


FDA
Home Page | CDRH Home Page | Search
| CDRH
A-Z Index | Contact CDRH
![]()
   |
| FDA
Home Page | CDRH Home Page | Search
| CDRH
A-Z Index | Contact CDRH
|
 |

|
U.S. Department of Health and Human Services
Public Health Service
Food and Drug Administration
Center for Devices and Radiological Health
OFFICE OF SCIENCE AND ENGINEERING LABORATORY DIVISIONS
Host Response: Tissue-Materials Interactions and Tissue-Device Interactions
Biological Risk Assessment
Biotechnology and Biomolecular Studies
Genomic and Genetic Devices
Materials Characterization and Polymer Degradation
Medical Imaging and Diagnostics
Fluid Dynamics and Ultrasonics
Radiation Bioeffects
Electrophysiology and Electrical Stimulation
Electrical, Electronics, and Software Engineering
Optical Physics – Diagnostics and Therapeutics
Optical Radiation Safety and Devices
Electromagnetics and Wireless Technologies
Radiological Health and Safety
Mechanics of Materials and Structures
Standards Management
APPENDIX A – OSEL Publications
APPENDIX B – OSEL Presentations
APPENDIX C – OSEL Academic Affiliations
APPENDIX D – OSEL Patents
APPENDIX E – OSEL-Sponsored Seminars
APPENDIX F – Interagency Agreements
APPENDIX G - FDA FY 2004 Award Grants for Collaborative Science Projects
APPENDIX H - Abbreviations and Acronyms
The mission of the Food and Drug Administration’s (FDA) Center for Devices and Radiological Health (CDRH) is to promote and protect the health of the public by ensuring the safety and effectiveness of medical devices and the safety of radiological products.
The Office of Science and Engineering Laboratories (OSEL), formerly the Office of Science and Technology (OST), is one of seven Offices within the Center for Devices and Radiological Health (CDRH). The seven CDRH Offices are comprised of six program offices (of which OSEL is one) and one administrative and technological support office. OSEL serves as the laboratory science nucleus for the Center. Specifically, OSEL supports the scientific basis for the Agency’s regulatory decision- making by developing independent laboratory information for regulatory and other public health activities of CDRH. In addition to providing consultation to the Center’s regulatory experts, OSEL researchers are involved in mission-oriented science activities including test methods development, risk assessments, forensic investigations, product evaluations, and technology forecasting.
From a science standpoint, OSEL conducts laboratory and field research in the areas of physical, life, and engineering sciences as related to the human health effects of medical devices. CDRH relies upon this work to support its efforts ensuring public safety in areas as varied as accredited mammography facilities, breast implants, or drug eluting stents.
Since mid-2003, the Office has undergone at least three major changes that have helped shape the new organization, the Office of Science and Engineering Laboratories (OSEL). The first was the move of the newly reorganized Division of Biology to the newly constructed FDA Life Science Laboratories in White Oak, Maryland. This move was the beginning of a planned consolidation of FDA facilities. The remaining OSEL divisions are expected to move to the White Oak facilities in 2007. The second change concerns the science prioritization process. In the beginning of 2004, the Office conducted a review of all 14 programs in an ongoing process to bring advice from the rest of CDRH and FDA to assist in developing the direction for the lab programs. The third and final major change is the reorganization itself. OST was formally reorganized in early 2004 to improve the overall operating efficiency of the Office and to better integrate it into the mission and functions of CDRH. This reorganization is expected to clarify the ongoing research within the Office for both FDA and outside scientists. The reorganization has created a new structure in which six new divisions have replaced the four former divisions in OST and has removed all designated branches. The new office is named the Office of Science and Engineering Laboratories (OSEL).
This reorganization has taken place at a crucial time. Over the past few years, with MDUFMA (Medical Device User Fee and Modernization Act of 2002) legislation and accompanying resources, the Office has had an opportunity to broaden and improve its scientific program. This gives the management an excellent incentive to increase the collaboration with other components of CDRH. Finally, with the recent move of the life sciences staff to White Oak and the impending construction of the engineering and physics building, the prospects for OSEL are promising.
OSEL long-term goals focus on the following:
The OSEL Annual Report provides current information about the Office’s organization and intramural science activities; provides a summary of the Office’s direct laboratory support for pre-market review and compliance cases; and provides a bibliography of scientific publications, presentations, and research seminars for the fiscal year. OSEL management welcomes comments on the programs described in this report. We hope you find this report useful and informative, and your comments are welcome.
For additional information, please contact us at 301.827.4777.
Larry G. Kessler, Sc.D.
Director
Office of Science and Engineering Laboratories
DB participates in the Center's mission by conducting research, participating in device review activities, developing consensus standards both domestic and international, developing regulatory guidance, testing forensic and regulatory samples, and providing educational programs in the area of biological sciences. Specifically, DB conducts research to support the Center’s mission to assure the safety and effectiveness and promote the improvement of medical devices in the areas of biological risk assessment, biosensors/nanotechnology, genomic and genetic technologies, infection control and sterility, tissue-device interactions, toxicity/biocompatibility, and radiation bioeffects. Through laboratory studies, researchers evaluate the potential adverse effects of medical devices on host biological systems and, in collaboration with engineering divisions, identify the source and impact of product degradation on organ systems both under acute and chronic conditions. The Division staff develops measurements methods and analytical procedures to characterize and evaluate devices and products, studies molecular and cellular mechanisms and bioeffects of biomaterials, and supports the Center’s enforcement and product testing activities.
The DB staff members are primarily biologists, chemists, and biomaterials scientists.
DIVISION OF CHEMISTRY AND MATERIALS SCIENCES (DCMS)
DCMS participates in the Center's mission by conducting research, participating in device review activities, developing consensus standards both domestic and international, developing regulatory guidance, testing forensic and regulatory samples, and providing educational programs in the area of chemistry and materials sciences. Specifically, the DCMS focus is on the developing experimental data, test methods and protocols for regulatory and scientific activities involving multicomponent mass transfer, reaction kinetics, absorption and swelling of network polymers, polymer processing, modeling of physiological processes, and materials degradation. Research conducted in the division includes polymer synthesis; synthesis of polymeric nanocomposite materials; sensors; thermodynamics; thermal transitions and phase stability; hydrogel and biopolymer synthesis and characterization; polymer formulation; separations; spectroscopy; small-angle x-ray and neutron scattering; and shelf-life and service life prediction. DCMS tests the performance of chemical processes of importance to medical devices, such as mass transfer through membranes used in dialysis and blood oxygenation, and manufacturing processes used to fabricate materials.
The technical disciplines of the DCMS staff include physical chemistry, chemical physics, polymer science, pharmacology, materials science, and biomedical and chemical engineering.
DIVISION OF ELECTRICAL AND SOFTWARE ENGINEERING (DESE)
DESE participates in the Center's mission by conducting research, participating in device review activities, developing consensus standards both domestic and international, developing regulatory guidance, testing forensic and regulatory samples, and providing educational programs in the area of electrical engineering and software. Specifically, the DESE works in the application of electronics, software engineering, and systems engineering body of knowledge to the regulation of medical devices and electronic products that emit radiation. The division addresses the cutting edge of medical devices through all phases of the product life cycle and all aspects of the product manufacturer’s business, from research and development through procurement, production, and ongoing customer support. DCMS hosts the following resources and capabilities: analog and digital circuit design, data acquisition and display, embedded microprocessor and PC-based systems, software-based virtual instruments, quality management and risk management as applicable to electronics and software, testing for hazards arising from the use of electrical and electronic technology in medical products, and electronic design including components, circuits, and analytical techniques for controlling high voltages and/or currents.
DESE staff members are primarily electronics engineers, physicists, biomedical engineers, and general engineers.
DIVISION OF IMAGING AND APPLIED MATHEMATICS (DIAM)
DIAM participates in the Center's mission by conducting research, participating in device review activities, developing consensus standards both domestic and international, developing regulatory guidance, testing forensic and regulatory samples, and providing educational programs in the area of medical imaging and applied mathematics. Specifically, DIAM provides scientific expertise and carries out a program of applied research in support of CDRH regulation of radiation-emitting products, medical imaging systems, and other devices utilizing computer-assisted diagnostic technologies. Medical imaging research encompasses ionizing and non-ionizing radiation from data capture through image display and observer performance. The computer-assisted diagnostics work of DIAM is focused on the appropriate mathematical evaluation methodologies for sophisticated computational algorithms used to aid medical practitioners interpret diagnostic device results. The Division is charged with developing and disseminating performance assessment methodology appropriate to these modalities. DIAM operates a calibration laboratory for ionizing radiation detection instruments and participates in a full range of programs in support of the Public Law 90-602 mission of the Center.
DIAM staff members are primarily physicists, mathematicians, and physical science technicians.
DIVISION OF PHYSICS (DP)
DP participates in the Center's mission by conducting research, participating in device review activities, developing consensus standards both domestic and international, developing regulatory guidance, testing forensic and regulatory samples, and providing educational programs in the area of physics. Specifically, DP conducts research and engineering studies to support the Center’s mission to assure the safety and effectiveness of medical devices and electronic products, and to promote their improvement. Scientific and technical specialties in the division include optical physics and metrology, sensors, fiber optics, electromagnetics, electromagnetic compatibility and electromagnetic interference, electrophysics and electrical stimulation technologies, electrophysiology, radiofrequency/microwave metrology, and minimally invasive optical and electromagnetic technologies. The Division develops measurement methods, instrument calibration capabilities and analytical procedures to characterize and evaluate devices and products, and supports the Center’s enforcement and product testing activities. DP evaluates interactions of electromagnetic and optical energy with matter, analyzes implications for the safety and effectiveness of devices and products, and develops and evaluates procedures for minimizing or optimizing human exposure from such devices.
The technical disciplines of DP staff include physics, mathematics, biophysics, biomedical engineering, electronics, and general engineering.
DIVISION OF SOLID AND FLUID MECHANICS (DSFM)
DSFM participates in the Center's mission by conducting research, participating in device review activities, developing consensus standards both domestic and international, developing regulatory guidance, testing forensic and regulatory samples, and providing educational programs in the area of solid and fluid mechanics. Specifically, the core responsibilities of this division involve issues for which mechanical interactions or transport are of primary concern, such as those involving motion; structural support, stabilization, or vibrations; device and material mechanical integrity; materials durability; and biologically relevant parameters of device and materials. The division has expertise in the areas of fluid dynamics, solid mechanics and materials, acoustics and ultrasonics. DSFM develops measurement methods, instrument calibration capabilities, and analytical procedures to characterize and evaluate devices, device materials, and products, and supports the Center's enforcement and product testing activities. The division staff also evaluate interactions of ultrasound energy with matter and the implications of these interactions on the safety and effectiveness of devices and products.
Technical disciplines of the DSFM staff include mechanical engineering, materials science, biomedical engineering, general engineering, and physics.
STANDARDS MANAGEMENT STAFF (SMS)
The SMS is responsible for developing, managing, and supporting standards used for regulatory assessments. SMS manages the participation of CDRH and other FDA staff in standards development. This involves working closely with the Standards Developing Organizations (SDOs), advertising standards liaison representative positions, facilitating a Center recommendation to serve on a particular standards activity, maintaining a standards database that provides access to established standards to all CDRH staff and field inspectors.
SMS increases the recognition of voluntary consensus standards for medical devices and radiation-emitting electronic products. The Standards Program was created as a result of the Food and Drug Administration Modernization Act (FDAMA) of 1997. Although CDRH had been involved in the development of medical device standards for decades, FDAMA formalized the process. As part of this responsibility, the staff publishes lists of recognized standards annually and consistently increases the list of available standards.
SMS supports participation in medical device standards committees. The staff accomplishes these tasks with the help of Standards Task Groups (STGs). Additionally, the SMS assists in setting teams in the development of guidance documents that help CDRH stakeholders in improving the quality of submissions as well as in faster approval of device applications.
MANAGEMENT SUPPORT STAFF (MSS)
MSS provides leadership and support to the Office of the Director, Division Directors, and laboratory professionals on all administrative, general management, and knowledge management issues. MSS is responsible for planning, developing, and implementing Center and OSEL programmatic matters concerning financial management, personnel, procurement, contracts, inter-agency agreements, employee training, and facilities.
MSS is tasked with the managing and administering OSEL resources designed to support ongoing programs. The staff ensures the proper distribution of operating and payroll dollars, facility plans, procurement and property, travel requests and ADP needs. MSS advises the Office of the Director on potential issues that may affect resources, staffing, and management issues to comply with policies and avoid potential conflicts. In addition, MSS directs and conducts special assignments or projects for the Center as well as the Office Director.
MSS is also tasked with Knowledge Management Support (KMS) for the office. The KMS team provides technical support for the acquisition, retrieval, and analyses of data supporting the office’s mission including developing specialized databases and related applications where needed. Additionally, the staff performs specialized activities associated with the development, design, installation, and administration of data processing systems, particularly those that are integral to laboratory functioning.
The KMS team collaborates with the Office of Systems and Management (OSM) and the Office of IT Shared Services (OITSS) in developing major initiatives involving OSEL, CDRH, and FDA data and systems. The KMS staff also coordinates OSEL activities with these offices to assure compliance with Center and FDA policies regarding data structure and format and with FDA initiatives to assure data consistency and compatibility.
This is an interconnected program of laboratory research, risk assessment, and standards development activities designed to provide a scientific basis for regulatory decision making in CDRH. The data on potential adverse effects of medical device materials and chemicals gathered from pre-clinical experimental approaches in this program are used to reduce uncertainties in assessing risks to patients exposed to physical and chemical insults, and protect their health.
BackgroundIn 1983, the Bureau of Radiological Health and the Bureau of Medical Devices were merged into the Center for Devices and Radiological Health. This merger presented the new Center with a disparity between the research programs devoted to radiation issues and those devoted to medical device issues. Additionally, a discontinuity existed between classical chemical toxicology and the potential adverse health effects posed by exposure to medical devices. To address this need, OSEL expanded the existing radiation research program to include medical device toxicology. More recently, the program has evolved to address the development of toxicological and microbiological approaches to risk assessment and investigation of biological issues relating to infection control and tissue-engineered medical products (TEMPs).
Program DescriptionThe “Host Responses: Tissue-Materials Interactions, Tissue-Device Interactions” research program encompasses two major areas: 1) Biological Effects of Chemicals and Medical Device Materials, and 2) Infection Control.
1) Biological Effects of Chemicals and Medical Device Materials
OSEL is conducting a wide range of projects designed to examine the biological effects of chemicals released, intentionally or unintentionally, from medical device materials or the tissue-device interactions themselves. The general goals of these studies are to evaluate the safety of these chemicals and materials and to develop or refine test methods that improve preclinical testing of device materials. Studies in this area fall into several subcategories:
Immunological/Inflammatory/Proliferative effects . OSEL scientists are conducting research to examine the immunological, inflammatory, and proliferative effects of materials and chemicals released from materials, including examination of the stimulation of chronic inflammation by particles using both in vitro and in vivo models, and the induction of allergic responses by material constituents, such as natural rubber latex proteins and metals. In addition, scientists are conducting research on the ability of compounds incorporated into a device (e.g., drug-eluting coronary stents) that are intentionally released in order to mitigate inflammatory or cell proliferative responses induced by the device.
Toxicity of compounds released from medical device materials . OSEL is involved in investigating the adverse effects of compounds (e.g., metals, DEHP, ethylene oxide, bisphenol A, endocrine disruptors) released from medical device materials using small and large animal models, developing toxicity tests specific for medical device materials (e.g., polymers that cure in situ), and developing biomarkers to detect early cell and tissue damage caused by compounds released from devices.
Biological effects of nanotechnology products and tissue engineered medical products (TEMPs). The development of TEMPs and nanoparticles in health care delivery is at the cutting edge of medical device technology. OSEL is developing test methods to examine the potential tissue interactions of these materials and medical devices, such as TEMPs scaffold materials and nanoparticles, in patients receiving the device and on the cells and tissues that are components of the device.
2) Infection Control
Infection at the site of an implanted device represents a potentially devastating event, often requiring surgical intervention to remove the device. Prevention of infection is the key to infection control and a wide range of CDRH-regulated devices are required to ensure sterility in surgical procedures. OSEL scientists have addressed the issues of infection control through the development of cleaning procedures for new and reusable devices; examination of disinfection and sterilization equipment and procedures; assessment of chemical sterilant residuals on devices; development of test methods to ensure that barriers such as surgical drapes, gowns and gloves are tested for effectiveness in preventing transmission of microorganisms; and evaluation of the impact of bacterial adherence to materials (biofilms and endotoxins) on infection risks.
Relevance to FDA and Public Health ImpactThe experimental studies in this laboratory research program generate independent data for assessing toxicological risks and for developing standards and guidance documents. OSEL remains at the forefront in medical device toxicology and for developing methods for risk assessment. Specifically, OSEL serves as an independent source of data on medical device toxicology and risk assessment for risk managers in CDRH Offices. These data and risk assessments provide a scientific basis for developing important pre-clinical and post-market activities, such as developing ASTM standards for testing biological responses to particles both in vivo (F1904-98) and in vitro (F1903-98), ISO standards (e.g., ISO 10993-17) for establishing tolerable intake values, Federal rule-making (e.g., for natural rubber latex protein content in gloves and condoms), and for risk management decision-making in the Center (e.g., FDA Public Health Notification for DEHP in medical plastics).
Program AccomplishmentsInteractions of Host and Interventional Therapeutics in Humans and Swine Models
There have been changes in the project planning that resulted from preliminary research findings. These research findings are the result of the two major technical accomplishments of the past year:
The adult domestic swine on a diet high in fat and cholesterol show de novo fatty lesions containing macrophages and foam cells. These lesions were especially prevalent in the proximal coronary arteries and most marked in the right coronary artery as compared to the left anterior descending and left circumflex artery. Additionally, coronary stenting of adult domestic swine on a diet high in fat and cholesterol showed delayed healing that is more like that seen with humans as compared to that in normal swine. Foam cell accumulation was increased in the intact male animals that also showed the most advanced de novo lesions and in-stent lesions. Moreover, the healing response in some of the animals fed this diet suggests development of vulnerable plaque in the target arteries. Since either of these findings would lead to more predictive animal models of vascular disease and intervention, the work with this model of disease has been shifted to a higher priority. Furthermore, the initial study of estrogen in normal animals has shown significant therapeutic effectiveness in reducing the adverse healing response post-intervention.
Given the potential clinical ramifications and the need for safety and pharmacokinetics data on the two drugs currently incorporated in drug eluting coronary stents, the use of the local drug delivery model has been supported as a primary research effort. The local drug delivery model and model of vascular disease induced by diet will be combined over the next year.
Vascular Genomics
Animal Models of Vascular Disease, Intervention and Local Drug Delivery
The objectives have been partially met.
Long-term objectives include 1) develop and establish test methods and models for evaluation of potential adverse effects of medical device materials, and medical devices, including elucidation of new, clinically relevant, and sensitive biomarkers to predict adverse effects in the preclinical stages of product development, and 2) characterize the potential adverse effects using pre-clinical laboratory models and utilizing the data to predict the likelihood of adverse effects in humans.
Risk assessment is the process of determining the extent of human health hazard relative to exposure conditions. Staff in the OSEL biological risk assessment program conduct research to address CDRH’s regulatory need for improved methods of detecting and quantifying risks associated with 1) chemical compounds released from medical device materials, 2) microorganisms associated with medical devices, and 3) exposure to radiation. Research in the program is designed to reduce uncertainties in the risk assessment process and to support risk management decision-making in the Center. The program is closely linked with the Tissue-Material Interaction Program and includes staff with expertise in toxicological, microbial, and radiation risk assessment.
Background
OSEL staff has long been responsible for conducting risk assessments of compounds released from medical device materials. These risk assessments have been directly used to support regulatory decision making in the Center (e.g., microbial risk assessment to support Sterility Assurance Levels, DEHP Safety Assessment to support the issuance of a Public Health Notification and draft labeling guidance). More recently, staff have been involved with the development of the ISO 10993-17 standard, Method for the Establishment of Allowable Limits for Residues Using Health Based Risk Assessment. Toxicity studies used for the risk assessment of compounds released from medical devices are almost always conducted using healthy animals; however, patients exposed to these compounds may be critically ill or injured.
A number of studies have demonstrated that the potency of some compounds is potentiated by conditions such as renal failure, liver failure, and sepsis. Therefore, a tolerable intake (TI) value derived for a compound in a study that uses healthy animals may not be adequately protective for a critically injured patient. In the ISO/DIS 10993-17 standard, the approach for deriving a TI involves application of a default Uncertainty Factor (UF) of 10 to account for inter-individual variability in the human population, in the absence of more specific data to identify sensitive subpopulations. However, it is not clear whether this default UF is adequate to protect critically ill or injured patients from the toxic effects of compounds released from medical devices. To address this broad issue, animal models of compromised health will be developed in our laboratory and used to examine whether the potency of compounds is increased in experimental animals with compromised health compared to healthy animals. These models will also be used to assess the impact of ultrasound contrast agents on the vascular endothelium and to develop new devices that can be used
Program DescriptionFDA's Center for Devices and Radiological Health (CDRH) is responsible for ensuring the safety and effectiveness of medical devices and eliminating unnecessary human exposure to man-made radiation from medical, occupational and consumer products. This broad mandate requires chemical, microbial, and radiation risk assessments to be performed to support regulatory decision making in these areas. Chemical risk assessment activities in CDRH focus on three areas: 1) the development and validation of new risk assessment methodologies, 2) bench-top research to provide information for the hazard identification and dose-response assessment stages of the risk assessment process, and 3) the application of risk assessment approaches to assist with regulatory decision making. Risk assessments have been used by CDRH to assist in reaching decisions on various issues that have received considerable attention in the media, including the safety of phthalate esters released from PVC devices, dioxin released from tampons, and 2,4-toluenediamine released from polyurethane-foam covered breast implants. The research component of the program is key in addressing uncertainties regarding the response of sensitive subpopulations to the effects of chemical compounds. Radiation risk assessment activities in CDRH focus on three areas: 1) assessing the risk of skin cancer, particularly malignant melanoma, from exposure to tanning lamps, 2) evaluating published results that state that there are possible health risks from exposure to radiofrequency radiation from cellular telephones, and 3) assessing any potential increases in risk from unnecessary ionizing or non-ionizing radiation from regulated medical devices and radiological products.
