should include the following information: (1) manufacturer name, (2) date of submission in the format of DD-MMM-YYYY, with DD and YYYY being numerical and MMM being the first three letters of the month (e.g., AUG for August), (3) title, including cc, STN, license number, product code(s) (if applicable), and type of lot, (4) electronic protocol filename(s), and (5) lot number(s) of the protocol(s). We provide examples of labeled media in Appendix 2 and 3 at the end of this guidance. We recommend that you consult the CD-ROM manufacturer before using felt-tip pens on CD-ROMs, as some pens contain dangerous solvents that may damage the CD-ROM.
You should package CD-ROMs carefully to ensure that they arrive in a usable condition. Jewel cases are less vulnerable when shipped in envelopes with bubble type protective material or stiff backing. Mailing envelopes padded with paper material only typically do not provide adequate protection for shipping CD-ROMs.
You should send electronic protocol(s) and test results, with or without lot release samples, to the following address. If you are sending lot release samples, you must send them by courier service (21 CFR 600.2(c)).
Sample Custodian (ATTN: HFM-672)
Food and Drug Administration
Center for Biologics Evaluation and Research
Bldg: NLRC-B, Room: 113
5516 Nicholson Lane
Kensington, MD 20895
To facilitate our review of your submission, you may contact Joseph Quander at the CBER, Product Release Branch, at (301) 594-6517, or (301) 594-6924 (fax) before switching from submission on paper to submission on electronic format.
A) Single Product Submission  |
B) Multiple Product Submission 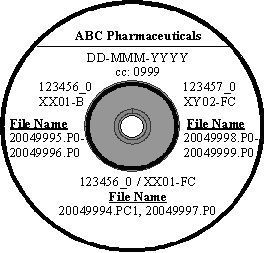 |
||||||||||||||||||||||||||||||||||||||
C) CD Jewel Case, inside cover for Disk A
|
D) CD Jewel Case, inside cover for Disk B
|
||||||||||||||||||||||||||||||||||||||
1 Note that FDA has issued guidance describing an interim policy of enforcement discretion relating to certain Part 11 requirements. http://www.fda.gov/cber/gdlns/prt11elect.pdf