Relevance to FDA’s and CDRH’s Mission, Program, and Public Health ImpactEfficient, science-based risk management is a component of the FDA Commissioner’s five-part strategic action plan to protect and advance the health of Americans (http://www.fda.gov/oc/mcclellan/strategic.html). Risk assessment is the first step in the risk management process. The OSEL program in risk assessment involves laboratory-based efforts to address risk assessment uncertainties, development and validation of new risk assessment methodologies, and use of risk assessment to support regulatory decision-making. A key laboratory-based effort is directed towards examining whether critically ill or injured patients represent a sensitive subpopulation and can be more susceptible to adverse effects of chemicals. Research is also being conducted to address data gaps identified in risk assessments performed by the research staff (e.g., studies on the pulmonary effects of DEHP). Risk assessment methods are being developed as part of the process to create consensus standards for the biological evaluation of medical devices under the auspices of ISO.
Program AccomplishmentsAnimal Model of Compromised Health for Biocompatibility and Risk Assessment
Objective 1: Develop and validate an animal model of subclinical renal injury (SRI).
The Critical Path initiative calls for a new product development toolkit containing powerful scientific and technical methods such as more predictive and clinically relevant animal models. Consistent with this goal, we have successfully developed and validated an animal model of SRI that is more sensitive to a low dose of nephrotoxicant compared to healthy control animals. The model features improvements over existing renal failure models:
The animal model:
Objective 2: Identify new biomarkers of early kidney injury.
Another component of the new product development toolkit is the development of new, sensitive and clinically relevant biomarkers of safety and effectiveness. Our work to develop new biomarkers of kidney injury is consistent with this critical path goal and has yielded two interesting results, in collaboration with CDER colleagues:
Objective 3: Develop an in vitro alternative model for renal injury biomarkers of nephrotoxicity. Seminar update to Office of Science and Health Coordination, CAFDAS,
The program’s laboratory research is designed to ensure that there are consistent testing requirements and that the Center is aware of and understands cutting-edge biological technologies in developing new devices or device materials.
BackgroundStudies are proposed to address safety concerns about current and impending submissions of combination products, including the importance of possible immunotoxic reactions to incorporated biomaterials. The goal of these studies is to develop critical issues in the emerging field of combination product (i.e., biological matrices used in wound healing); issues in cardiovascular surgery; and issues addressing cell encapsulation. These studies will also address the potential of combination products to cause chronic inflammation.
Program DescriptionLaboratory investigations will address intravascular catheters that are coated or impregnated with antimicrobial or antiseptic agents. It is important to assess microbial contamination of catheters and other medical devices and to compare and evaluate the efficacy of antimicrobial treatments. These studies use biosensors that are tools for effective real-time biological analysis. Bioluminescence is explored as the technology for such analyses.
Other studies explore the detailed mechanism of action of taxol or sirolimus in drug elutes stents. Mechanisms of action of platelets and delayed endothelial healing leading to thrombosis will be investigated. Laboratory in vivo and in vitro exploration of neointimal hyperplasia leading to in-stent re-stenosis, as well as clarifying the factors that contribute to fatalities, are planned in these studies. Techniques using differential display will be developed for these analyses.
Relevance to FDA’s and CDRH’s Mission, Program, and Public Health ImpactUsing new technology in developing medical devices for pre-market approval is anticipated as the trend of products generated by creative research and biotechnological progress. Much of the laboratory work at CDRH evaluates production of leachables released from devices. Questions about whether a finished device, device material, or extract of the device produce adverse biological effects can be addressed using new technology. Output from this work would appear in guidance documents, in review consults, and via scientific exchange. Reviewers can use these resources for rapid access to cutting-edge information.
Program AccomplishmentsMethods Development to Assess Endocrine Disruptor Properties of Medical Device
Technical accomplishments
The project examines the potential hormone-like effects of materials found in certain medical devices, focusing on bisphenol A, a basis for polycarbonates and a plasticizer. Bisphenol A has been found in dental sealants (where it can make up to 50% of the product), kidney dialysis filter beds, labware, and flexible tubing. It has been shown to be an endocrine disruptor and mimics the actions of the hormone estrogen; thus, it has the potential to affect women's health, reproductive capabilities, and fetal development.
Our efforts in the last 4 years on this project demonstrated that bisphenol A mimics the hormone estrogen in the murine uterus.
This year’s efforts were focused towards a different assay system, examining effects in neurological tissue that is responsive to estrogens, the hippocampus of fetal rats. The hippocampus is a major integration area of the brain and controls memory and many of the hormonal responses of the body. This tissue is responsive to estrogens but not to other hormones and compounds that might interfere with the assay. We looked at up-regulation of gene expression by bisphenol A and estrogen. A major technical accomplishment involved the isolation of hippocampi from fetal rat brains. These cells were grown in
tissue culture, subsequently exposed to bisphenol A or estrogen, and cellular RNA was harvested. The RNA was used in a "gene microarray" system to identify those genes that were up-regulated. These results were compared in order to determine if bisphenol A was a "pure" estrogen, i.e., exhibiting the same responses as estrogen, or if it elicited responses for activating different genes, but genes that resulted in the same phenotypical effect as the genes activated by estrogen.
Gene array technology is not a fully developed technique, but is extremely useful in providing information for hypothesis development in further studies. The results are subject to numerous confounders, and false positives and negatives are common. Therefore, it is necessary to confirm all array results with additional techniques, such as reverse transcriptase polymerase chain reaction (RT-PCR) assays.
We selected eight genes from the array results and subjected them to RT-PCR. These eight genes were significantly affected by low (physiological) levels of BPA. The genes are involved in nerve cell growth and differentiation and include caldesmon, fos-related antigen, complement C3, cyclin D1, calpastatin, calpain 3, microtubule associated protein and retrovirus-related gag protein. Based on the results of the microarray analyses, these genes provided the main focus for RT-PCR.
Chemical Analytical Methods: Measuring Drugs and Devices In Vivo
Objective: Measure in vivo the real time release of chemicals from devices intentionally:
I (i.e., drug eluting stents), or unintentionally II.(i.e., plasticizers).
Because drugs used on stents are expensive, sensitive to light and moisture, surrogate compounds were used.
The revolution in human genetics and the sequencing of the human genome have created new opportunities for advances in public health and new challenges for FDA. Opportunities include the integration of genetic information into routine medical practice, e.g., for use in optimizing individual therapies. The new technologies can also be used to address the issues of safety and effectiveness of products undergoing pre-market review and to address post-marketing issues such as adverse responses. The challenges arise for CDRH as the Center responsible for approving new genetic and genomic diagnostic devices. A major challenge is establishing approaches for review and approval of novel diagnostic devices that provide hundreds or thousands of data sets generated simultaneously on microarrays. A microarray is a small piece of glass, plastic, silicon, or other material on which thousands of samples of DNA (e.g., oligonucleotide probes) or other receptor material (e.g., antibodies, tissues) have been attached in tiny pinpoint samples in a two- or three-dimensional format.) Microarrays are used to screen a biological sample for the presence of thousands of genetic sequences, proteins, or other targets at once, for example, to study gene expression. The need for genetic diagnostic tests that rapidly identify agents of bioterrorism and genomic human responses to these agents will add a level of urgency to the pre-market review responsibility faced by the center.
BackgroundProjects under this program are designed to provide hands-on knowledge and experience using the new technologies. A major effort is being devoted to the evaluation of microarray technology and device performance, in order to contribute substantively to standards development for genetic and genomic diagnostic devices. Although the technologies are new in some respects, they can still be validated against known surrogates. Projects are chosen that are informative for a particular device issue, such as factors related to microarray performance, a new approach to safety or effectiveness evaluation, or data that contribute to understanding and preventing adverse events. Projects are also designed to provide a basis for keeping up with the technologies as they evolve.
There are two basically different types of devices representing the new technology coming to CDRH for review: genetic and genomictesting devices.
A genetic test determines the presence or absence of a particular, targeted DNA sequence already known (or suspected) to be related to a health outcome. Examples are mutations in the cystic fibrosis gene (human genetic disease), in a drug metabolizing gene (efficacy of a given class of drug), in the p53 gene of a tumor (prognosis and therapeutic stratification), or in genes related to susceptibility to cardiovascular disease or cancer. One expectation is that pharmaceutical companies will submit applications for approval of combination products, e.g., a genetic diagnostic test along with a drug, in order to stratify a clinical trial population, or to include or exclude a certain segment of the population. Another use of genetic tests is to rapidly identify pathogens that may be used in terrorist attacks. Genetic testing is an established technology. It involves a query for a mutant sequence, usually within one gene. Microarrays can be used for parallel queries of many potential mutations or sequence identities.
The genomictype of diagnostic test involves gene expression, usually measured by mRNA or a surrogate, often in comparison to a reference set of expressed genes. Genomic technology involves analysis of many hundreds or thousands of genes that are up-regulated or down-regulated either constitutively or in response to a stimulus. The result is a pattern of gene expression that is designed to be diagnostic or characteristic of a condition. Examples would be patterns related to toxic responses, characteristic of a disease subset or representative of a human response to a pathogen. Diagnostic devices can also be used to analyze expression profiles of proteins, the end-products of gene expression (i.e., Proteomics). Microarrays can be used to generate, and bioinformatics used to analyze, the complex patterns generated by genomic/proteomic and genetic devices. Whole genome analysis is relatively new and experimental, and methods and analytical approaches are still being developed.
Program DescriptionBecause genetic and genomic devices are based on different technologies, projects are underway in each area. Issues are described below.
Project: Beta testing of new genomic technologies (keep pace with new developments)
Project: Microarray detection of drug-resistant strains of M. tuberculosis;
These projects provide a base for genomic and genetic device performance evaluation, and for continuously updating our capability in new technologies as they evolve. The knowledge and experience gained including methods design and development, as well as device performance evaluation, will enable OSEL scientists to do the following: 1) make informed regulatory decisions by critically evaluating data obtained with diagnostic devices based on genomic and genetic technology, and 2) contribute to writing standards and guidance documents. The projects will also demonstrate some of the ways in which new genetic and genomic approaches can enhance public health. Mastery of genomic and genetic technologies involved in these projects will prepare us for possible future projects involving the rapid detection of microorganisms and human host responses associated with biodefense. The projects in this program support the CDRH Strategic Plan, especially the Total Product Life Cycle and Magnet for Excellence. Additionally, collaborations within FDA, with other government organizations, academia, and industry have provided ample opportunity for significant leveraging of resources and expertise.
Relevance to FDA and CDRH Mission, Program, and the Public Health impactScientists in this program area are developing and performing laboratory projects that give them hands-on experience with emerging microarray-based and related molecular technologies. The knowledge and experience gained, including methods design and development, as well as device performance evaluation, will enable OSEL scientists to 1) participate effectively in the CDRH regulatory review of pre-market device applications, 2) make informed regulatory decisions by critically evaluating data obtained with diagnostic devices based on genomic and genetic technology, 3) contribute to writing standards and guidance documents. The projects will also demonstrate some of the ways in which new genetic and genomic approaches can enhance public health. Mastery of genomic and genetic technologies involved in these endeavors will prepare us for possible future projects involving the rapid detection of microorganisms and human host responses associated with biodefense. In addition, collaborations within FDA, with other government organizations, and with academia and industry have provided ample opportunity for significant leveraging of resources and expertise.
Program AccomplishmentsThe primary focus of the laboratory was organizing and implementing the OSHC inter-center project on “Prioritizing variables in genomic microarray data.” Other projects were “[missing end quote]Microarray analysis of SNP’s (genetics), and analysis of pathogens using microarrays (genetics).” The inter-Center microarray project addresses the following variables:
Figure 1. Fractional Factorial Design
A (array surface) |
Printer lab 1 |
Printer lab 2 NCTR |
Printer lab 3 CBER |
||||||
|---|---|---|---|---|---|---|---|---|---|
Test lab 1 (CDRH) |
S1 | S2 | S3 | S1 | S2 | S3 | S1 | S2 | S3 |
| A1 (codelink) |
XY | Y | X | Y | X | XY | |||
| A2 (superamine) |
X | XY | Y | XY | Y | X | |||
| A3 (Gap) |
Y | X | XY | X | XY | Y | XY | Y | X |
| A4 (Super epoxy) |
|||||||||
| A5 (polylysine) |
Y | X | XY | X | XY | Y | |||
Test lab 1 (NCTR) |
S1 | S2 | S3 | S1 | S2 | S3 | S1 | S2 | S3 |
|---|---|---|---|---|---|---|---|---|---|
| A1 |
Y | X | XY | X | XY | Y | |||
| A2 |
XY | Y | X | Y | X | XY | |||
| A3 |
X | XY | Y | XY | Y | X | Y | X | XY |
| A4 |
|||||||||
| A5 |
X | XY | Y | XY | Y | X |
Test lab 3 (CBER) |
S1 | S2 | S3 | S1 | S2 | S3 | S1 | S2 | S3 |
|---|---|---|---|---|---|---|---|---|---|
| A1 |
X | XY | Y | XY | Y | X | |||
| A2 |
Y | X | XY | X | XY | Y | |||
| A3 |
XY | Y | X | Y | X | XY | X | XY | Y |
| A4 |
|||||||||
| A5 |
XY | Y | X | Y | X | XY |
All Labs |
S1 | S2 | S3 | S1 | S2 | S3 | S1 | S2 | S3 |
|---|---|---|---|---|---|---|---|---|---|
| A1 |
XXYY | XXYY | XXYY | XXYY | XXYY | XXYY | |||
| A2 |
XXYY | XXYY | XXYY | XXYY | XXYY | XXYY | |||
| A3 |
XXYY | XXYY | XXYY | XXYY | XXYY | XXYY | XXYY | XXYY | XXYY |
| A4 |
|||||||||
| A5 |
XXYY | XXYY | XXYY | XXYY | XXYY | XXYY |
X corresponds to dye-variety configuration (Cy3->V1, Cy5->V2).
Y corresponds to dye-variety configuration (Cy3->V2, Cy5->V1).
Figure 2. Print design for prioritization of microarray variables
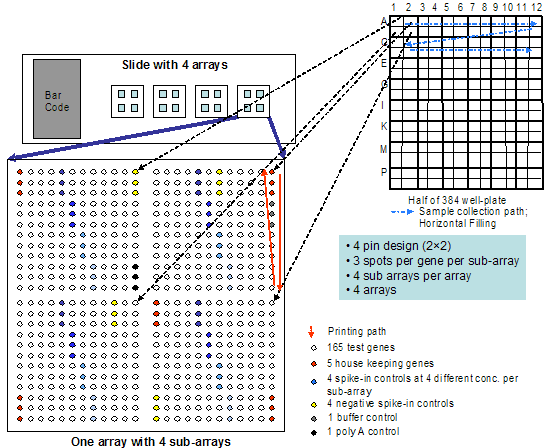
Figure 3. Genomic Profile of Irradiated and Unirradiated TK6
cells
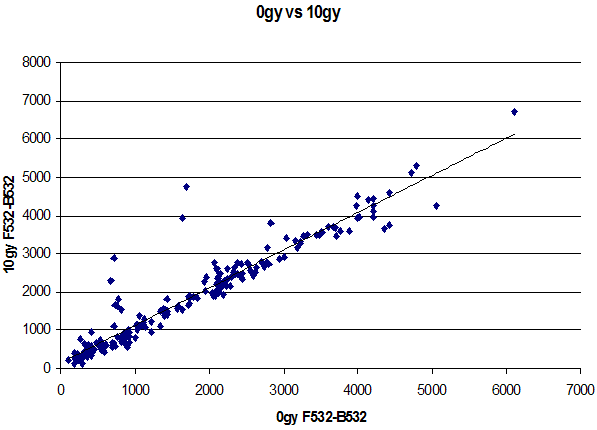
In genomic analyses, the goal is to resolve increased or reduced expression of whole genes (copied as RNA) targeted by ~70 base pair regions (qualitative and quantitative information). In the genetics projects, on the other hand, the goal is resolution of multiple single base changes in DNA as a yes or no query. Because single base pair resolution by hybridization is exquisitely sensitive to DNA sequence context, this is a challenge. Conditions are often optimal for one set of base pair hybridizations but not for others. Our microarray SNP project used a model human gene, p53, important in cancer diagnosis, prognosis and treatment stratification expected to be a major focus in personalized medicine. We chose 10 hot spot mutations found in human tumors, and experimentally addressed the factors important to successful resolution of these mutations. The most important factors not already well known were oligonucleotide probe purity (often subject to n-1 contaminants in commercial samples, due to the mechanism of manufacture) and shearing of the target DNA to the appropriate size. Control of both of these factors dramatically improved resolution of human DNA SNP’s.
Five-Year Objectives
This program conducts research and testing in support of the Center's mission related to materials characterization, degradation, and materials-tissue interactions as they affect medical devices. The program was established to provide CDRH and other FDA Centers the scientific and engineering capability to test and evaluate medical device materials safety and effectiveness in the total product life cycle (TPLC). The testing and evaluation services include development of instrumentation and testing protocols, procurement of appropriate research and regulatory device/materials samples and providing recommendations for the product performance criteria, accuracy, precision, and safety of medical devices. The program's two laboratories are structured to assist other components of CDRH on materials science aspects of both premarket evaluations and postmarket surveillance of medical devices. The research aspects of the program are intended to provide the Center with independent data as well as intramural knowledge and experience concerning the use of preclinical in vitro and in vivo studies for the evaluation of medical device materials safety and performance. The major output of the laboratory specialty areas include independent assessment of manufacturers' claims and data, test methods, standards, regulatory guidance, and publications related to the public health impact of medical device materials design and safety.
The program's research and testing activities contribute to evaluating medical device materials safety and effectiveness in TPLC. These activities are directed not only toward solving the specific device-related regulatory issues, but also in finding ways to apply the knowledge gained to publish in medical device peer-reviewed journals. Research efforts have been focused on the development of in vitro science and engineering studies suitable for the characterization, degradation, and biomaterials applications.
The program addresses materials synthesis, processing, and fabrication as they influence medical device performance. These processes are affected by the molecular structure, phase, and ultimately the physical-chemical interactions in materials. The research includes characterization of residue and contamination analysis, purity, chemical structure and formulations, thermal stability, phase stability and transformation, transport and thermodynamic properties, and viscoelastic and adhesive properties.
The laboratories are capable of testing the performance of physical and chemical processes of importance to medical devices, such as mass transfer through membranes used in dialysis and manufacturing processes used to fabricate materials. This program provides the Center with independent data as well as intramural knowledge and experience concerning the use of preclinical/postmarket studies for the evaluation of medical device materials safety and performance. Additionally, the program evaluates the degradation of materials in storage or use in vivo or ex vivo, identifying potential materials issues related to failure modes, and also contributes to the development of regulatory guidance and test methods to ensure the safety and effectiveness of medical devices and their material components.
The materials characterization area focuses on surface and interface chemistry, bulk and surface morphology, bulk composition, and chemical/physical parameters for structure determination. The materials degradation area evaluates the chemical, thermal, and environmental degradation of materials and the affect of degradation on medical device performance and safety. The polymer/materials degradation area focuses on materials integrity, materials interactions; chemical, physical, and thermal degradation; in-vitro and in-vivo studies for medical device/materials; and shelf and service-life. This work includes post-market evaluations of device failures and forensic investigations such as unidentified particles in PVC blood bags, defective IV set fabrication, adhesion barriers, and counterfeit hernia repair mesh.
This program's experimental pathology laboratory is designed to evaluate the explant pathology of medical devices utilizing gross pathology, histopathology, immunohistochemical staining and molecular pathology studies. This research program has provided independent data identifying heart valve failure modes associated with emerging polymeric and tissue-derived materials as well as identifying a mechanism for the loss of cuspal cells in viable allograft heart valves following implantation. The research results have supported the regulatory decisions and recommendations made concerning four generations of replacement valves.
The materials-tissue interactions area conducts experimental research in support of pre-clinical models for evaluating dental, orthopedic, and cardiovascular device applications with respect to calcification and other phenomena. There is new focus is on materials processing, and materials science-related issues relevant to in-vitro diagnostic devices. The research is directed toward developing and establishing the in vitro and in vivo studies and models suitable for evaluating materials-tissue interactions, failure modes and effects analysis, the assessment of medical device-related pathology, peer-reviewed basis for regulatory guidance recommendations and standards development.
FDA's CDRH mission is to assure the safety and effectiveness of medical devices. The materials characterization, polymer degradation, materials-tissue interactions programs play a pivotal role in pre-market evaluation, and post-market monitoring activities. Materials will continue to be an essential component of medical devices, and OSEL's laboratory capability to evaluate materials will help the Agency make regulatory decisions based on the best available expertise and independent scientific information. It is anticipated that a focused program in which the materials characterization, degradation, and tissue-materials interactions laboratories will help determine product performance criteria, accuracy, precision, reliability and safety of medical devices which will help the Center in its mission in every phase of TPLC. This program's activities will help ODE, OC, OSB and other FDA Centers to develop guidance documents and a substantial number of standards. The research in this program area is directed toward the development and establishment of in-vitro and in-vivo studies and models suitable for the evaluation of medical device materials safety. The peer reviewed findings of these research projects serve as the scientific basis for regulatory guidance recommendations and standard development. The quality of new device materials must be assured by the appropriate pre-market testing and post-market surveillance. The goal of the Chemistry and Materials Science Program is to develop the quality regulatory science base to meet the new challenges.
The following are examples of current research projects and accomplishments during the year.
1. Computational Kinetics and In-Vitro Evaluation of Stent-Based Drug Delivery
Systems
This project's main objectives were to identify and quantify the factors
that control the coating morphology in a polymeric controlled release drug
coating, relevant to drug eluting stents and develop suitability and robustness
of currently evolving dissolution methods to characterize coating integrity.
Based on this information, the project's objectives were to focus on how
the manufacturing process affects the microstructure of polymeric controlled
release coatings.
2. Screening for an Intraocular Lens Material's Predisposition to Vacuole Formation
The intraocular lens (IOL) industry has been struggling with the "glistening" problem for several years. Glistening (fluid appearing in micro vacuoles that enlarge over time) results from vacuoles that form in the lens after implantation. Patients with this problem experience glare and shadows especially at night. The cause of this effect is unknown.
3. Characterizing Cross-linked Viscous Abdominal-Pelvic Adhesion Barriers
Adhesion formation after peritoneal surgery is a major cause of postoperative bowel obstruction, infertility and chronic pelvic pain. Ironically, cross-linked hyaluronic acid with 0.5% ferric ion or ferric hyaluronate (Fe-HA) viscous solution is a new class of adhesion barrier that was recently approved in preventing and reducing postoperative adhesions. After the initial distribution of this product, it was voluntarily withdrawn from the market after reports of two cases of hysterectomy and several cases of "worsening of adhesions and/or abdominal pain.” These adverse events may have been attributed to poorly cleared gel causing noninfectious peritonitis and severe abdominal pain. Previously, the common way to assess the clearance of adhesion barriers in patients has been exploratory surgery. This project proposed to develop test methods to characterize the factors that affect the absorption, distribution and clearance of ironically cross-linked hyaluronic acid. The goal of this project was to identify biomarker' as an alternative to secondary exploratory surgery. This information would be useful to substantiate the current Guidance for Resorbable Adhesion Barriers for future pre-market applications.
4. Breakage of Blood Bags Used Off-Label to Store Hematopoietic Stem Cells
Blood bags are used off-label to store hematopoietic stem cells. In this application, the bags are stored at cryogenic temperatures and are subject to mechanical failure when they are handled. Blood bag failure for storage of stem cells can have severe consequences, e.g., partial or complete loss of a patient's unique contents which could eliminate a patient's last chance for cure or remission. After initial reports of this problem, the major suppliers of blood bags wanted to recall their product. This project was designed to test a cross-section of bags commercially available to identify the factors that contribute to mechanical failure. The objective was to identify the features of the polymer chemical structure, polymer morphology, and bag design which will provide some useful data to improve the utility of these bags for their intended use. It is anticipated that this work will contribute towards improving the guidance documents for reviewing and manufacturing these products.
5. Assessment of Calcium Phosphate Deposition Mechanisms in Dental, Orthopedic, and Cardiovascular Device Applications
The use of calcium phosphate compounds in both dental and orthopedic applications has long been established. Recently, however, emphasis has been placed on the potential of these materials, especially amorphous calcium phosphate, to help remineralize both bone and dental defects. The work outlined in this project was aimed at gaining an understanding of the ion release characteristics of proposed new materials and developing potential test methodologies for assessing their effectiveness. Current materials research is looking at the remineralization potential of several "bioactive" calcium phosphate (CaP) compounds or their mixtures. It is speculated that the sustained release of calcium and phosphate ions over the time that healing process occurs will provide supersaturation conditions necessary for mineral re-deposition and will promote new hard tissue formation. In a related process, dystrophic calcification of polymeric and bioprosthetic heart valves is the major cause of structural deterioration of these products. In an effort to control this mineralization process, heart valve manufacturers have developed various anti-calcification treatments with unknown long-term clinical effectiveness.
Currently, there are no accepted in-vitro or in-vivo test protocols to validate or quantify the re-mineralization claims for restorative materials. In the case of cardiovascular materials, the lack of fundamental understanding of the calcification process that lead to failure of these devices makes it impossible to develop meaningful in-vitro test protocols for assessing anti-mineralization techniques. The objectives of this project are (1) to establish baseline dynamics and, possibly, standardized test protocols for dental restoratives, and (2) to gain better understanding of the growth kinetics of cardiovascular deposits and the effects that various anti-calcification treatments have on this unwanted mineralization.
6. The Effects of Preimplantation Processing of Cardiovascular Tissue-Derived Biomaterials
The objective of this project was to identify appropriate morphologic and tissue mechanics endpoints as well as "pass/failure" criteria for the assessment of cardiovascular tissue-derived biomaterial structural integrity. Morphologic and tissue mechanics studies were conducted evaluating preimplantation tissue processing of decellularized extracellular matrix (ECM) materials used in the cardiac reconstructions of the ventricular outflow tract. Surfactant decellularized aortic valve conduits were investigated as a prototype tissue-derived biomaterials, since aortic root replacement using a decellularized heart valve conduit represented the "worst case" situation for a tissue-derived biomaterial with compromised structural integrity.
The main objectives of this project were (1) assess morphologic features of allograft valve conduit decellularized techniques, (2) evaluate biomechanical properties of fresh and decellularized allograft valve conduits, and, (3) study decellularized allograft valve conduit explant pathology following ventricular outflow tract implantation in sheep.
A wide variety of new digital imaging and display devices are under development by academia and industry, with a broad range of performance characteristics. The Center thus requires new/improved guidance for the evaluation of such devices. To this end, OSEL scientists are developing evaluation methodologies for diagnostic medical imaging systems such as mammography and fluoroscopy, computed tomography, nuclear medicine, diagnostic ultrasound, and magnetic resonance imaging, as well as novel soft-copy display devices for viewing medical images. This program is located within the Division of Imaging and Applied Mathematics (DIAM).
The Medical Imaging Program at CDRH was initiated in the early 1970s by its predecessor, the Bureau of Radiological Health (BRH). The goal was to go beyond the traditional BRH laboratory approach of simply measuring the level of radiation emitted by an electronic or diagnostic modality to measurement of the level of imaging performance as well. Laboratory measurement methods were developed for assessing the performance of contemporary and new technologies in the field of radiography, mammography, computed tomography, diagnostic ultrasound, radioisotope imaging, magnetic resonance imaging, and innovations in digital detectors and displays. The program led to contributions to consensus measurement methodology and international standards that are used here today in the approval process for new technologies, in particular, digital radiography and mammography, and diagnostic ultrasound. In-house research and collaboration with academic investigators have also led to laboratory and clinical systems that optimize the ratio of imaging performance to radiation exposure in mammography.
DIAM scientists are developing appropriate methods for evaluating medical imaging system performance and dose. Investigations take the form of theoretical analysis, numerical simulation of the entire imaging chain, and experimental validation. In some instances improved/optimized system designs are validated through actual system construction and clinical evaluation. Measurement and analysis procedures are also being developed to evaluate the performance of new soft-copy display devices that can have dramatically different light-emitting structures and associated performance characteristics, whose impact on the image interpretation process is currently unknown. Our researchers provide reliable, quantitative laboratory measurements of imaging system characteristics to the imaging research community. These scientists are also elucidating the fundamental mechanisms underlying the interaction between the image-forming radiation and the anatomy being imaged.
The expertise developed through this program is being applied to the review of PMAs for ultrasound bone sonometers and new digital radiographic imaging systems, the development of amendments to the diagnostic x-ray performance standard, the development of an advisory pertaining to pediatric CT exposures, and the joint planning of a consensus development conference on CT with NIH. The x-ray spectral measurements program provides a source of otherwise unavailable data to the entire mammography research community. Finally, investigating computer-assisted diagnosis devices provide the Center with the scientific basis to effectively regulate this fast growing field.
The Medical Imaging and Diagnostics Program is involved in developing and applying methodologies for the characterization and assessment of imaging systems and diagnostic devices. During 2004 its activities were divided into four main program areas as outlined below:
1. Image Acquisition
The 2004 objectives of this project were to model x-ray imaging systems, compute the impact of design parameters on physical and diagnostic performance measures, and to validate the results obtained with physical measurements and observer performance studies. In pursuit of these goals, the joint NIBIB/CDRH Laboratory for the Assessment of Medical Imaging Systems (LAMIS) was established through an interagency agreement (IAG). Through NIBIB-funded ORISE positions, two postdoctoral fellows were recruited to work on modeling and experimental investigations of high-dimensional x-ray imaging systems. Principal milestones for this project include the following:
2. Computer-Aided Diagnosis
The objective of this project is to develop an understanding of computer-aided diagnostic medical devices in order to better participate in the Center's regulatory activity in this area. In addition to previously initiated work with x-ray imaging modalities, the project has been extended to include the development and evaluation of CAD methods for immunohistochemical (IHC) and fluorescence in situ hybridization (FISH) data sets. The development of such CAD methods has the potential for improving the clinical utility while reducing the variability of these important molecular medicine methods. An NIBIB-funded post-doctoral fellow was recruited through participation in LAMIS and an MDFP undergraduate co-op student was recruited for the project.
A primary accomplishment this past year was starting the acquisition of clinical data for use in the CAD projects. This was accomplished with the collection of mammographic and CT lung cases from the University of Michigan along with CT colonography (CTC) cases from the military. We are also continuing to work directly with the NIH/NCI Lung Imaging Database Consortium (LIDC) and expect to start receiving CT lung cases from that source within the next 2 months.
The availability of clinical cases is a key requirement for this CAD project; whenever possible we obtain such cases through strategic clinical collaborations, which also gives us access to clinical expertise that is critical for the success of the project. The case collection process will continue in FY-2005, expanding our collection of CT lung and digital mammography cases. Some accomplishments:
Data acquisition
Investigations
3. Image Display
The objective of this project is to address new and existing display technologies, which are becoming increasingly complex and which have a major impact on the diagnostic efficacy of digital medical imaging systems. The following milestones were accomplished during 2004:
Technical accomplishments:
During 2004, the results of this research project resulted in eight technical publications on specific aspects of medical displays as well as three more general publications in the area of medical display. Optical probes were designed to interface with the new instruments recently purchased. A viewing angle observer study with 11 readers was carried out. A computational observer that incorporates the contrast sensitivity performance of human visual system was developed.
4. Multivariate Statistical Analysis
The objective of this project is to adapt the techniques of multivariate statistical analysis to the Center's regulatory programs in medical imaging systems and other diagnostic devices.
In the process of validating our software for bootstrap-based nonparametric ROC analysis, it was discovered that the second-order approach to the bootstrap that we have been using tends to be too conservative with regard to rejecting the null hypothesis (e.g., testing the equivalence of competing diagnostic tests or imaging systems). The flip side of this is a concomitant loss of statistical power. This discovery led us to carry out an extensive study of higher-order bootstrap and permutation-related methods (first through sixth order). This study has demonstrated a very slow convergence toward optimum performance, proceeding from the lowest through the highest-order approach. The details of this work are being prepared for presentation at the SPIE Medical Imaging 2005 conference and several manuscripts for submission to peer-reviewed journals. After peer review, these new computational techniques will be incorporated into our DIAM software. The new Beowulf computer cluster has been essential to all of these computationally intensive tasks.
Simulations of the use of an expert panel as a surrogate for a gold standard of truth are on-going. One of the first results was a demonstration of how a diagnostic test can “apparently outperform” the ideal Bayesian observer for a given level of noise in the test. This apparent performance results from the bias of using a panel of experts reading the very same test images to determine the unavailable truth. This work was presented at the annual meeting of the Radiological Society of North America in November 2004 and will be updated at SPIE Medical Imaging 2005.
Program staff has participated in all of the meetings of NCI’s Lung
Image Database Consortium (LIDC) and prepared and presented the design of a
pilot study that can be used to size a database for the development of algorithms
for the detection or classification of a specified disease condition. The next
step is to obtain candidate algorithms from the LIDC members, format the early
database data for our own algorithms, and run the pilot experiment. In addition,
imaging program staff has contributed to all of the meetings of the new NCI/FDA/Industry
special-interest groups on use of computer aids for both detection and diagnosis
of disease as well as monitoring of therapeutic regimens.
A major landmark has been achieved with the development of a computationally
intensive approach to the estimation of uncertainties in the assessment of
a classical discriminant or artificial neural network. The approach is based
on a synthesis of the literature on the influence function and a generalized
approach to cross-validation based on statistical resampling techniques.
The method has been validated for some common classes of discriminants using
Monte Carlo simulations. The significance of the approach is that the uncertainties
from the finite number of trainers and the finite number of testers can be
estimated from a single given data set. The next step is to validate its
application for neural networks and more complicated classifier architectures.
The work has been submitted to the journal Pattern Recognition Letters. This
project has immediate applicability to the wide range of new technologies
that use high-dimensional data for diagnostic or prognostic medicine, including
DNA micro arrays, protein micro arrays, and many applications of computer-aided
diagnosis in medical imaging.
Technical accomplishments
5. Communication (Summary of communication with stakeholders)
Regular Thursday morning research meetings are held to communicate research progress and work out any issues. The Medical Imaging and Diagnostics Laboratory hosts Thursday afternoon seminars that are widely advertised and presented by inside and outside speakers on topics related to current projects. We are corresponding members of the AAPM task group working on the development of a CT noise metric. OSEL staff participate in conferences as authors and program committee members, and our work on review teams also serve to communicate the work of this project to stakeholders.
The previous objectives of this project were to model x-ray imaging systems, compute the impact of design parameters on physical and diagnostic performance measures, and to validate the results obtained with physical measurements and observer performance studies. While our emphasis has been on planar mammographic imaging, the project will be moving to 3D/4D methods in the coming year (fluoro, CT, tomosynthesis), with the concomitant need for modeling methods relevant to the physics of higher energy radiation and relevant experimental validation approaches. Higher dimensional imaging geometries and simulations of higher energy physical processes have put increased requirements on our Beowulf computing cluster.
The Medical Imaging and Diagnostics program has made fundamental contributions to the field of statistical analysis of diagnostic imaging and systems for computer-aided diagnosis. We would like to exploit this work and validate its range of utility through extensive computer simulations. In the process, we would be seeking the most efficient or statistically powerful approaches to the evaluation of medical imaging and computer-assist decision modalities. An ultimate goal is the development of a multiple-reader (e.g., multiple radiologists, multiple pathologists) multiple-case (MRMC) version of our current software for ROC analysis in the absence of ground truth (i.e., without a gold standard). Development of such a system would address one of the most difficult yet most common assessment problems in the field of diagnostic medicine.
The rapid development of medical devices employing minimally invasive technologies has revolutionized modern health care. Diseases that once required invasive surgery for diagnosis and treatment are now routinely addressed on an outpatient basis. The goal has been a reduction in health care costs and an increase in patient safety. In addition, many diseases can now be diagnosed much earlier, resulting in more effective treatment.
The continuing dearth of natural donor organs, the inherent shortcomings of existing prosthetics, and significant advances in the understanding of the fundamental requirements of artificial organs all fuel significant research, development, and regulatory activity that drive the fluid dynamics research projects. The medical products in this area are among the most complex that the Center evaluates, and their public health significance is often profound. Similarly, the rapid growth of diagnostic techniques and minimally invasive therapies drives our current work in ultrasonics.
The maintenance of blood transport is a major focus of the fluid dynamics program, as heart failure is the prime cause of death in this country. Prosthetic heart valves, ventricular assists, total heart replacements, grafts, stents, bypass pumps, hemodialysis systems, and oxygenators all must avoid placing unusual hydrodynamic loads on the body; they must avoid damaging the cellular components of blood, and they must minimize the activation of platelets that initiates the clotting cascade. The requirements imposed by the above constraints are primary concerns of the fluid dynamics research effort.
In ultrasonics, the variable of ultimate interest is that of tissue temperature, as temperature profiles largely determine cell viability. Thermal injury is highly desirable for therapies intended to shrink or ablate tumors. However, injury is usually highly undesirable in the case of diagnostic imaging. Either way, the ability to predict the temperature-time response requires accurate knowledge of the ultrasound fields and how they are absorbed. This becomes particularly important in the relatively new technology of high intensity-focused ultrasound ablation where energy levels are high and the targets may be deep within the body.
A variety of approaches are employed depending upon the specifics of the issue and the devices in question.
High-intensity focused ultrasound (HIFU) holds the potential for radically advanced surgical techniques, including ablation of both malignant and benign lesions and cessation of internal bleeding in injured vessels and organs. Although some clinical success has been achieved, the lack of standardized methods to assess the acoustic and thermal characteristics of the focused beam is one factor that has hampered general understanding and acceptance and has slowed the regulatory review process, especially in the preclinical phase. The objective of OSEL current research is first to develop methods for assessing the acoustic beam characteristics of HIFU devices. Then, temperature profiles will be calculated using computer models. Finally, the results of these two tasks will be validated by measuring the temperature distributions of HIFU beams under various exposure conditions using thermal test objects and other tissue-equivalent materials.
For prosthetic heart valves, the effective orifice area and regurgitation are macroscopic quantities that provide key descriptions of the valve operation. Researchers have worked with industry and academia via standards development to define useful characterization tests for prosthetic heart valves. Current work in this area centers on the need to remove the dependence of test results on the specific pulse duplicator hardware employed. Other recent work involving prosthetic heart valves examined the physical stresses inherent in the fluid-solid interaction. These stresses are an important predictor of valve life. For mechanical valves, they are determinants of potential crack propagation and of cavitation. Either effect can lead to valve failure.
Thrombosis is a major concern for any device with direct blood contact. The clotting cascade is an extremely complex process; however, flow stagnation is often involved in triggering platelet activation and initiating the cascade. Tiny regions that allow flow stagnation in or around medical devices can act as seeds for the development of thrombus. OSEL is developing and employing advanced flow visualization techniques to allow evaluation of the potential for thrombus formation. Current work involves evaluating the clot trapping and flow characteristics of vena cava filters. These techniques are also needed for evaluating regions of high shear stress, where mechanical damage to red blood cells and platelet activation may occur. Scientists are also performing systematic testing to identify and control the primary variables that determine the mechanical fragility of blood. Researchers anticipate that these tests of mechanical hemolysis and flow visualization would be incorporated into the preclinical screening of any device for which such testing would be beneficial.
HIFU shows promise for localized destruction of a targeted tissue volume with minimal damage to the surrounding region. Although some clinical success has been achieved, the lack of standardized methods to assess the acoustic and thermal characteristics of the focused beam is one factor that has challenged the regulatory review process, especially in the pre-clinical phase. Therefore, the goal of this project is to develop standardized test procedures for measuring the properties of HIFU beams, and for predicting the spatial distribution of temperature in tissue.
The project is divided into three tasks. Progress under each task is described below.
Task 1: Acoustic output and beam profile measurements of high intensity focused ultrasound (HIFU) transducers
Task 2: Mathematical models for calculating temperature profiles
Finite-element modeling of thermal effects due to ultrasound absorption contributed significantly in the analysis of the Ex-Ablate uterine fibroid ablation system (PMA submitted by InSightec). Computations were performed to verify the feasibility of temperature estimates submitted by the manufacturer, in the case of absorption by the sacral bone during an ablation procedure. The risk of thermal damage to nerves located various distances from the bone/soft-tissue interface was computed. A previous FDA thermal model was extended to include the effect of blood flow; this extension was important for predicting thermal effects over long time periods.
FDA’s ability to simulate ultrasound propagation at high intensities was enhanced with the acquisition of the source code for the KZK propagation model. The code has been installed and tested and used to illustrate nonlinear propagation effects such as pulse steepening. The KZK model will be used in thermal analyses during 2005.
Task 3: Validation of the beam output measurements and mathematical modeling results
As an initial step, several commercial tissue-mimicking material samples were obtained, and broadband attenuation measurements using the laboratory’s time delay spectrometry system were made to verify the manufacturers’ specifications and to evaluate for possible use as HIFU phantom materials.
Diagnostic ultrasound used for ophthalmic applications, as well as new diagnostic ultrasound techniques under development by industry, generate temperature rises that are not appropriately dealt with by the steady-state models assumed and criteria utilized by current safety standards and FDA guidance. This project is designed to research these problems so that appropriate standards and industry guidance can be developed. The project is divided into two modules. Progress for each module is described below.
Module 1- Ophthalmic diagnostic applications:
Module 2 – Radiation force imaging:
The evaluation of prosthetic heart valve performance relies on the use of a left heart model (pulse duplicator). Manufacturers and test centers have developed various models and test instrumentation in order to evaluate hydrodynamic performance. Recent interest in the rewriting of the FDA Heart Valve Guidance available to manufacturers and the revision of the ISO 5840 International Standard for cardiac valve prosthesis has prompted a study of the test technology. The study is to evaluate interlaboratory consistency of current test technology for the determination of a broad range of pulsatile flow prosthetic valve parameters, including the Bernoulli relationship. This collaboration includes several manufacturers and test centers in addition to the inclusion of two left heart models in our own lab.
The research will support the Center’s mission to assure the safety and effectiveness of prosthetic heart valves. Accomplishments are as follows:
Technical Accomplishments
This is a combined CDRH and CBER project. Scientists at CBER are investigating the effectiveness of the WHO protocols with an animal model. The effects of these protocols on surgical instruments were investigated by CDRH scientists. The results have been published and are summarized in the publication below:
Full Article: Stanley A. Brown, Katharine Merritt, Terry O. Woods, and Deanna N. Busick. The Effects on the instruments of the World Health Organization- recommended protocols for decontamination after possible exposure to transmissible spongiform encephalopathy- contaminated tissue. J Biomedical and Materials Research Part B, 72B: 186-90, 2005.
Long-term planning guides the decisions about major equipment, infrastructure, and personnel investments. OSEL anticipates that the use of mechanical organ replacement and assists will continue to grow over the time period in question. Advances in genetics and tissue engineering that would render mechanical assists or artificial organs obsolete are not yet on the horizon. These alternate approaches are not likely to reduce the regulatory workload for mechanical organ assists and replacements in the coming decade. Advances in electronics, computer technology, and materials will allow for devices of increased capability, complexity, and yet smaller size. However, a fundamental shift away from technologies currently employed is unlikely. OSEL will continue to maintain a heavy investment in life-extending/saving technologies, principally those in the cardiovascular area. The interactions of blood with the body form the primary limitations for these devices and, thus, this is where the most improvement is likely to come.
This program will continue to examine new ultrasonics devices and technologies for safety and effectiveness. New policies, regulations, standards and guidance will be needed to enable individuals to safely operate and maintain these devices. This information will be obtained from independent laboratory studies and from in-house research that is conducted to anticipate new directions of this technology. Once obtained, the information will be disseminated in the form of peer-reviewed publications, consultative reviews, and guidance documents.
Numerous medical devices and consumer products regulated by CDRH emit radiation: ionizing (x-rays) or non-ionizing (ultraviolet radiation [UV], radio frequency, or acoustic radiation). In order for the public to enjoy the benefits of these products and technologies, it is necessary to establish safe limits for the exposures to these emissions (whether intentional or incidental) or provide scientific basis for reducing the exposures. The goal of the radiation bioeffects program is to develop scientifically based criteria for evaluating radiation-emitting medical devices and consumer products and for developing relevant CDRH/FDA guidelines and standards.
Currently over 100 million Americans use wireless phones. Data relating to the safety of radiation from wireless phones are inadequate; however they suggest that exposures to radio frequency radiation at levels relevant to wireless phone use may cause biological effects. In this area, the OSEL bioeffects project serves as the coordinator of independent research conducted in several laboratories.
These laboratories have been selected by CDRH, and OSEL is extensively involved in these investigations. The new findings together with other published data are being evaluated by OSEL for inclusion in appropriate FDA guidelines and standards.
OSEL currently has a number of active programs to investigate (1) the safety of cell phones, (2) the biological effects of radiation emitted by radiation therapy devices for treating cancer or dermatological conditions, and (3) radiation emitted by or transmitted through consumer products such as tanning equipment or cosmetics (in collaboration with CFSAN, NCTR, and NCI). OSEL evaluates the bioeffects of acoustic (ultrasound) radiation to ensure safety of the diagnostic and therapeutic use of this modality. The bioeffects program also collaborates with other agencies, such as the National Toxicology Program, to initiate and coordinate broader programs such as a national study of the possible carcinogenicity of radio frequency radiation (nominated for study by members of the OSEL bioeffects program). In past years, the radiation bioeffects program has been involved in extensive collaborative research efforts with other national agencies in the areas of electromagnetic fields involved in the transmission and use of commercial electric power. In the future, the bioeffects program expects to become increasingly involved in the support of homeland defense and counter-terrorism that may involve radiological incidents. Currently the program is developing collaboration with the Department of Defense to study methods for identifying and testing new drugs to protect people from the effects of radiation such as might be released in a terrorist act. In another counter-terrorism area, our involvement will be needed for evaluating the radiation-emitting equipment proposed for security control at the entrances to sensitive areas (e.g., metal detectors at airport terminals).
The radiation bioeffects program is currently undergoing a shift in emphasis away from electromagnetic fields research, which was successfully completed with external funding from the National Electromagnetic Fields research program funded by Congress and administered by NIEHS. The currently strong program of UV research will continue to pursue new problems relating to UV radiation/tissue interactions and tanning equipment and is partnering with CFSAN and NCTR to investigate problems related to the interaction of UV with cosmetics. The program is expected to begin collaborating with CDER in the area of sunscreen testing, especially regarding UVA protection. The ionizing radiation program is being strengthened with the recent relocation to the White Oak campus where facilities are available for the installation of upgraded ionizing radiation sources. Operating these new radiation sources is critical to the strengthening of the radiation bioeffects program. The program is thus becoming less involved with the risk assessment of electromagnetic fields (which formed the backbone of the program in the 1990’s) and more oriented toward the radiation-emitting medical devices and consumer products, as well as the radiation-related issues in the area of counterterrorism.
Medical devices that rely on electrophysiology and electrical stimulation for safety and effectiveness cut across all medical specialties. The obvious examples are devices that work in the nervous system and heart including cardiac pacemakers, defibrillators, heart monitors, brain stimulators (for Parkinson’s disease, pain, motor function, hearing), electroconvulsive therapy, cochlear implants, spinal cord stimulators, electroencephalography, vagus nerve stimulators, peripheral nerve stimulators (including those for locomotion, breathing, bladder and bowel control) and magnetic nerve stimulators. The less obvious examples are devices for the electrical detection of cancer (from breast, colon, and cervix), the transdermal electrical extraction of glucose for monitoring, and a number of “complementary and alternative medicine” devices. The scientific discipline of electrophysiology forms a unified basis for the scientific evaluation of all of these devices. The scientific issues involve the basic electrophysiology of a number of body systems and the biomedical engineering of the devices.
There is large and increasing interest in the scientific and medical communities in the use of electrophysiology and electrical stimulation in diagnosis and treatment of diseases and disorders. Between 1998 and 2002 electrophysiological devices comprised 22% of all PMAs and 31% of all IDEs for CDRH. The need for specialized skills from OSEL scientists related to electrical stimulation has increased. To this end, OSEL shares three positions with the Office of Device Evaluation (ODE): a cardiac electrophysiologist, a retinal electrophysiologist, and a computational neuroscientist. The requirement within ODE for scientific skills in these areas is substantial. The American College of Cardiology recently advised that the patient population to be implanted with automatic defibrillators be expanded, and there are a host of new electrical stimulation device applications being submitted for approval. Retinal stimulators have become devices of major public interest because of their potential to treat the blindness afflicting millions of Americans. Electrical stimulation devices are being submitted for urological conditions and even for the diagnosis of breast cancer. There are a host of brain and nerve stimulators along with the long-standing need for a guidance document for electroconvulsive therapy device submissions.
The diversity of devices and the large number of regulatory applications has focused OSEL’s laboratory work broadly on the basic mechanisms by which these devices exert their effects. Understanding basic mechanism, especially regarding safety, is a unique primary concern for CDRH. These studies permit expert consultations for preclinical device reviews in every area of CDRH activity; they provide the basis for guiding clinical trials and guidance documents; they prove useful as part of the approach that assists firms with the least burdensome route to approval; and they generate publications that draw the attention of the scientific community to issues of safety. Current staff perform in-house laboratory studies encompassing cellular neurophysiology, cardiac electrophysiology, and visual science.
OSEL’s investigations of electrophysiology and electrical stimulation focus on clarifying the mechanisms of interaction of the technology with the body. The work is specifically aimed at forming the scientific basis for regulatory decisions, developing guidance documents that speed device approvals, and establishing industry safety standards for electrical stimulation. Specific areas of investigation are the cellular basis of electrical stimulation safety in nerve and heart, cardiac electrophysiology and defibrillation, and retinal electrophysiology and stimulation. These areas map onto the anticipated regulatory needs of the Center in this broad area of medical devices. Current areas of investigation and accomplishments are listed below:
Present studies are focusing on the effects of high-frequency stimulation. These studies are being performed with computational modeling of action potential propagation, with confirmatory studies in animal central nervous system. The results demonstrate that high-frequency stimulation is likely to fatigue nerve and produce conduction block (even for subthreshold stimulation). This work is serving as a basis for requesting post-market studies of certain devices to include human neuropathological studies.
Computer and Laboratory Modeling to determine MRI Heating Compatibility of Medical Devices
Optimizing Electrical Stimulation by Retinal Prostheses
We have constructed a compact optical system and recording setup for extracellular recordings from ganglion cells in the superfused in-vitro retina of rabbits and rats. A computer-driven digital micromirror projector provides the visual stimulus to the retina using a Python-driven visual stimulator program, the VisionEgg program (freeware).
NEURON modeling of mammalian retinal ganglion cell data:
We have started to generate neural models based on the anatomy and physiology of cat retinal ganglion cells. We used a Neurolucida reconstruction system (courtesy of NIH/NICHD) to generate 3D digital representations of seven ganglion cells. We are using the conversion program CVAPP to convert these 3D representations into computational models for modeling in NEURON. Using the neural modeling program, we generated dendritic branch models of the major cat retinal ganglion cell types in order to compare to our future physiological results using electrical stimulation. To date we have reconstructed the morphologies of 2 ON-X, 2 OFF‑X, 1 ON-Y, and 2 OFF-Y retinal ganglion cells. We have also generated NEURON models of retinal ganglion cell axons from the anatomical data. In collaboration with the Miller laboratory at the University of Minnesota, we have obtained the voltage-gated ganglion cell channels at physiological temperatures (5-channel model) inserted into a model ganglion cell with an equivalent dendritic cylinder.
Electrical Stimulation Safety in Heart and Blood
Our accomplishments in the basic science of electric shock effects demonstrate that defibrillator shocks cause changes to ion channels (in addition to possible electroporation), and these findings stem from calcium measurements from heart cells exposed to the shocks. In this study, isolated cardiac heart cells were cultured from the chicken embryo.
Preliminary studies are underway that are measuring electrical impedance from heart cells exposed to shocks. Early results show a brief drop of impedance, which implies electroporation followed by a long period of cardiac arrest, which implies ion channel changes.
Versatile Platform for the Stu dy of Cardiac Stimulation
One of the models for the myocardium that will be explored is a two-dimensional analogue of the heart – neonatal cardiomyocyte monolayers. This is essential for conducting cardiac electrodynamic experiments for two reasons: 1) the heart is best modeled as a syncitium, or an electrically continuous tissue; and 2) the cardiac electrodynamics mechanisms of interest – reentrant action potential propagation – is know to occur on length scales of centimeters. Although less organotypic than the myoballs currently used in the laboratory, the cardiomyocyte monolayers are on the scale of centimeters and not hundreds of micrometers. The techniques used in culturing neonatal chick cardiomyocytes have been optimized for the consistent formation of continuous cardiomyocyte monolayers.
Instrumentation for Optical Mapping Experiments Instrumentation hardware and software is being assembled, tested, and debugged.
Thermal Tissue Damage Modeling of Radiofrequency Ablation Devices
Optimization of UV Exposure Patterns
Thirteen subjects completed the 7-week study. A total of 37 subjects have been enrolled to date. Four subjects could not complete the study due to scheduling conflicts. Six subjects have undergone the protocol using the 2 nd radiation source (Cosmolux VLR lamps). The first 30 subjects were completed by May 2004.
A paper describing the results of the pilot study is in progress.
The use of electrical stimulation and electrophysiology in medical devices will continue to grow for the foreseeable future. Our focus will be on developing research that will have general applicability to the regulatory process including providing the scientific expertise for reviews, guidance document development, and contributions to industry standards. This program will continue to examine new devices and technologies for safety and effectiveness. The information will be obtained from independent laboratory studies and from in-house research conducted to anticipate new directions of this technology and will be disseminated in the form of peer-reviewed publications, consultative reviews, and guidance documents. It will assist in speeding the rapid movement to market of safe and effective medical devices benefitting the U.S. public.
The specific plan includes developing test methods and guidance documents (with the necessary supportive research and extramural interactions) for devices that stimulate the nervous system and heart with electrical current.
This program, based in the Division of Electrical and Software Engineering, provides highly specialized technical support for Center and Agency regulatory activities in the areas of electrical/electronics engineering, software engineering, and systems engineering. While the bulk of our work in recent years has been in the medical device arena, the Division of Electrical and Software Engineering traces its roots to the Bureau of Radiological Health and continues to devote a portion of its efforts in support of the radiological health mission of the Center.
This program, based in the Division of Electrical and Software Engineering, provides highly specialized technical support to Center and Agency regulatory activities in the areas of electrical/electronics engineering, software engineering, and systems engineering. While the bulk of our work in recent years has been in the medical device arena, the Division of Electrical and Software Engineering traces its roots to the Bureau of Radiological Health and continues to devote a portion of its efforts to support of the “rad health” mission of the Center.
The program focuses on product realization, a term used by engineers to describe the process of converting a design concept into a viable product. At times, in the effort to focus on the cutting-edge science that makes a device possible, more prosaic technology concerns that make the product viable are overlooked. This program addresses that oversight.
Product realization encompasses all phases of the product life cycle and all aspects of the product manufacturer’s business, from research and development through procurement, production, and ongoing customer support. 1 We examine the adequacy of the manufacturer’s documented processes, the extent to which the documented processes are being followed in practice and, in particular, the decisions arising from the application of those processes. We assure that the processes are grounded in established quality management and risk management principles, and that design decisions affecting safety and effectiveness of the product are consistent with established scientific and engineering principles. Our goal is to ensure that products consistently perform as intended, fulfilling the requirements of customers and other stakeholders.
As a result, this program emphasizes analysis, laboratory testing, and educational activities over basic and applied research. Another important reason for this emphasis is that most regulatory issues involving electronics and/or software have historically arisen from misapplication of these technologies, rather than any inherent limitations.
Our research program currently focuses on two specific needs: objective methods to assess the performance of intelligent medical devices (specifically, those that provide clinical interpretation of physiological waveforms), and formal methods of software verification.
The Division also routinely provides engineering support services to customers in CDRH, in other FDA Centers, and to those outside of FDA. These services include developing custom electronic instrumentation, designing and fabricating mechanical components and assemblies, procuring specialized electrical and electronic components, and maintaining and calibrating test equipment.
Electronics and software are enabling technologies for many, if not most, classes of medical devices. Devices that incorporate these technologies are inherently complex and require that engineers must be able to skillfully peel back many layers of abstraction from the underlying mathematical models that govern device operation, to their hardware and software realizations, and down to the physical characteristics of component parts.
A large body of knowledge has developed within the electronics, software, and systems engineering communities to assure successful application of these technologies. The mission of the Division of Electrical and Software Engineering is to apply this body of knowledge to the regulation of medical devices and electronic products that emit radiation.
The breadth of these engineering disciplines poses a significant challenge. The body of knowledge is segmented into numerous areas of specialization. Within industry, large manufacturers typically have sizable organizational components to address those engineering segments (specialties) having most relevance to their needs. Small manufacturers typically have specialists in just a few key areas and rely on consultants or other external resources to augment their in-house capabilities.
As regulators, we have followed a similar approach, building depth in those key areas that repeatedly surface as regulatory concerns and augmenting our in-house capability by leveraging additional “just-in-time” knowledge from our colleagues in academia, other government laboratories (e.g., NASA, NIST, JPL), and the standards community.
Our strategy for maintaining the required depth is to recruit senior engineers from industry, each having broad experience in a number of engineering specialties. While each staff member brings a unique mix of engineering skills and experience, we strive to maintain enough overlap to maintain critical mass in the key areas. We maintain a suite of special-purpose, computer-aided engineering tools and laboratory facilities having broad applicability to medical device electronics and software, and we rely on external sources for specialized capabilities that are needed on an occasional basis.
Regulatory Support. While many groups within the Center have the need for electronics or software expertise, very few have sufficient need to justify a full-time engineering staff. By building a relatively small team of engineers who are highly qualified and making this critical mass of expertise available throughout the Center, we are able to focus as much effort as is needed on particular problems on an ad-hoc basis, thereby freeing the Center of the need to place engineering specialists in every Office of the Center. Co-locating the engineers in this fashion also facilitates communication and collaboration, ensuring that analyses and opinions rendered by the group have the benefit of extensive peer interaction and review. Furthermore, centralizing this function makes it possible to provide effective logistic support (laboratory facilities, supplies, test equipment, specialized software, and support personnel) to the team.
Education. Given the sheer numbers of medical devices that incorporate electronics and/or software, there is no practical way to ensure their safety and effectiveness through a program of retrospective reviews and inspections. The manufacturer must design safety and effectiveness. Established manufacturers generally do a good job of this; but some lack the requisite technical expertise and fail miserably.
Over the long term, we believe that the principles and practices of product realization need to be better integrated into the core engineering curriculum at both the undergraduate and graduate levels. We have had some promising conversations with a number of educators and this is an initiative that bears further investment.
Standards Development. Consensus standards represent another excellent tool for leveraging industry to improve the quality of medical devices, as well as streamlining the review process. We are participating directly in the development and/or maintenance of several of the high-profile “horizontal” standards —notably as members of the committees responsible for the IEC 60601 series and ISO 14971—as well as several “vertical” standards (e.g., apnea monitors, pulse oximeters).2 We played a leadership role in developing a new AAMI/IEC standard covering software life cycle processes and we have continued to encourage AAMI, IEEE, and ISO/IEC to work jointly to develop additional guidance for the medical device software community.
Engineering Support Services. Originally, the development of custom electronic instrumentation and test methods in support of FDA research and compliance activities was the principal mission of our staff. We have earned a number of patents for our innovative designs. Today, support services constitute a relatively small, but still important, component of our mission.
We view this work as important for two reasons. First, these support services are all based on capabilities that are essential to fulfilling our mission, and it is cost-effective to extend these capabilities to our colleagues who have similar needs.3 Second, and more importantly, similar engineering support services play a key role in the manufacture of medical devices. There is a substantial synergy to be realized from applying the expertise of our engineering services staff to a wide variety of regulatory problems in CDRH.
Research. Our research program is aimed, not at developing new technologies, but at improving our capabilities in measurement and analysis. This is an avenue that medical device manufacturers are understandably reticent to pursue, even though it is clearly in the public interest to do so.
By contrast, manufacturers enthusiastically embrace our research into formal methods of software verification because it will lead to tools that they can use for software development, help them to establish the safety and effectiveness of their devices, and streamline the regulatory approval process.
The Division of Electrical and Software Engineering has long devoted substantial resources to reducing the knowledge deficit. We administer or contribute to a variety of CDRH Staff College courses in the principles of medical instrumentation, software review practices, electromagnetic compatibility, design controls, and risk management. We also develop formal FDA guidance to industry on many of these topics, present at trade conferences, publish articles and papers, and serve as faculty in courses offered to industry by trade associations such as AAMI and AdvaMed.
Our research involving test methods is aimed at improving FDA’s ability to objectively compare the performance of diagnostic medical devices, even though those devices may use different modalities to sense the clinical conditions of interest.
Historically, many device problems arise at the confluence of hardware and software, the user, the manufacturing process, and the use environment. We apply a broad range of analytical tools to develop an understanding of these problems and engage the manufacturer in voluntarily seeking a solution. When the manufacturer is not responsive, we may test the device in question in our laboratory to develop evidence justifying unilateral action by FDA to remedy the problem.
Premarket Review. We performed engineering reviews of over 200 premarket submissions during the past year, the majority of them focusing on software. A small number of our reviews uncovered significant deficiencies in the product design. More frequently, we identified minor deficiencies in the design or design processes, leading to corrective action on the part of the manufacturer. We always strive to inform and educate the reviewers and manufacturers we work with, focusing attention on the underlying causes of deficiencies so that mistakes will not be repeated in future development efforts.
Postmarket Support. We generally become involved only when a significant problem is already suspected. In the past year, we performed substantive analysis and/or participated in on-site investigations in 20 compliance cases and provided informal guidance to investigators in numerous other situations. Several of these cases required a sustained effort over a period of weeks or months. While a few of these investigations are still ongoing, our analysis has helped to bring about product recalls in a number of egregious situations and induced manufacturers to correct major quality system deficiencies in other cases. In a few instances, our findings have supported the manufacturer in disputes with FDA investigators.
Standards Development. We are contributing to major revisions of several of the high-profile “horizontal” standards —notably the IEC 60601 series and ISO 14971.4 We played a leadership role in developing a new AAMI/IEC standard covering software life cycle processes, and also led the development of an AAMI Technical Information Report addressing Software Risk Management.
Engineering Support Services. We provided engineering support services to other divisions of OSEL, including an ongoing major redesign of calibration control systems in the X-ray Calibration Laboratory. We developed a data acquisition system in support of a multi-center clinical study at NIH. Our Machine Shop completed a total of 81 assignments to design, fabricate, and/or modify lab equipment for OSEL researchers. We have undertaken a new assignment to develop LabView control software for an air-burst test fixture at the FDA Winchester Engineering and Analytical Laboratory.
Training/Outreach Activities. DESE staff served on the faculty of several courses sponsored by AAMI and AdvaMed on the topics of risk management and software risk management. We also gave presentations at professional meetings sponsored by professional associations such as ASQ. These forums provide the opportunity to increase awareness of the principles of risk management among practitioners in industry, and obtain feedback concerning the experience of manufacturers in applying these principles.
Wireless Program. A DESE engineer is co-chair of the AAMI EMC Committee, and Secretary/Co-Convenor of IEC SC62A MT23, and has been playing a leading role in revising IEC 60601-1-2, the seminal standard for medical device EMC. DESE engineers contributed to a number of premarket reviews and compliance activities involving medical device EMC, and also engaged with outreach activities with industry groups, such as the Mobile Healthcare Alliance, that are working on EMC issues.
Some examples of the more interesting assignments we completed are as follows:
Expertise in the areas of software engineering and risk management is to be expanded since they are becoming of critical importance to the increasingly sophisticated medical devices for which the Center bears regulatory responsibility. Of particular importance is developing educational initiatives. OSEL believes that every CDRH and ORA employee should be familiar with the principles of quality management and risk management. Because of our systems engineering orientation, we have been at the forefront of efforts to increase staff awareness of these topics. Educational outreach to industry and academia is also being explored.
In the risk management arena, investigations into methods for establishing acceptability criteria for evaluating risk, performing risk-benefit evaluations, using post-production information, and evaluating drug/device combinations must be initiated. Work is under way within CDRH to establish a uniform risk-based approach to device regulation. Guidance must be developed for performing risk management and reporting risk management results in pre-market submissions, establishing risk management as a component of a quality system, integrating risk management with design controls for both device design and design of manufacturing processes, and reconciling post-production information with prior risk management results.
1The FDA Critical Path report uses the term industrialization to describe the same concept.
2IEC 60601 is a mature and widely cited family of standards addressing requirements for safety and performance of all medical electrical devices. ISO 14971 is a seminal new standard that delineates a risk management process for medical devices. In CDRH jargon, these are examples of horizontal standards that apply to a wide range of medical devices across clinical boundaries, while vertical standards apply to one or a limited number of categories of devices within a single clinical specialty.
3With the imminent consolidation of FDA Headquarters components at White Oak, we foresee additional opportunities to provide engineering support services to other FDA Centers, with attendant benefit to our regulatory program.
4IEC 60601 is a mature and widely cited family of standards addressing requirements for safety and performance of all medical electrical devices. ISO 14971 is a seminal new standard that delineates a risk management process for medical devices. In CDRH jargon, these are examples of horizontal standards that apply to a wide range of medical devices across clinical boundaries, while vertical standards apply to one or a limited number of categories of devices within a single clinical specialty.
5http://www.fda.gov/cdrh/comp/guidance/1553.pdf
6http://www.ncvhs.hhs.gov/041119p1.htm
7http://www.fda.gov/cdrh/safety/bedfires.pdf
The rapid development of medical devices employing minimally invasive optical technologies is revolutionizing modern health care. Diseases that once required invasive surgery for diagnosis and treatment are now routinely addressed on an outpatient basis. The goal is a reduction in health care costs and an increase in patient safety and comfort. In addition, many diseases can now be diagnosed much earlier, resulting in more effective treatment. For many of these devices, reliable test methods and guidance documents are not available. The Optical Physics laboratory program is directed at early identification of (1) key scientific questions, (2) safety and effectiveness issues, and (3) the mechanisms of interaction for new optical diagnostic and therapeutic technologies. This information should facilitate the development of relevant evaluation criteria early in the review process.
There is increased interest in the scientific and medical communities in the use of optical technologies for the diagnosis and treatment of disease. Minimally invasive optical devices are rapidly becoming commonplace in clinical settings. For example, techniques that use light to measure bilirubin levels in neonatal skin, monitor oxygen saturation in blood and detect precancers in the colon and lungs have already been approved by the FDA. Furthermore, novel diagnostic approaches based on optical phenomena such as coherence and fluorescence are being studied in laboratories and hospitals throughout the world, and are likely to have a significant impact on modern medicine. As a consequence, CDRH is receiving an ever-increasing number of submissions in these areas. OSEL is investigating a number of high-priority, optical technologies in order to assist Center reviewers in the timely assessment of manufacturer’s submissions. Much of this research focuses on developing reliable standardized test methods that examine specific device attributes.
There is a lack of basic scientific knowledge on the mechanisms of interaction of optical devices with tissues. Understanding the mechanisms of interaction, especially regarding safety, is critical to CDRH's ability to make science-based regulatory decisions for this growing class of products.
In a separate, but related area, CDRH has the responsibility for evaluating and approving implanted devices designed to improve human vision. OSEL maintains laboratory instrumentation to evaluate the quality of intraocular lens implants (IOLs). In the past, this activity has aided in the establishment of standard test methods and guidance for product reviews. Currently, new types of IOLs are being developed for which present test methods are not appropriate. Further, new test methods need to be developed to evaluate the safety of new IOL materials and designs.
The mission of the Optical Physics Laboratory is to perform forward-looking research on FDA-specific topics relevant to medical devices that CDRH will be called on to evaluate for safety and effectiveness now, and in coming years. In order to achieve a solid understanding of these technologies, we are analyzing the physical mechanisms of these approaches as well as evaluating device performance and the factors that influence them. Results of these activities have been used, and will continue to be used, in guidance and standards for device performance.
OSEL’s investigations focus on clarifying the mechanisms of interaction of optical physics technology with the body and on developing meaningful performance assessment procedures. In the area of optical diagnosis, our scientists are developing analytic techniques to identify optical tissue properties by using diffuse reflectance data, evaluating fiber optic probes used in optical diagnosis, and developing mathematical models to assist in quantifying the distribution of optical energy within tissues. OSEL is also studying laser therapy devices in order to elucidate the mechanisms of interaction in order to maximize treatment effectiveness. Examples of other products currently being investigated include optical coherence tomography (OCT) for high-resolution imaging, dual-modality fluorescence spectroscopy and spectral reflectance spectroscopy for early diagnosis of disease, ablative lasers used for ophthalmic, cardiovascular, and dermatologic tissues, and optical biosensors for detection of glucose, oxygen, and pharmaceuticals.
OSEL already maintains laboratory instrumentation to evaluate the quality of intraocular lens implants (aphakic IOLs). However, new phakic intraocular lenses (IOLs) are being developed for the correction of myopia and hyperopia; these new IOLs require development of new test methods for product evaluation. Additionally, many patients have reported both temporary and permanent glare resulting from implanted IOLs, in some cases requiring IOL explantation and replacement; again, reliable test methods are needed.
There is a lack of standardization of terminology and parameters, and standardized test methods for evaluating many optical medical devices. In some cases there is even a lack of basic scientific knowledge on the mechanisms of interaction of optical energy with tissue. This program addresses the current gap in scientific knowledge by continuing laboratory studies of optical medical devices, developing test methods, and using our data as input to guidance and standards for streamlining the review of these products in CDRH.
Mechanisms of Optical Spectroscopy-Based Diagnostic Devices for Neoplasia Detection
In FY 2004, our group arranged for CDRH science seminars by highly regarded published research engineers from companies which are developing novel optical technologies such as fiberoptic confocal microscopy for in vivo pathology ( Sacha Loiseau, Ph.D., Mauna Kea Technologies, February 24, 2004), fluorescence spectroscopy for diabetes diagnosis (Woody Ediger, PhD, InLight Technologies April 28, 2004) and optical coherence tomography for ophthalmology (Dan Hammer, PhD, PSI, Inc., May 12, 2004). These presentations provided opportunities for OSEL researchers to meet ODE stakeholders and discuss technical and regulatory issues relevant to novel optical diagnostic devices.
Optical Nanobiosensors for Minimally Invasive Intracellular Monitoring
Minimally Invasive Optical Imaging of Biological Tissue
The use of optical medical devices will continue to grow for the foreseeable future since they can be used with far less discomfort, pain, and inconvenience than the more conventional methods used to detect and treat disease. Also, the use of optical devices will involve individuals more directly in their own health care. For example, there is an emergent need for more frequent and convenient monitoring of chronic disease conditions, giving individuals warnings of problems early so that medical intervention or prevention measures can be initiated. This program continues to examine new devices and technologies for safety and effectiveness. New policies, regulations, standards and guidance will be needed to enable individuals to safely operate and maintain devices. This information will be obtained from independent laboratory studies and in-house research that is conducted to anticipate new directions of this technology and will be disseminated in the form of peer-reviewed publications, consultative reviews, and guidance documents.
This project will provide laboratory support for optical radiation-emitting products for which the FDA has performance standards and provides input to national and international voluntary standards. In addition, this project provides dosimetry support for ongoing inter-Center projects (see below).
CDRH has the responsibility for enforcing three optical radiation performance standards for Lasers, Sunlamps, and Mercury Vapor Lamps under the Radiation Control for Health and Safety Act. In support of this activity, OSEL maintains FDA’s primary instrumentation, with calibrations traceable to NIST, for measurements of the wide variety of laser products on the market today. OSEL also maintains FDA’s instrumentation and standards for measurements of non-coherent optical sources.
OSEL calibrates laser measurement instruments, maintains laser field instrumentation kits used by FDA inspectors, tests regulated products, and provides technical consultation and measurement support for enforcement activities. Optical radiation measurements are used to support risk assessments and to evaluate potential adverse effects of optical radiation-emitting medical devices and consumer products.
OSEL provides spectral dosimetry measurements and consultation to 1) WEAC for spectral measurements of sunlamp products, 2) NCTR to assist them in maintaining an FDA-wide phototoxicity testing center, 3) CDER to assist in the development of the Final Monograph for Sunscreen Testing and the Test Method for determining the UVA Protection of OTC Sunscreen Products, 4) CFSAN to assist in phototoxicity and photocarcinogenicty testing of cosmeceuticals, and 5) ODE for hazard analysis in the approval of innovative medical devices which use optical diagnostic techniques. In addition, OSEL provides measurement and optical engineering assistance to the following multi-center Photosciences Projects:
Voluntary national and international standards have been developed and continue to be developed for the optical radiation safety of medical devices and radiologic products which emit optical radiation under ANSI Z311, Z136, IEC 60335-2-27 and ISO TC 172 SC 7 and SC 9. The international standards for laser products (IEC 60825-1) and sunlamp products (IEC 60335-2) have been revised, and CDRH continues to move forward in the revision of the FDA performance standards for these products. OSEL has been and will continue to participate in the harmonization of the IEC standards with the CDRH product performance standards. OSEL has been and will continue to maintain leadership roles in national and international standards committees, allowing CDRH viewpoints to be expressed and considered in the formulation of the developing standards.
The major technical accomplishments included work to measure the dioptic power of IOLs and the scattering and transmission properties of fog filters to be used in an ODE-sponsored driver simulation study as described in the following paragraphs.
Fig. 1. Principal optical design of the confocal fiber-optic laser method for IOL power measurement. The LASER is a 12-W-power 632.8-nm-wavelength intensity stabilized He-Ne laser The DETECTOR is a precise digital optical power meter. The system uses a 2 ´ 1 50/50 single-mode fiber coupler; O, an infinity corrected microscope objective; the IOL test to be tested, and a total reflectance mirror; f IOL,. A typical Gaussian beam profile formed by the single-mode fiber couple is shown in the figure. |
Testing the optical power of some IOLs presents a significant problem. Some of the IOLs have a high negative power that cannot be accurately measured. Also, measurements of diotic power of some IOLs have to be done with the IOL in a balanced salt solution making measurements particularly difficult. To overcome these problems a novel fiber optic confocal based optical system was developed. The fiber optic based system developed (figure 1) provides high accuracy (exceeding 1 mm) in spatially locating the IOL focal point and therefore in measuring the IOL dioptic power. Most importantly, this method provides FDA and the medical device community with a means for the accurate measurement of a wide range of both positive and negative powers including high-magnification IOLs with power greater than ± 20 diopters. Such accurate measurements are not achievable with present day methods. In addition, this new testing method provides the CDRH/FDA with an independent source of measurement data and information for evaluating the effectiveness and safety of novel IOL products. Without this new system, FDA would not be able to assess the optical power of new high power negative and positive lenses.
The new optical power measurement system was used to evaluate the dioptic power of regulatory samples of new IOL designs. In the low dioptic power ranges, the system was used to confirm the labeled dioptic power of low power negative phakic IOLs. The new testing system was also used to measure the power of high power negative IOLs from several different manufacturers. Finally, the system has been used to evaluate official samples of IOLs on regulatory hold for questions about the accuracy of the labeled dioptic power. The data obtained with this system was needed for making regulatory decisions. This novel system for measuring dioptic powers of IOLs has proven to be more accurate than any other present day system
Early in 2004, colleagues in ODE requested that fog filters with different degrees of scattering be characterized to determine which filters are appropriate for an ODE sponsored driver simulation study at the University of Iowa . A number of tests were developed and conducted to characterize the scattering properties of the filters. They included tests of the spectral transmittance, the spatial power distribution, the homogeneity of the scattering across the face of the filters and the effect of the scattering on a Gaussian laser beam to assess the effect of the scattering on image distortion.
The experimental set-up used to evaluate the scattering properties of the fog filters are shown in figure 2. The set-up included a Helium-Neon laser beam operating at a wavelength of 632.8 nm, an optical isolator, I, a fiber coupling microscope 10x objective, O1, a single-mode fiber, a collimating 20x microscope objective, O2, a 2 mm diameter diaphragm, and a power meter. The tip of the single-mode fiber was located at the focal point of objective O2 so that light from O2 was a collimated Gaussian beam.
Fig 2. Experimental set-up used to measure the spatial power distribution of the light scattered from the Fog filters. Step Index fiber with an operating wavelength of 630 nm, a core diameter of 4.3 microns, an NA of 0.11, and a cut-off wavelength of 580 + 40 nm. The power meter used is a silicone based instrument, Newport Optical Power Meter Model 840 with Newport detector Model 818 ST. The detector was fitted with a 2 mm diameter aperture and was located on a rotating stage which allowed for rotating the detector around the filters at the center of the rotating stage. For these measurements, the detector was located at a distance of 15 mm from the center of the fog filters and data was obtained at 1 degree increments. |
The single-mode fiber used is a Newport Model F-SV/F-SV-C. The same experimental setup was used to determine the effect of scattering on three-dimensional Gaussian laser beam profiles transmitted through the Fog Filters except that the power meter was replaced with a Spiricon PC Laser Beam Analyzer Model LBA-PC Series with a Pulnix 745 CCD camera. For these measurements, the fog filters were placed directly against the face of the CCD camera. A Shimadzu Model UV-3101PC UV-VIS-NIR dual beam Scanning Spectrophotometer was used to measure the spectral transmittance of the fog filters.
The effect on the Gaussian laser beam was assessed by evaluating the effect of the scattering on the shape of the Gaussian laser beam with the use of CCD camera imaging. Three filters were initially tested to determine which filters would be appropriate for the driver simulation study. The CCD camera images showed that all three fog filters would not distort the images to be viewed. All filters had significant forward scattering within +10 degrees. Finally, all three filters were homogeneous over the face of the filters tested. On the basis of this testing, it was concluded that Fog Filters 1 and 2 would be appropriate for the study. The scattering from Fog Filter 3 was too large for the study. On the basis of this testing ten additional filters were evaluated for the study. All ten additional filters were found to be acceptable for the study. A technical report was prepared which described the study and the results of the study.
In addition to the above, ray trace analysis (ZEMAX) was performed to predict the implanted power of a phakic IOL based on a patient’s data. This work showed that the predicted power of the IOL differed from that implanted and resulted in correct labeling for these phakic IOLs. Finally, ODEs ray trace analysis (ZEMAX) system was upgraded.
Finally, late in CY 2004 when funds became available, a high magnification microscope was purchased for the project to study the phenomenon of glistenings in IOLs.
Lasers
In conjunction with OC colleagues, and as a short training session for an Electro-Optics Specialist (EOS), laboratory evaluation of a laser product was accomplished. Evaluation confirmed that the product was correctly classified. However, this work also indicated that since classification was under the IEC 60825-1 scheme, additional measurement techniques will be needed once the CDRH is harmonized with the IEC standard.
Milestones met are as follows:
Non-coherent Optical Radiation Program
Successfully migrated spectroradiometer control from old laptop and software to new laptop and software.
This program focuses on the various issues associated with medical devices that utilize or are affected by electromagnetic (EM) fields. One issue concerns addressing the rapid deployment of wireless technology around and into medical devices, and the safety and effectiveness concerns associated with electromagnetic interference (EMI) disruption of medical devices and the deposition of the electromagnetic energy in the human body.
Another issue is developing methods to evaluate medical devices used for ablation of body tissues and the measurement and evaluation of EM heating and the evaluation of devices used intentionally to hear (heat?) body tissues. A principle goal of this effort is to develop standard techniques for measuring and evaluating RF heating for both high- and low-frequency electromagnetic devices.
Additionally, CDRH has the responsibility in the federal government to study and assess the risks of exposure to humans from electromagnetic non-ionizing radiation from radio frequency and microwave-emitting electronic products. This is a complex and challenging field. OSEL scientists work vigilantly to stay abreast of the many hundreds of papers produced annually on this subject. The Division of Physics within OSEL performs measurements and dosimetry to evaluate the most common emitters of em fields, e.g., cellular phones and MRI devices.
Responding to numerous adverse event and other reports, OSEL evaluated many types of medical devices for their susceptibility to interference from electromagnetic-field emitting sources such as wireless (cellular) telephones and magnetic-field emitting security devices. OSEL has already found the causes of several specific EMI problems and published results in peer-reviewed literature. Additionally, OSEL was assigned by CDRH to lead the Center’s Electromagnetic Compatibility (EMC) group that develops Center-wide EMC solutions for medical device EMI problems.
CDRH has been involved in responding to a number of concerns expressed by numerous groups about the safety of human exposure to electromagnetic radiation emitted by hand-held wireless (cellular) telephones and other wireless devices. OSEL began addressing this issue by chairing or actively contributing to several international standards-setting groups. The groups are developing wireless phone measurement standards. A well-defined measurement standard is necessary if both the manufacturers of wireless devices and the regulatory agencies that protect public health are to agree on compliance with existing FDA and FCC radiation emission standards or set new standards for wireless devices. Further, research into the potential biological effects of non-ionizing RF radiation is highly dependent on the amount (dose) of absorbed RF radiation. Accurate and repeatable measurement of the RF radiation dose (dosimetry) is critical for laboratory and epidemiological studies. The thermal injury that results from tissue heating for electromagnetic devices needs to be tested. Given the wide variety of device designs and tissues in which the devices are used make it difficult to generalize the dosimetric and thermal heating patterns of medical devices.
This program area develops standard techniques for measuring and evaluating RF heating for both high-and low-frequency electromagnetic devices.
OSEL has an active ongoing program of testing high-risk medical devices for susceptibility to electromagnetic interference (EMI) emitted by a wide variety of common sources of electromagnetic fields. Examples of sources of EMI include wireless personal communications devices (e.g., cellular phones) and radio or TV broadcast towers. In addition to laboratory work, OSEL researchers routinely perform regulatory reviews of pre-market submissions awaiting approval by FDA as well as post-market assessments of EMI on medical devices.
This program covers a wide range of medical device areas that include essentially all electrically powered devices, as well as the human exposures and energy deposition from a wide range of commonly used radio frequency emitters (e.g., cell phones, wireless computer links, security systems).
The wireless technology revolution, together with a flood of new medical devices incorporating sensitive microelectronics, is leading to a highly unstable situation. Dangerous malfunctions and numerous patient injuries have been induced in medical devices via electromagnetic interference (EMI) from electromagnetic fields emitted by wireless equipment. This equipment includes cellular phones, magnetic-field-emitting security devices (such as airport metal detectors) and other medical devices such as shortwave diathermy and magnetic resonance imaging (MRI). OSEL leads the FDA effort to make all electrically powered medical devices electromagnetically compatible (EMC) with the electromagnetic environment where they are used. In addition to EMC, the public and news media continually express concerns about the possible harmful effects of exposure to radio frequency (RF) electromagnetic fields (also known as non-ionizing RF radiation) from hand-held wireless (cellular) telephones and other wireless personal communications devices.
The objective of this program is to develop independent data, measurement and computational techniques, and test methods that will serve as solid scientific foundations for regulatory guidance, proposals for national and international standards, and peer-reviewed technical publications. All of the work is driven to promote the public health by developing and coordinating vital information that is unavailable elsewhere. The program utilizes the unique OSEL expertise and facilities built-up over several years of successfully performing research and taking active leadership in addressing the hazards from medical device EMI and human exposure to electromagnetic non-ionizing energy.
Wireless Technology EMC for Medical Devices
Developed simulation tool for Bluetooth and IEEE 802.11b wireless technology to study data loss and corruption, latency, through-put, and coexistence with other wireless signals. Presented project report to TATRC.
OSEL continues to study the general issue of safety, data integrity, and risks to patients in the clinical or home being monitored by wireless medical device technology. Researchers evaluate potential EMI/EMC problems associated with various wireless technology products (e.g., cellular phones, two-way radio transmitters) and wireless-connected palm/pocket computers that are increasingly being deployed in hospitals. OSEL will also evaluate medical devices with wireless interfaces (Bluetooth, IEEE 802.11b) as sources and victims of EMI.
Researchers will continue to evaluate the measurement and computer modeling of human exposure to radio frequency non-ionizing radiation emitted by wireless electronic products worn on the body and others. In each of these areas, scientists will perform independent laboratory experiments and make measurements in the clinical environment to develop independent data on each subject area. This information will be disseminated in the form of peer-reviewed publications, input to national and international standards efforts, consultative regulatory reviews, and guidance documents.
Scope:
The scope of this program is to provide laboratory and technical support to the Center’s Radiological Health mission. FDA serves as a reference laboratory in the national measurement system for safety from radiation-emitting electronic products. OSEL maintains measurement and calibration facilities for x-ray, laser, non-coherent optical sources, and microwave measurements. These calibration laboratories provide traceability for standards enforcement measurements, facilitate uniformity of measurements, and provide metrology expertise for pre- and post-market issues.
The program began in the early seventies with the implementation of mandatory performance standards for electronic product radiation. With nationwide compliance testing of x-ray equipment, it was necessary that measurements be consistent. The program provided field inspectors with uniform instrumentation that was accurate but simple to use. A state-of-the-art calibration laboratory was developed in order to provide the Bureau of Radiological Health (later CDRH) with a large volume of high-quality, low-cost calibrations at a time when such calibrations where not available elsewhere. Operating its own calibration lab gave the Bureau complete and independent control over the traceability of field measurements. This facilitated the validation of compliance measurements when they were challenged, provided uniformity of data for analysis, and eliminated possible conflicts of interest.
In the nineties, with the implementation of the Mammography Quality Standards Act (MQSA), the laboratory workload increased as FDA began annual inspections of mammography facilities. The laboratory was instrumental in developing the national calibration standard for mammography x-ray beams maintained by NIST. The CDRH X-ray Calibration Laboratory contributed to many of the standards for calibrations of ionizing radiation measuring instruments. In 1992, the laboratory was the first to receive accreditation from NIST's National Voluntary Laboratory Accreditation Program for this type of calibration. Through the years, the laboratory has provided FDA and agreement-state agencies with reliable ionizing radiation calibrations and metrology support.
The Radiological Health and Safety Program performs the following functions:
Through the operation of the CDRH X-ray Calibration Laboratory, OSEL provides the necessary traceability to national standards for instruments used by FDA and state inspectors to measure x-ray exposures from FDA-regulated products. The calibration laboratory is accredited by the National Voluntary Laboratory Accreditation Program (NVLAP) and complies with ISO Standard 17025.
In FY 2004, OSEL’s Ionizing Radiation Measurements Laboratory (IRML) spent over $200,000 on equipment and supplies for the RCHSA and MQSA field programs and to keep the calibration laboratory updated and traceable.
The IRML staff
Additionally, the laboratory staff made significant updates to the calibration laboratory’s automation systems, including fabrication of new electro-mechanical controllers and the near completion of new Labview software to control the calibration process.
OSEL continues to contribute significantly to the Center’s effort regarding the safety of x-ray security screening systems. In FY 2004 OSEL staff participated in discussions with other agencies, users, and manufacturers on the need for new radiation safety standards. OSEL was instrumental in the April 2004 formation of Task Group N43.16 to develop a new ANSI standard on cargo screening systems. Currently, OSEL and OCER are facilitating the exchange of information by the different security agencies through the Interagency Steering Committee on Radiation Standards.
In addition to the present level of support, the x-ray program expects increased activity in support of the field programs during the next 5 years. The increases are necessitated mainly by two new requirements: 1) new metrology support and calibration needs due to proliferation of x-ray security screening systems; 2) need to replace the main field instrument for testing medical diagnostic x-ray equipment and adaptation to states as partnership customers. Calibration support for the MQSA and diagnostic x-ray field inspection programs is expected to continue at or near the present level.
Necessary new activities in support of OC and ORA for screening system safety include working with instrument manufacturers to produce field instruments capable of making appropriate radiation measurements, developing new calibration techniques and procedures for these instruments, working with OC in developing field testing procedures, increased number of routine calibrations of survey instruments in low-intensity x-ray fields, and involvement in developing new performance standards for non-medical x-ray equipment.
Providing the calibration service to the states as a successful leveraging tool requires an increase in the types of instruments accepted for calibration. It also presents new bookkeeping and logistics challenges. New program activities will involve testing instruments, writing specifications, developing and implementing new calibration facilities and writing new procedures.
The x-ray program also expects to complete the upgrade of all its facilities to modern computer equipment and to install new x-ray equipment for non-invasive kV meter calibrations.
For the laser program, state-of-the-art measurement equipment is needed to provide support to the Office of Compliance and the Office of Device Evaluation when testing is needed to verify product classification or to obtain data for risk assessment of new laser devices. Amendments to the FDA laser standard will implement new requirements that will necessitate changes in measurement techniques.
Medical device performance and safety requires reliable and safe use of materials. The synthesis, processing, and fabrication of materials affect the molecular structure, phases and, ultimately, the physical, chemical, and mechanical properties, and biocompatibility of devices used in medical applications. Failure can result from improper material selection, inadequate stress analysis during device design, manufacturing errors, or misuse/abuse of devices. Materials degradation not only affects performance: it can also produce toxic substances that can cause serious injury or death to the patient. However, degradation is not always undesirable. It may be by design, as with resorbables. Thus, materials characterization must always by done keeping end use in mind.
The Mechanics of Materials and Structures program is structured to help CDRH understand materials issues of concern in both pre-market evaluations and post-market reported adverse events. The materials of interest include synthetics like metals and polymers, materials of biological origin, and those used in tissue engineered medical products (TEMPs). OSEL has the capabilities to measure mechanical properties ranging from the tensile strength of sutures and medical glove materials to the fatigue strength of total joint prostheses. Besides purely mechanical characterizations, our measurement capabilities for TEMPs constructs and scaffolds include quantification of phenotypic stability and the histomorphology of TEMPs relevant cell types. The combined output of this effort includes improved critical review of manufacturers’ claims and data, test method development, material and methods standards development, and publications related to the public health impact of medical device materials design, fabrication, or failure.
Activities in this program may be triggered within any phase of the product life cycle. In general, the activities of this group are directed not only towards resolving the specific issue that provided the trigger, but also in finding ways to apply the knowledge gained to future device problems. A few examples of these activities are provided in the following paragraphs.
Compatibility issues involving magnetic resonance imaging (MRI) systems and implants or support equipment have existed since this imaging technology was introduced. CDRH has received reports of adverse events through both its post-market monitoring system and the scientific literature describing deaths, burns, and other injuries from dislodged aneurysm clips, failed pacemakers, hurtling oxygen bottles, and brain stimulators. In addition, pre-market clearance of devices likely to be exposed to MRI has been a continuing problem. Some implants can be used near the magnet but not in the magnet. Other implants cease to function temporarily in the magnet but restart when the device is removed. Still, other devices fail completely in MRI. Some devices interfere with imaging but are immune from damage. And, in some cases, the device can produce RF heating when placed within the MRI system, resulting in serious burns. In response, OSEL scientists performed a number of experiments supporting their lead in the development of four ASTM International standards on MRI compatibility that are now utilized in pre-market reviews.
As a result of the recent new health care industry practices to reuse single-use devices (SUDs), OSEL scientists first evaluated the post-market device performance of balloon angioplasty catheters after single use at area cardiology centers. As a result of these and other studies, the issues of reuse have become an integral part of the pre-market review of reprocessed SUDs. Results of OSEL investigations provided vital information used in formulating Agency guidance on SUDs and "opened but not used" (OBNU) devices and has been used to develop training for field inspectors.
A potential problem was detected during pre-market review when an ODE reviewer observed that a plasma spray coating on a total hip implant could be scraped off with a credit card. Because there were no reliable tests or acceptance criteria for abrasion resistance, all devices of this type were subjected to required post-market surveillance. Industry responded by improving the quality of the coatings. OSEL put together a research team which developed a test method, directed and participated in a round robin, and wrote an ASTM standard (F1978) for abrasion testing of thermal sprayed coatings. An OSEL, OSB, and ODE team was assembled to develop a guidance document for rescinding the required postmarket surveillance. The companies used the method to document the improved abrasion resistance and surveillance was rescinded. Pre-market concerns in ODE also recognized the need to standardize the characterization of the alginate, chitosan, and collagen materials used in TEMPs as scaffolds. Staff in this program area led the standards development effort which, to date, has resulted in the approval of three standards for characterizing these materials. This also has led to laboratory and standards development for characterizing natural materials after exposure to cells.
As technology advances in the medical materials arena, OSEL scientists strive to maintain expertise in these areas. The field of nanotechnology is presenting exciting challenges in composite materials. TEMPs present a variety of material issues as well as cellular response issues. To address the broad scope of materials, we have also worked with other FDA centers (CFSAN, CDER, and CBER) on a diverse range of products, such as blood filters, imaging agents, adhesives and packaging materials, as well as the decontamination of instruments that may have contacted Creutzfeldt-Jakob Disease (CJD). We are also piloting some laboratory work on the effects of repeated sterilization on resorbable polymers, which we hope to develop in the near future into a full project.
Since the inception of the FDA Medical Device program, this program has been heavily involved with voluntary device standards organizations, such as ASTM International. Participation in these standards activities has leveraged Agency resources with industry and academia, creating lasting consensus solutions to these regulatory issues once the laboratory studies have been completed.
Characterization of Mechanical and Material Properties of Tissue Engineered Medical Products (Temps) Using A Standardized Cell-Based Test Method
Digital Particle Image Velocimetry (DPIV)/Computerized Fluid Dynamics (CFD) evaluation of velocity and wall shear stress in a proposed ASTM standard test method for cell adhesion for tissue engineered medical products (TEMPs): DPIV experimental flow visualization and CFD numerical modeling were investigated with promising results for inclusion into a proposed ASTM standard test method for cell adhesion.
Atomic force microscopy (AFM) to perform small-scale mechanical measurements of alginate gels. The visco-elastic properties (Tan Delta or Loss modulus/storage modulus) were measured and calculated for alginate gels cross-linked for various periods of time. This measure will be used to compare the mechanical properties of the construct to those of the natural material it is intended to replace.
Figure 1
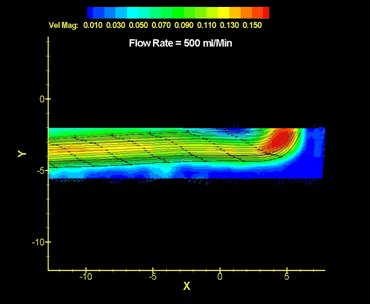
Figure 2
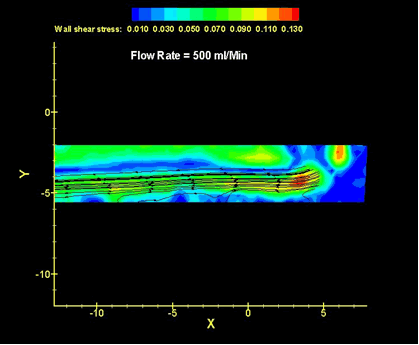
Medical glove and condom manufacturers continue to receive inquiries from consumers regarding whether or not certain lubricants are compatible with their products. The American Society for Testing and Materials (ASTM) formed Task Group D11.40.07 to address this issue. The Task Group developed a Round Robin test protocol for evaluating the compatibility of various consumer lubricants with natural rubber latex gloves and condoms, with Round Robin #3 occurring in FY 2004. FDA/CDRH/OSEL participated in this interlaboratory study, which tested three lubricants commonly used by medical glove and/or condom users (Keri® Lotion, Astroglide® personal lubricant, and Monistat® suppositories), as well as a positive control (Vaseline®), for compatibility with natural rubber latex (“latex”). The lubricants were applied to latex glove specimens, conditioned for 60 minutes, and removed from the specimens prior to tensile testing.
Development of a Test Method to Determine Glove Durability
Medical gloves are currently made from materials that behave differently from one another during actual use, yet there is no standard test method for evaluating glove durability. The American Society for Testing and Materials (ASTM) formed Task Group D11.40.02 to develop such a test method. It is hoped that the resulting durability ratings and/or specifications will assist consumers in choosing gloves according to the task at hand (e.g., a “heavy duty” task would require a more durable glove than a “light duty” task). Therefore the TG developed a Round Robin test protocol for evaluating the durability of an entire glove using a combination of abrasion testing and water leak testing.
OSEL subjected 2 sets of vinyl exam gloves to abrasion and water leak testing and found that 27 of 32 gloves leaked in the first set, and 20 of 32 gloves leaked in the second set. None of OSEL’s 32 control gloves leaked (control = water leak test only). Among TG participants the repeatability within each lab was high; however, there was wide variation in results from one laboratory to the next, and as a result the TG decided to adjust the protocol. Round Robin #4 will include exam gloves made of three different materials (vinyl, nitrile, and latex).
Materials issues will continue to play a major role in the overall safety and effectiveness of medical devices. History will repeat itself: things will continue to degrade, wear, and break. Additionally, we must be able to anticipate new areas of development and areas where problems may arise. New materials and new technologies, such as nanophase composites, hydrogels, biointeractive surfaces and TEMPs will see future applications in the universe of new medical devices. Also, challenges presented by custom-designed components and the development of ever smaller-scale minimally invasive and nanodevices will create a need for more sensitive and miniaturized methods. The features that limit the usefulness of these materials in these applications need to be identified to prevent injuries and also insure that post-market problems are handled correctly.
The mechanical quality of new device materials must be assured by the appropriate pre-market testing and post-market surveillance. The appropriate test methods and measurements, and their limitations need to be identified. OSEL is working to incorporate these methods into national and international standards, which will result in the use of uniform, described and accepted methods, as well as increase efficiency, quality, and uniformity of product reviews. The goal of the mechanics of materials and structures program is to develop the regulatory science base to meet these new challenges.
The Standards Management Staff develops and manages the standards used for regulatory assessments. Staff in this area facilitate the participation of CDRH and other FDA staff in developing standards. This involves working closely with the Standards Developing Organizations (SDOs), advertising standards liaison representative positions, facilitating a Center recommendation to serve on a particular standards activity, and maintaining an appropriate standards database providing access to established standards to all CDRH staff and field inspectors.
36 new standards
60 standards that were withdrawn and new versions were recognized
76 changes to the existing recognized standards
1 standard was withdrawn
27 were transferred from one Specialty Task Group (STG) to a more appropriate STG
In June 2004, the Center recognized:
9 new standards
11 standards that were withdrawn and new versions were recognized
27 changes to the existing recognized standards
1 standard was withdrawn
13 were transferred from one Specialty Task Group (STG) to a more appropriate STG
In October 2004, the Center recognized:
53 new standards
82 standards that were withdrawn and new versions were recognized
25 changes to the existing recognized standards
10 standards were withdrawn
17 were transferred from one Specialty Task Group (STG) to a more appropriate STG
October 1, 2003 – December 31, 2004
Journal Articles
Al-Khaldi SF, Myers KM, Rasooly A, Chizhikov V. Genotyping of Clostridium perfringens toxins using multiple oligonucleotide microarray hybridization. Cellular and Molecular Probes 18:359-67, 2004.
Armato SG, McLennan G, McNitt-Gray MF, Meyer C, Yankelevitz D, Aberle DR, Henschke CI, Hoffman EA, Kazerooni EA, MacMahon H, Reeves AP, Croft BY, Clarke LP, Dodd L, Gur D, Petrick N, Staab E, Sullivan DC, Wagner RF, Bland, PH, Brautigam K, Brown MS, De Young B, Engelmann RM, Enquobahrie AA, Floyd CE, Guo J, Husain A, Laderach, GE, Metz CE, Mullan B. The lung image database consortium (LIDC): Developing a resource for the medical imaging research community. Radiology, 232(3):739-748, 2004.
Badano A, Flynn MJ, Martin S, Kanicki J. Angular dependence of the luminance and contrast in medical monochrome liquid crystal displays. Medical Physics 30(10):2602-2613, 2003.
Badano A. Optical blur and collection effciency in columnar phosphors for x-ray imaging. Nuclear Instruments and Methods in Physics Research, Part A508(3):467-479, 2003.
Badano A, Gagne RM, Gallas BD, Jennings RJ, Boswell JS, Myers KJ. Lubberts effects in columnar phosphors. Medical Physics, 31(11):3122-3131, 2004.
Badano A , Fifadara DH. Comparison of Fourier-optics, telescopic, and goniometric methods for measuring angular emissions from medical liquid-crystal displays. Applied Optics 43(26):4999-5005, 2004.
Badano A, Fifadara DH . Goniometric and conoscopic measurements of angular display contrast for one-, three-, five-, and nine-million-pixel medical liquid crystal displays. Medical Physics, 31(12):3452-3460, 2004.
Badano A. Display systems. Radiographics 24:879-889, 2004.
Badano A, Drilling S, Imhoff B, Jennings RJ, Gagne RM, Muka E. Noise in flat-panel displays with sun-pixel structure. Medical Physics 31(4):715-723, 2004.
Badano A. AAPM/RSNA Tutorial on Equipment Selection: PACS equipment overview: display systems. Radiographics, 24(3):879-889, 2004.
Bassen HI, Casamento JP. High resolution computations and measurements of potential EMI with models of medical implants and radiating sources. IEEE EMC Symposium 2004, IEEE Electromagnetic Compatibility (EMC) Society, Santa Clara, CA, August 9-13, 2004.
Beard BB , Kainz W. 2004 Review and standardization of cell phone exposure calculations using the SAM phantom and anatomically correct head models BioMedical Engineering OnLine 3 34.
Beiden SV, Maloof M, Wagner RF. A general model for finite-sample effects in training and testing of competing classifiers. IEEE Transactions in Pattern Analysis Machine Intelligence 25:1561-1569, 2003.
Brown SA, Merritt K, Woods TO, Busick DN. The effects on the instruments of the WHO recommended protocols for decontamination after possible exposure to TSE contaminated tissue Journal of Biomedical and Materials Research, Applied Biomaterials (published on line 24 Sept 04)
Brown SL, Todd JF, Luu HMD. Breast implants adverse events during mammography: reports to the Food and Drug Administration. Journal of Women’s Health 13:1-8, 2004.
Bryans TD, Braithwaite C, Broad J, Cooper JF, Darnell KR, Hitchins VM, Karren AJ, Lee PS. Bacterial endotoxin testing: A report on the methods, background, data, and regulatory history of extraction recovery efficiency. Biomedical Instrumentation and Technology 37:73-78, 2004.
Buist DS, Newton KM, Miglioretti DL, Beverly K, Connelly MT, Andrade S, Hartsfield CL, Wei F, Chan KA, Kessler L. Hormone therapy prescribing patterns in the United States . Obstetrics and Gynecology 104(5):1042-50, November 2004.
Byrnes K, Barna L, Chenault V, Waynant R, Ilev I, Longo L, Miracco C, Johnson B, Anders J. Photobiomodulation improves cutaneous wound healing in an animal model of type II diabetes. Photomedicine and Laser Surgery22:281-290, 2004.
Chang I, Mikityansky I, Wray-Cahen D, Pritchard WF, Karanian JW, Wood BJ. Effects of perfusion on radiofrequency ablation in swine kidneys. Radiology 231(2):500-5, May 2004.
Chang IA. FEA software enables study of tissue ablation dynamics NASA Tech Briefs 5:48-49, May 2004.
Chen T, Small DA, McDermott MK, Bently WE, Payne GF. Enzymatic methods for in situ cell entrapment and cell release. Biomacromolecules4:1558-1563, 2003.
Desta AB, Owen RD, Cress LW. Non-thermal exposure to radiofrequency energy from digital wireless phones does not affect ornithine decarboxylase activity in L929 cells. Radiation Research 160:488-491, 2003.
Dodd LE, Wagner RF, Armato SG, McNitt-Gray MF, Beiden S, Chan HP, Gur D, Metz CE, Petrick N, Sahiner B, Sayre J. Assessment methodologies and statistical issues for computer-aided diagnosis of lung nodules in CT: Contemporary research topics relevant to the Lung Image Database Consortium. Academic Radiology, 11(4):462-475, 2004.
Dornish M, Kaplan D. Regulatory aspects and standardization of chitin and chitosan. Advances in Chitin Science, Volume VII, 152-156, 2004.
Fernandez-Figares I, Shannon AE, Wray-Cahen D, Caperna TJ. The role of insulin, glucagon, dexamethasone, and leptin in the regulation of ketogenesis and glycogen storage in primary cultures of porcine hepatocytes prepared from 60 kg pigs. Domestic Animal Endocrinology 27(2):125-40, August 2004.
Fifadara DH , Averbukh A, Channin DS, Badano A. Effect of viewing angle on luminance and contrast for a five- and a nine-million-pixel medical liquid crystal display. Journal of Digital Imaging, http://springerlink.metapress.com/openurl.asp?genre=article&id=doi:10.1007/s10278-004-1021-7, 2004.
Gagne RM, Boswell JS, Myers KJ. Signal detectability in digital radiography: spatial domain figures of merit. Medical Physics 30(8):2185-2193, 2004.
Gallas BD, Boswell JS, Badano A, Gagne RM, Myers KJ. An energy- and depth-dependent model for x ray imaging. Medical Physics, 31(11):3132-3149, 2004.
Gammell PM, Harris GR. IGBT-based kilovoltage pulsers for ultrasound measurement applications. IEEE Transactions in Ultrasonics, Ferroelectrics, and Frequency Control 50(12): 1722-1728, 2003.
Goodsitt MM, Chan HP, Lydick JT, Gandra CR, Chen NG, Helvie MA, Bailey JE, Roubidoux MA, Paramagul C, Blane CE, Sahiner B, Petrick N. An observer study comparing spot imaging region selcted by radiologists and a computer for an automated stereo spot mammography technique. Medical Physics 31(6): 1558-1567, 2004.
Hanigan MD, Crompton LA, Reynolds CK, Wray-Cahen D, Lomax MA, France J. An integrative model of amino acid metabolism in the liver of the lactating dairy cow. Journal of Theoretical Biology 228(2):271-89, May 2004.
Harris GR, Gammell PM, Lewin PA., Radulescu E. Interlaboratory evaluation of hydrophone sensitivity calibration from 0.1 MHz to 2 MHz via time delay spectrometry. Ultrasonics 42:349-353, 2004.
Helvie MA, Hadjiiski LM, Makariou E, Chan HP, Petrick N, Sahiner B, Lo SCB, Freedman M, Adler DD, Bailey JE, Blane CE, Hoff D, Hunt K, Joynt L, Klein K, Paramagul C, Patterson SK, Roubidoux MA. A non-commercial CAD system for breast cancer detection in screening mammograms achieves high sensitivity – A pilot clinical trial. Radiology 231(1):208-214, 2004.
Herman BA, Harris GR. Response to “An extended commentary on ‘Models and regulatory considerations for transient temperature rise during diagnostic ultrasound pulses’.” Ultrasound in Medicine & Biology 29(11):1661-1662, 2003.
Hilbert SL, Boerboom LE, Livsey SA, Ferrans VJ. An explant pathology study of decellularized carotid artery vascular grafts. Journal of Biomedical Materials Research 62A:197-204, 2004
Hilbert SL, Yanagida R, Souza J, Wolfinbarger L, Linthurst Jones A, Krueger P, Stearns, Bert A, Hopkins RA. Prototype anionic detergent technique used to decellularize allograft valve conduits evaluated in the right ventricular outflow tract in sheep. Journal of Heart Valve Disease 13:831-840, 2004.
Howell MD, Tomazic VJ, Truscott W, Meade BJ. Immunomodulatory effect of endotoxin on the development of latex allergy. Journal of Allergy & Clinical Immunology 113:916-924, 2004.
Ilev I, Waynant R, Byrnes K, Anders J. A fiber-optic approach for in vivo minimally-invasive study of tissue optical properties, SPIE 5317:147-150, 2004.
Johnson J, Jinneman K, Stelma G, Smith BG, Lye D, Messer J, Ulaszek J, Evsen L, Gendel S, Bennett RW, Swaminathan B, Pruckler J, Steigerwalt A, Kathariou S, Yildirim S, Volokhov D, Rasooly A, Chizhikov V, Wiedmann M, Fortes E, Duvall RE, Hitchins AD. Natural atypical Listeria innocua strains with Listeria monocytogenes pathogenicity Island 1 genes. Applied and Environmental. Microbiology 70:4256–4266, 2004.
Jones PL, Hislop D, Lee J, Pearce F, Van Albert S. Special section: analysis of requirements for a medical device – the computer-assisted resuscitation algorithm (CARA) experience. International Journal on Software Tools for Technology Transfer 5(4) May 2004 (published online February 19, 2004).
Kessler L , Ramsey SD, Tunis S, Sullivan SD. Clinical use of medical devices in the “Bermuda Triangle.” Health Affairs (Millwood) 23(1):200-7, January-February 2004.
Krauthamer V, Smith T. Acute effects of adrenergic agents on post-defibrillation arrest time in a cultured heart model. Cellular and Molecular Life Sciences 61:3093-3099, 2004.
Landry R, Wolffe M, Burrows C, Rassow B, Byrnes G. Study of the effect of involuntary user movement on the potential light hazards from some ophthalmic instruments. Applied Optics 43(8):1643-1647, March 2004.
Lee S-J, Badano A, Kanicki J. Monte Carlo modeling of the light transport in polymer
light-emitting devices on plastic substrates. IEEE Journal on Selected Topics in Quantum Electronics 10(1):37-44, 2004.
Lopez H, Beer JZ, Miller SA, Zmudzka BZ. Ultrasound measurements of skin thickness after UV exposure: a feasibility study. Journal of Photochemistry and Photobiology, B:Biology 73(3):123-132, 2004.
Lucas AD, Merritt K, Hitchins VM. The effects of gamma and electron beam radiation and ethylene oxide gas sterilization on office, personal use, and over the counter medical devices. Biomedical Instrumentation and Technology 38(6):476-484, 2004.
McDermott MK, Paradiso NA, Byrne ML, Balsis SL, Schroeder LW, Briber RM. Mechanical properties of polyurethane film exposed to solutions of nonoxynol-9 surfactant and polyethylene glycol. Journal of Applied Polymer Science 91(2):1086-1096, January 15, 2004.
McDermott MK, Chen T, Williams CM, Markley KM, Payne GF. Mechanical properties of biomimetic tissue adhesive based on the microbial transglutaminase-catalyzed crosslinking of gelatin. Biomacromolecules 5(4):1270-1279, July 12, 2004.
Miller SA, Landry R J, Byrnes GB. Endoilluminators - an evaluation of potential hazards. Applied Optics 43(8):1648-1653, 2004.
Myers MR. Transient temperature rise due to ultrasound absorption at a bone/soft-tissue interface. Journal of the Acoustical Society of America 115(6):2887-2891, June 2004.
Nguyen U, Brown S, Chang I, Krycia J, Mirotznik M. Numerical evaluation of heating of the human head due to magnetic resonance imaging. IEEE Transactions in Biomedical Engineering (51)8:1301-1309, August 2004.
Obuchowski NA, Beiden SV, Berbaum KS, Hillis SL, Ishwaran H, Song HH, Wagner RF. Multireader, multicase receiver operating characteristic analysis: an empirical comparison of five methods. Academic Radiology 11:980-995, 2004.
Paquerault S , Yarusso LM, Papaioannou J, Jiang Y, Nishikawa RM. Radial gradient-based segmentation of mammographic microcalcifications: observer evaluation and effect on CAD performance. Medical Physics 31(9):2648-57, September 2004.
Passerini A, Polacek DC, Shi C, Francesco NM, Manduchi E, Grant G, Pritchard WF, Powell SJ, Chang G, Stoeckert C, Davies PF. Coexisting proinflammatory and antioxidative endothelial transcription profiles in a disturbed flow region of the adult porcine aorta. Proceedings of the National Academy of Sciences , USA , 101(8):2482-2487, February 24, 2004 .
Peterson ED, Hirshfeld JW Jr, Ferguson TB, Kramer JM, Califf RM, Kessler LG. Part II: Sealing holes in the safety net. American Heart Journal 147(6):985-90, June 2004.
Petrick N, Badano A, Chan HP, Gagne RM, Sahiner B, Hadjiiski LM. Digital indirect detection x-ray imaging with a prototype microlens focusing layer between the screen and photodetector: effects on swank information factor. RSNA 89th Scientific Assembly and Annual Meeting, Radiological Society of North America (RSNA), November 30, 2003, Chicago, IL, Radiology, 223(P):392, 2003.
Pfefer TJ, Matchette LS, Drezek RA. Influence of illumination-collection geometry on fluorescence spectroscopy in multi-layer tissue. Medical and Biological Engineering and Computing 42(5):669-673, 2004.
Pfefer TJ, Matchette LS, Agrawal A, Drezek RA. Computational analysis of beveled-tip fiber probes for selective detection of subsurface fluorophores in turbid media. Proceedings of SPIE 5317:206-213, 2004.
Pritchard WF, Wray-Cahen D, Karanian JW, Hilbert S, Wood BJ. Radiofrequency cauterization with biopsy introducer needle. Journal of Vascular and Interventional Radiology 15(2, Part 1):183-187, February 2004.
Samei E, Siebert JA, Andriole K, Badano A, Crawford J, Reiner B. General guidelines for purchasing and acceptance testing of PACS equipment. Radiographics 24:313-334, 2004.
Sapsford KE, Rasooly A, Taitt CR, Ligler FS. Rapid detection of Campylobacter and Shigella species in food samples using an array biosensor. Analytical Chemistry 76:433-40, 2004.
Sergeev N , Volokhov D, Chizhikov V, Rasooly A. Simultaneous analysis of multiple Staphylococcal enterotoxin genes by an oligonucleotide microarray assay. Journal of Clinical Microbiology42(5):2134-43, May 2004.
Stewart SFC , Lyman DJ. Effects of an artery/vascular graft compliance mismatch on protein transport: a numerical study. Annals of Biomedical Engineering 32:991–1006, 2004.
Stewart SFC , Herman BA, Nell DM, Retta SM. Effects of valve characteristics on the accuracy of the Bernoulli equation: a survey of data submitted to the U.S. FDA. Journal of Heart Valve Disease 13:461-466, 2004.
Taitt CR, Golden JP, Shubin YS, Shriver-Lake LC, Sapsford KE, Rasooly A, Kulagina N, Ligler FS. A portable array biosensor for detecting multiple analytes in complex samples. Microbial Ecology 47:175-85, 2004.
Tomazic-Jezic VJ , Lucas AD and Sanchez BA. Binding and measuring of NRL proteins on glove powder. Journal of Immunoassay & Immunochemistry 25:109-123, 2004.
Volokhov D, Pomerantsev A, Kivovich V, Rasooly A, Chizhikov V. Identification of Bacillus anthracis by multiprobe microarray hybridization.Diagnostic Microbiology and Infectious Disease 49:163–171, 2004.
Wagner RF , Beam CA , Beiden SV. Reader variability in mammography and its implications for expected utility over the population of readers and cases. Medical Decision Making 24:561-572, 2004.
Walsh DL , Schwerin MR, Kisielewski RW, Kotz RM, Chaput MP, Varney GW, To TM. Abrasion resistance of medical glove materials. Journal of Biomedical Materials Research Part B: Applied Biomaterials 68B: 81-87, 2004.
Waynant R , Ilev I, Mitra K. Are ultrashort X-ray pulses likely to be less carcinogenic? SPIE 5312:267-272, 2004.
Wear KA . Measurement of frequency dependence of scattering from cylinders using focused transducers. Journal of the Acoustical Society of America115:66-72, January 2004.
Wei J, Chan H-P, Helvie MA ,Roubidoux MA, Sahiner B,.Hadjiiski LM, Zhou C, Paquerault S, Chenevert T, Goodsitt MN. Correlation between mammographic density and volumetric fibroglandular tissue estimated on breast MR images. Medical Physics 31(4):933-42, April 2004.
Wray-Cahen D , Fernandez-Figares I, Virtanen E, Steele NC, Caperna TJ. Betaine improves growth, but does not induce whole body or hepatic palmitate oxidation in swine (Sus scrofa domestica). Comparative Biochemistry and Physiology:Part A, Molecular and Integrative Physiology 137(1):131-40, January 2004.
Xing Y, He Z, Warnock, JN, Hilbert SL, Yoganathan AP. Effects of constant static pressure on the biological properties of porcine aortic valve leaflets. Annals of Biomedical Engineering 32:555-562, 2004.
Xing Y, Warnock JN, He Z, Hilbert SL, Yoganathan AJ. Cyclic pressure effects the biological properties of porcine aortic valve leaflets in a magnitude and frequency dependent manner. Annals of Biomedical Engineering 32:1461-1470, 2004.
Books
Badano A, Flynn MJ, Kanicki J. High-fidelity medical imaging displays. Tutorial Texts in Optical Engineering (Series). SPIE Press, 2004.
Barrett HH, Myers KJ. Foundations of Image Science, John Wiley & Sons, Inc., New York, 2004.
Schutte E, Picciolo GL, Kaplan D. (Eds.,) Tissue Engineered Medical Products (TEMPs). ASTM STP1452. ASTM International, West Conshohocken , PA. 2004.
Book Chapters
Anderson JM, Schoen FJ, Brown SA, Merritt K. Implant retrieval and evaluation. In: Biomaterials Science, (Eds) Ratner, Hoffman, Schoen, Lemons, Elsevier, San Diego, 2004, pp 771-782.
Badano A. Principles of cathode-ray tube and liquid crystal display devices. In: Advances in Digital Radiography: RSNA Categorical Course in Diagnostic Radiology Physics. RSNA, pp. 91-102, 2003.
Badano A. Medical display metrology. In: Annual Meeting of the Society for Information Displays, A. Lakatos, ed., Palissades Press, Seattle, 2004.
Brown SA. Let's not repeat history; good examples of bad ideas. In: Medical Device Materials S. Shrivastava (ed) ASMInternational, Materials Park OH, 2004, pp.3-7
Brown SA, Merritt K. Biocompatibility, metal ions, and corrosion products. In: Medical Device Materials S. Shrivastava, (ed) ASMInternational, Materials Park OH, 2004, pp 172-175.
Chen T, Small DA, McDermott MK, Bentley WE, Payne GF. Biomimetic Approach to Biomaterials: Amino Acid-Residue-Specific Enzymes for Protein Grafting and Crosslinking. In: Polymer Biocatalysis and Biomaterials, chapter 8, American Chemical Society (publisher), 2004.
Divine KK, Goering PL. Tantalum. In: Elements and Their Compounds in the Environment – 2nd edition, M Anke, M Ihnat and M Stoeppler, (eds.), Wiley-VCH, Weinheim Germany, Vol. 2, Chapter 21, pp. 1087-1097, 2004.
Goering PL, Zielger TL. Niobium. In: Elements and Their Compounds in the Environment – 2nd edition, M Anke, M Ihnat and M Stoeppler, (eds.), Wiley-VCH, Weinheim Germany, Vol. 2, Chapter 19, pp. 1039-1046, 2004.
Goering PL. The Lanthanides. In: Elements and Their Compounds in the Environment – 2nd edition, M Anke, M Ihnat and M Stoeppler, (eds.), Wiley-VCH, Weinheim Germany, Vol. 2, Chapter 14, pp. 867-878, 2004.
Hilbert SL, Schoen FJ, Jones M, Ferrans VJ. Allograft heart valves: morphologic, biomechanical and explant pathology studies. In: Cardiac Reconstructions with Allograft Tissues, 2 nd Edition, Hopkins RA (ed.) Springer-Verlag, New York, Section IV, Chapters 21-26, pp 193-231, 2004.
Hilbert SL, Hopkins RA. Tissue engineered heart valves: the ultimate challenge. In: Cardiac Reconstructions with Allograft Tissues, 2 nd Edition, Hopkins RA, (ed.) Springer-Verlag, New York, Chapter 65, pp 612-620, 2004.
Kaplan DS. Development of standards for the characterization of natural materials used in TEMPs. Tissue Engineered Medical Products (TEMPs), ASTM STP 1452, G.L. Picciolo, E. Schutte and D. Kaplan, Eds., ASTM International, West Conshohocken, PA. pp. 172-175. 2004.
Madden EF, Anderson CJ, Goering PL. Indium. In: Elements and Their Compounds in the Environment – 2nd edition, M Anke, M Ihnat and M Stoeppler, (eds.), Wiley-VCH, Weinheim Germany, Vol. 2, Chapter 12, pp. 801-809, 2004.
Schroeder LW, Walsh DL, Schwerin MR, Richardson DC, Kisielewski RW, Cyr WH. Standard quality control testing, virus penetration, and glove durability. In: Protective Gloves for Occupational Use, Boman A, Estlander T, Wahlberg JE, Maibach HI (eds). CRC Press, Boca Raton, FL, pp 89-109, 2004.
Tomazic VJ. In vitro testing for NRL allergy and allergens. In: Latex Intolerance: Basic Science, Epidemiology and Clinical Management. MM Chowdhury and HI Maibach, (eds.), CRC Press, Boca Raton FL, pp. 67-86, 2004.
Witters Jr D, Caroline A. Campbell CA. Wireless medical telemetry: addressing the interference issue and the new wireless medical telemetry service (WMTS). Clinical Engineering Handbook Joseph Dyro (editor) (ISBN: 0-12-226570-X), pages 482-497, 2004.
Wolbarst AB, Badano A. Electronic cameras and displays. In: Physics of Radiology,.Medical Physics Publishing Corporation., 3rd edition, 2004.
Zielger TL, Divine KK, Goering PL. Gallium. In: Elements and Their Compounds in the Environment – 2nd edition, M Anke, M Ihnat and M Stoeppler, (eds.), Wiley-VCH, Weinheim Germany, Vol. 2, Chapter 9, pp. 775-786, 2004.
Proceedings, Abstracts, Letters, Book Reviews, etc.
Alderson N, Ali SN, Debrabant A, Elkins CA, Fahmy R, Faustino PJ, Grant MA, Johannessen J, Khan AS, Kulka M, Lucas AD, Manjanatha M, Stern SH, Trinh H, Ward JL, Wood GE. Committee for the Advancement of FDA Science (CAFDAS): The FDA scientist’s liaison to the Commissioner’s Office. FDA Science Forum, Abstract M-11, p. 121, Washington DC, May 18-19, 2004.
Alderson N, Ali SN, Debrabant A, Elkins CA, Fahmy R, Faustino PJ, Grant MA, Johannessen J, Khan AS, Kulka M, Lucas AD, Manjanatha M, Stern SH, Trinh H, Ward JL, Wood GE. Committee for the Advancement of FDA Science (CAFDAS): Leveraging Activities. FDA Science Forum, Abstract M-12, p. 122, Washington DC, May 18-19, 2004.
Asher DM, Pomeroy KL, Taffs RE, Merritt K, Brown SA. Evaluating methods to eliminate TSE agents (Prions) from contaminated durfaces. Abstr C-36 at FDA Science Forum, Washington, DC, May 19, 2004.
Badano A. Sub-pixel structure and spatial noise in medical active-matrix liquid crystal displays. Proceedings of the IDRC, 23:165-168, 2003.
Beer JZ, Hearing VJ, Yamaguchi Y, Tadokoro T, Zmudzka BZ, Coelho SG, Miller SA. UV responses in the epidermis of different races: melanocytes, melanin and DNA damage. American Society for Photobiology Annual Meeting, Seattle WA, July 10-14, 2004, p. 40.
Blustein JN, Hitchins VM, Woo EK. Diffuse lamellar keratitis, endotoxin, and ophthalmic sponges. Letter to the Editor, Journal of Cataract Refractive Surgery 30:2027-2028, 2004.
Brown RP, Madden EF, Goering PL. Benchmark dose modeling of mercury-induced acute renal failure in Sprague-Dawley rats with renal insufficiency compared to healthy controls. Society of Toxicology Annual Meeting, Toxicological Sciences 78 (S-1), Baltimore MD, March 21-25, 2004.
Brown RP, Madden EF, Goering PL. Benchmark dose modeling of mercury-induced acute renal failure in Sprague-Dawley rats with renal insufficiency compared to healthy controls. FDA Science Forum, Abstract K-12, p. 110, Washington, DC, May 18-19, 2004.
Bursac N, Aguel F, Tung L. Multi-arm spirals in 2-dimensional heart substrate. Proceedings of the National Academy of Sciences 101:15530-15534, 2004.
Coelho SG, Miller SA, Beer JZ. The use of digital photography to document and quantify erythema and pigmentation induced in human skin exposed to tanning lamps. FDA Science Forum, Abstract F-11, p. 90, Washington, DC, May 18-19, 2004.
Coelho SG, Kornhauser A, Wei RR, Miller SA, Beer JZ. The use of digital photography in clinical studies on interactions of hydroxyl acid-containing cosmetics with UV exposure. American Society for Photobiology 32 nd Annual Meeting, Seattle, WA, July 10-14, 2004.
Coelho SG, Miller SA, Beer JZ. The use of digital photography for objective assessment of UV responses in skin of different racial/ethnic background. American Society for Photobiology Annual Meeting, Seattle, WA, July 10-14, 2004.
Cygnarowicz T, Baker K, Malshet V, Kane J, Regnault W, Jaffee S, Weininger S, Jorgens J, Wollerton M, Koustenis G, Hoban M, Beard B, Woods T, Casamento J, Mann E. From concept to reality: FDA’s recommendations for the design, manufacturing, clinical evaluation, and postmarket surveillance of class iii implantable middle ear hearing devices. 10 th Annual FDA Science Forum, May 18-19, 2004, p. 104.
Dermody NC, Woods TO, Graham JH. Mechanical characterization of calcaneus bone. 2004 FDA Science Forum, Washington, DC, May 18-19, 2004.
Dornish M, Kaplan DS. Hyaluronan in tissue engineered medical products: The ASTM Guide for the Characterization and Testing of Hyaluronan, Hyaluronan 2003 Meeting, Cleveland, OH, October 11-16, 2003.
Elespuru RK, Jenning SM. Molecular epidemiology of esophageal cancer using the IARC p53 human tumor database. Society of Toxicology Annual Meeting, Baltimore, MD, March 21-25, 2004.
Elespuru RK, Ranamukhaarachchi D, Fuscoe J, Puri R, Morris S, Leighton J, Pennello G, Roayaei J, Chen J, Wang S-J, Martinsky T, Mendrick D. Prioritizing sources of variability in genomic profiling data for standards and guidance development: draft statistical design. Genomics (Microarray) Biostatistics Workshop, PhRMA/FDA, University of Maryland – Shady Grove, Rockville, MD, April 15-16, 2004.
Elespuru RK, Fuscoe J, Puri R, Ranamukhaarachchi D, Pennello G, Roayaei J, Morris S, Martinsky T, Mendrick D, Leighton JK, Chen J. Prioritizing sources of variability in genomic profiling data for standards and guidance development. FDA Science Forum, Abstract J-14, p. 105, Washington, DC, May 18-19, 2004.
Elespuru RK, Jenning SM. Molecular epidemiology of human lung cancer: analysis using the IARC TP53 database. Environmental Mutagen Society Annual Meeting, Pittsburg, PA, October 2-6, 2004.
Godar DE. UVA1-mediated receptor and cytokine changes of transformed lymphocytes. FDA Science Forum, Abstract B-PO-01, p. 51, Washington, DC, May 18-19, 2004.
Godar DE. UV doses worldwide and beyond. FDA Science Forum, Abstract M-01, p. 119, Washington, DC, May 18-19, 2004.
Godar DE. UV doses worldwide and beyond. American Society for Photobiology Annual Meeting, Seattle, WA, July 10-14, 2004.
Gur D, Wagner RF, Chan HP. On the repeated use of databases for testing incremental improvement of computer-aided detection schemes. Academic Radiology 11:103-105, 2004.
Hadjiiski LM, Helvie MA, Sahiner B, Chan HP, Roubidoux MA, Nees A, Petrick N, Blane CE, Paramagul C, Bailey JE, Patterson S, Klein K , Adler DD, Foster M, Shen J. ROC study of the effects of computer-aided interval change analysis on radiologists' characterization of breast masses in two-view serial mammograms. In: Proceedings of SPIE Medical Imaging, 5370:51-58, 2004.
Harris GR. High-intensity focused ultrasound for tissue thermal ablation: Potential applications and critical path opportunity. 2004 FDA Science Forum, Washington, DC, May, 2004.
Hearing VJ, Yamaguchi Y, Tadokoro T, Zmudzka BZ, Miller SA and Beer JZ. Physiological regulation of melanocyte proliferation and differentiation in human skin following ultraviolet radiation. Pigment Cell Research17: 440-441, 2004.
Hilbert SL, Yanagida R, Krueger P, Linthurst-Jones A, Wolfinbarger L, Hopkins R. A comparison of the explant pathology findings of anionic and non-ionic detergent decellularized heart valve conduits. Proceedings of Cardiovascular Tissue Engineering: From Basic Biology to Cell-Based Therapies, p 43, 2004.
Hitchins VM, Merritt K. Production of IL-6, IL-1 beta, TNF-alpha, and nitric oxide by murine macrophages, murine bone cells, and human bone cells exposed to alginates of different MG ratios and different purities. FDA Science Forum, Abstract B-24, p. 48, Washington, DC, May 18-19, 2004.
Hutter JC, Richardson DC, McDermott MK, Chen ET, Crowe J, Platek F, Witkowski M, Poindexter B, Vostal J. Materials testing of recalled particle contaminated blood bags (poster), 10 th Annual FDA Science Forum, Washington DC, May 18-19, 2004.
Ilev I, Waynant R, Romanczyk T, Gorman E, Moore C, Anders J. In-vivo precise brain tissue ablation using smart infrared optical fiber. LEOS 2003 – Annual Meeting of the IEEE Lasers and Electro-Optics Society, Tucson, AZ, October 26-30, 2003.
Ilev I, Waynant R. Noninvasive optical monitoring of smoking-induced effects on exhaled nitric oxide. National Conference on Tobacco or Health, Boston, MA , December 1012, 2003.
Kaplan DS, Hitchins VM. Role and use of guidance documents and standards for submission of applications for combination and human cell, tissue, and cellular and tissue-based products to the Food and Drug Administration. Society for Biomaterials Meeting on Biomaterials in Regenerative Medicine: Advent of Combination Products, Philadelphia, PA, October 16-18, 2004.
Karanian JW, Wray-Cahen D, Ashby AE, Hilbert SL, Pritchard WF. Laboratory of Preclinical Studies Program overview. FDA Science Forum, Abstract B-01, p. 43, Washington, DC, May 18-19, 2004.
Karanian JW, Wray-Cahen D, Ashby AE, Virmani R, Kologie F, Hilbert SL, Pritchard WF. High cholesterol-high fat diet in swine leads to atherosclerotic lesions and a delayed healing response following coronary interventions. FDA Science Forum, Abstract B-03, p. 43, Washington, DC, May 18-19, 2004.
Karanian JW, Virmani R, Wray-Cahen D, Ashby AE, Hilbert SL, Pritchard WF. Local delivery of estrogen inhibits stenosis following angioplasty balloon injury in a swine model: A preliminary report on the safety and effectiveness of a trans-vascular needle injection catheter. FDA Science Forum, Abstract B-05, p. 44, Washington, DC, May 18-19, 2004.
Ketchedjian A, Cooper G, Hilbert S, Yanagida R, Krueger P, Lukoff J, Hopkins R. Chronic sheep implant study of small intestinal submucosa: fabricated pulmonary and mitral valve replacements. Journal of the American College of Surgeons 199:Suppl; S28, 2004
Lee S-J, Badano A, Kanicki J. Monte Carlo modeling of organic polymer light-emitting devices on flexible plastic substrates. Proceedings of the SPIE, 4800:156-163, 2003.
Lee S-J, Badano A, Kanicki J. Monte Carlo simulations and opto-electronic properties of polymer light-emitting devices on flexible plastic substrates. Proceedings of the IDRC, 23:26-29, 2003.
Leeper DB, Davies CL, Pollard MD, O'Hara MD. The effect of hyperglycemia plus MIBG and 42°C-hyperthermia on the cellularity of murine bone marrow. 9th International Congress on Hyperthermic Oncology (ICHO 2004), St. Louis, MO, April 20-24, 2004.
Leeper DB, Burd R, Davies CL, Pollard MD, Canter RJ, Zhou R, Kesmodel SB, Fraker DL and Glickson JD, O'Hara MD. Acute acidification by MIBG + hyperglycemia sensitizes melanoma xenografts but not bone marrow to hyperthermia and melphalan suggesting therapeutic gain. The Kadota Fund International Forum, Awaji Yumebutai International Conference Center, Japan, June 15-18, 2004.
Loparev VN, Gonzales A, Deleon M, Sergeev N, Lopareva E, Chumakov K, Shmid S. Identification and rapid detection of the three major genotypes of Varicella zoster virus in global circulation using combined PCR-microarray (CPM) analysis, snapshot and FRET-based probe hybridization (FBPH). International Conference on Emerging Infectious Diseases, Atlanta, GA, February 29-March 3, 2004.
Madden EF, Brown RP, Goering PL. Development and validation of a gentamicin-induced subclinical renal injury model in the Sprague-Dawley rat. American College of Toxicology Annual Meeting, Washington, DC, November 2-5, 2003.
McGuiggan PM, Deng Y, Simon Jr. CG, Wiederhorn SM, Lawn BR, Vegella T, Kaplan DS. Dynamical adhesion force of PSA film and cells. Materials Research Society (MRS) Fall Meetings, Boston, MA, December 1-5, 2003.
McDermott MK , Markley KM, Williams CM, Chen T, Janjua R , Steidl SM, Payne GF. Biomimetic approach for soft tissue adhesives. 2004 FDA Science Forum, Sigma Xi Poster Session, Poster F-03, page 88, Washington Convention Center, Washington, D.C., May 18-19, 2004.
Nguyen U, Brown S, Chang I, Krycia J, Mirotznik M. Numerical evaluation of heating of the human head due to magnetic resonance imaging. IEEE Transactions of Biomedical Engineering 51(8):1301-1309, August 2004.
Pandey AK, Chang IA, Myers MR, Banerjee RK. Radiofrequnecy ablation with a gaussian heat source in a realistic reconstructed hepatic geometry. Proceedings for the International Mechanical Engineering Conference and Exposition, Washington, DC, November 16-20, 2003.
Paquerault S , Yarusso LM, Papaioannou J. Potential improvement of computerized classification for malignant versus benign mammographic microcalcification clusters with the use of special views. SPIE International Symposium on Medical Imaging, San Diego , CA , Proceedings of SPIE Medical Imaging, 995, 2004.
Petrick N, Badano A, Chan HP, Sahiner B, Hadjiiski LM. Digital indirect-detection x-ray imagers with microlens focusing: effects of Fresnel reflections from the microlens layer. Proceedings of the SPIE, 5030:504-510, 2003.
Petrick N, Badano A, Chan HP, Gagne RM, Sahiner B, Hadjiiski LM. Digital indirect detection x-ray imaging with a prototype microlens focusing layer between the screen and photodetector: effects on swank information factor Radiology 223(P):392, 2003.
Petrick N. Computer aided diagnosis (CAD): the current state and future for this technology. FDA Science Forum (invited talk), May 2004.
Petrick N, Gallas BD, Samuelson F, Wagner RF, Myers KJ. Using a panel of experts as truth surrogate in reader studies: an initial look at the effects of panel size and expert skill on panel performance in defining truth. Radiological Society of North America (RSNA), Chicago, IL,2004.
Pritchard WF, Friedman MA, Wray-Cahen D, Karanian JW, Dewhirst MW, Ashby AE, Neeman Z, Hvizda J, Kanter P, Patel S, Wood BJ. Heat-sensitive liposomes as carriers for doxorubicin increase local drug deposition during radiofrequency ablation. Radiological Society of North America, Chicago, IL, November 30- December 5, 2003.
Pritchard WF, Passerini AG, Shi C, Francesco NM, Manduchi E, Grant GR, Karanian JW, Wray-Cahen D, Chun P, Ashby AE, Stoeckert CJ, Davies PF. Impact of gender, diet, and regional hemodynamics on arterial endothelial cell gene expression profiles in a swine model of vascular disease. FDA Science Forum, Abstract B-02, p.43, Washington, DC, May 18-19, 2004.
Ranamukhaarachchi D, Langone J, Elespuru RK. Evaluation of target cell and mRNA detection limits and RNA isolation in molecular diagnostics of disease. Chips-to-Hits: Microarray technologies and their use in drug discovery and development, Boston, MA, October 28-31, 2003.
Ranamukhaarachchi D , Langone J, Nandanie A, Chen F, Schneider M, , Elespuru R. Analysis of microarray expression signals generated by RNA labeled with fluorescent molecules compared with resonance light scattering nano-particles. FDA Science Forum, Abstract E-21, p.76, Washington, DC, May 18-19, 2004.
Ranamukhaarachchi D, Tinsworth M, Nandanie A, Langone J, Elespuru R. Gene expression profiling of type I latex allergy by fluorescent differential display PCR. FDA Science Forum, Abstract E-24, p.77, Washington, DC, May 18-19, 2004.
Robinson RA, Malinauskas RA, Yazdani S, Das S, Yabut D, Wozniczka J, BusharG, Stewart SFC, Grossman LW. In vitro steady flow evaluation of clot capture efficiency and clot location for five commercial vena cava filters. FDA Science Forum 2004, Washington, DC, May 18-19, 2004.
Sahiner B, Chan HP, Hadjiiski LM, Helvie MA, Roubidoux MA, Petrick N. Computerized detection of microcalcifications on mammograms: improved detection accuracy by combining features extracted from two mammographic views. Radiology 223(P):389, 2003.
Schwerin MR, Richardson DC. Abrasion occurring during emergency response can hasten loss of glove integrity, FDA Science Forum, May 18-19, 2004.
Sergeev N, Distler M, Chizhikov V, Rasooly A. Microarray analysis of Bacillus cereus group virulence factors. FDA Science Forum, Abstract C-16, p.55, Washington, DC, May 18-19, 2004.
Sergeev N, Distler M, Courtney S, Al-khaldi SF, Volokhov D, Chizhikov V, Rasooly A. DNA microarray for food safety analysis. FDA Science Forum, Abstract A-68, p.40, Washington, DC, May 18-19, 2004.
Shallcross JC, Lyle DB, Hitchins VM, Langone JJ. Nitric oxide detection in cell types involved in vascular restenosis. FDA Science Forum, Abstract B-07, p.44, Washington, DC, May 18-19, 2004.
Shallcross JC, Lyle DB, Langone JJ. Determination of optimal conditions for detecting nitric oxide involved in adverse reactions to cardiovascular devices. FDA Science Forum, Abstract B-17, p. 47, Washington, DC, May 18-19, 2004.
Stratmeyer ME. Book Review: Biological Safety & European Medical Device Regulations. In: Journal of Medical Device Regulation, pp. 75-76, November 2004.
Summers RM, Bitter I, Petrick N. Virtual colonoscopy [comment]. JAMA292(4):432-433, 2004.
Tang X, Langone JJ, Morris S, Bockstahler LE. Detection of Mycobacterium tuberculosis (MTB) drug-resistant strains using microarrays and allele-specific PCR. FDA Science Forum, Abstract A-35, p. 32, Washington, DC, May 18-19, 2004.
Thacker SC, Glick SJ, Badano A. Monte Carlo simulation of a CsI-based flat panel imager for mammography. Proceedings of the SPIE, 5368:411-419, 2004.
Triesel I, Manion M, Sergeev N, Shapiro B, Rasooly A. Hand-held microfluidics multi-channel immunosensor for detection of multiple microbial toxins simultaneously. FDA Science Forum, Abstract A-44, p.35, Washington, DC, May 18-19, 2004.
Volokhov DV, Peredelchuk M, Sergeev N, Hitchins AD, Pomerantsev A, Rasooly A, Chizhikov VE. Development of DNA microarrays for environmental, food and clinical microbiology. International Conference on Emerging Infectious Diseases, Atlanta, GA, February 29-March 3, 2004.
Wei J, Sahiner B, Petrick N, Chan HP, Hadjiiski LM, Helvie MA. Computer-aided diagnosis system for mass detection: comparison of performance on full-field digital mammograms and digitized film mammograms. Radiology 223(P):424, 2003.
Wei J, Sahiner B, Hadjiiski LM, Chan HP, Petrick N, Helvie MA, Zhou C, Ge Z. Computer-aided detection of breast masses on full-field digital mammograms: false positive reduction using gradient field analysis. In: Proceedings of SPIE Medical Imaging 5370:922-998, 2004.
Wood SC, Pettiford J, Langone JJ. Organ culture of coronary arteries: an in vitro system to study cardiovascular device performance. FDA Science Forum, Abstract B-31, p.50, Washington, DC, May 18-19, 2004.
Wray-Cahen D, Elsasser T, Wilkins B, Hilbert SL, Pritchard WF, Ashby AE, Karanian JW. Response of reactive nitrogen components of the nitric oxide cascade to angioplasty balloon injury in coronary arteries is gender- and hormonal-dependent in a swine model. FDA Science Forum, Abstract B-04, p.43, Washington, DC, May 18-19, 2004.
Yarusso YM, Nishikawa RM, Paquerault S, Schmitz J. Physical characteristics of full-field digital mammography and digitized screen-film mammography. The 7th International Workshop on Digital Mammography, Chapel Hill, North Carolina, Proceedings of IWDM-2004 (2004).
Zmudzka BZ, Beer JZ, Bushar HF, Coelho SG, Hearing VJ, Miller SA, Tadakoro T, Yamaguchi Y. Constitutive melanin, MED and UV-induced DNA damage in different racial/ethnic groups. FDA Science Forum, Washington, DC, May 18-19, 2004.
Technical Reports
Kelley EF, Badano A. Characterization of luminance probe for accurate contrast measurements in medical displays. Technical Report NISTIR 6974, NIST, 2003.
October 1, 2003 – December 31, 2004
Badano A . Perspectives on current medical display research. Annual Meeting of the American Association of Physicists in Medicine, Pittsburg , PA , 2004, and the High Information Content Display Systems Conference, Arlington , VA , 2004.
Badano A . Light transport in x-ray imaging detectors using DETECT-II, University of Michigan , 2004.
Bassen HI , Casamento JP. High resolution computations and measurements of potential EMI with models of medical implants and radiating sources. IEEE EMC Symposium 2004, IEEE Electromagnetic Compatibility (EMC) Society, 2004 Santa Clara, CA, August 9-13.
Beer JZ , Yamaguchi Y, Zmudzka BZ, Miller SA, Hearing VJ. Early post-UV-exposure dynamics of DNA damage in human skin. FDA/NCI Photobiology Symposium, Rockville , MD , March 9, 2004 .
Beer JZ . Overview of ongoing human studies at the FDA Photosciences Facility. FDA Science Forum, Washington , DC , May 18-19, 2004 .
Cyr WH , Desta AB. Radiofrequency and cell phone research. Bioelectromagnetics Society , Washington , DC , June 24, 2004 .
Cyr WH , Desta AB. Update on the cell phone CRADA. 2004 Joint Workshop on Mobile Telephony and Health. FCC Headquarters, Washington , DC , June 28, 2004 .
Cyr WH , Miller SA. Update on FDA regulation of the indoor tanning industry. Conference on sunlight, tanning booths and vitamin D. Sponsored by the American Academy of Dermatology, Washington , DC , August 2004.
Desta AB , WH Cyr. Update on studies at the national toxicology program. 2004 Joint Workshop on Mobile Telephony and Health. FCC Headquarters, Washington DC , June 28, 2004 .
Dornish M, Kaplan DS. Hyaluronan in tissue engineered medical products: The ASTM guide for the characterization and testing of hyaluronan, Hyaluronan 2003 Meeting, Cleveland , OH , October 11-16, 2003 .
Elespuru RK . FDA perspective and updates on molecular diagnostics and personalized medicine. Molecular Diagnostics and Personalized Medicine, International Business Communications (IBC), Princeton NJ , June 2-4, 2004 .
Fitzgerald B . Cybersecurity in healthcare networks. The Information Security Officers annual meeting of the Veterans Administration, Atlanta , GA , June 23, 2004 .
Fitzgerald B . Cybersecurity in healthcare networks. The SC62a WG22 JWG4, Budapest , Hungary July 26, 2004 .
Fitzgerald B . Cybersecurity in healthcare networks. The System Administrator Officers Annual Meeting of the Veterans Administration, Austin , TX, August 10, 2004 .
Fitzgerald B . Cybersecurity in healthcare networks. The Biomedical Technicians Annual Meeting of the Veterans Administration, Reno , NV, August 23, 2004 .
Fitzgerald B , Murray J. Cybersecurity in healthcare networks. Joint Armed Forces Working Group on Cybersecurity, Martinsburg , WV , September 20, 2004 .
Fitzgerald B , Jones P. Cybersecurity in healthcare networks. Inova Healthcare Systems, Reston VA , September 21, 2004 .
Fitzgerald B . Device Risk Management and Premarket Submissions, annual convention Biomedical Division ASQ, Santa Anna, CA, October 6, 2004.
Godar DE , Dowdy JC, Sliney DH, Coelho SG, Streicher JJ, Landry RJ, Ley RD, Cyr HW. Outdoor and indoor solar UV radiation: contributions toward skin cancer. American Society for Photobiology, Seattle , WA , July 10-14, 2004 .
Goering PL . Mechanisms of metal toxicity. American Chemical Society Continuing Education Course: Chemical Mechanisms in Toxicology, Philadelphia , PA , November 2003.
Harris GR , Maruvada S, Gammell PM. Two efficient methods for measuring hydrophone frequency response in the 100 kHz to 2 MHz range. International Conference on Advanced Metrology for Ultrasound in Medicine, Teddington, Middlesex , UK , April 2004.
Harris GR . High-intensity focused ultrasound for tissue thermal ablation: Potential applications and critical path opportunity. 2004 FDA Science Forum, Washington , DC , May 2004.
He Z, Grimm S, Wagner RF, Wear KA, Jannicky E, Huston D, Garra BS. Dependence of tissue characterization features on region of interest (ROI) size: studies on phantoms and simulations. IEEE Ultrasonics Symposium, Montreal , Canada , August 2004.
Hilbert S , Yanagida R, Krueger P, Jones AL, Wolfinbarger L, Hopkins R.
A comparison of the explant pathology findings of anionic and non-ionic detergent decellularized heart valve conduits. 8 th Annual Hilton Head Workshop, Cardiovascular Tissue Engineering: From Basic Biology to Cell-Based Therapies, Hilton Head Island , SC , March 6-10, 2004 , p 47.
Hutter JC , Luu HMD, Kim CS. Dynamics of Bisphenol A dosimetry in the neuroendocrine organs of rats. International Conference on Health Sciences Simulation, San Diego , CA , January 18-22, 2004 .
Hutter JC , Richardson DC, McDermott MK, Chen ET, Crowe J, Platek F, Witkowski M, Poindexter B, Vostal J. Materials testing of recalled particle contaminated blood bags. 10 th Annual FDA Science Forum, Washington , DC , May 18-19, 2004 .
Hutter JC , Luu MD, Kim CS. Dynamics of Bisphenol A distribution in the neuroendocrine tissues. 10 th Annual FDA Science Forum, Washington , DC , May 18-19, 2004 .
Ilev I . Wave-front ophthalmic sensors for improving vision. LABQUEST FDA/CDRH-USAF Meeting, White Oak, Silver Spring , MD , July 16, 2004 .
Ilev I . Modern optical biometrology: The science of measurement and data validation, ECI-NAALT 2004 Meeting on Light Activated Tissue Regeneration and Therapy, Kona, HI, August 25, 2004.
Jones PL . Medical device HCSS research projects and the FDA. HCSS Research Agenda Minutes, National Coordination Office for Information Technology Research and Development, Washington , DC , December 2003.
Jones PL . Risk management in the design of medical device software systems. International Quality & Productivity Center (IQPC), Washington , DC , March 15, 2004 .
Jones PL . Regulatory interests in certifying medical device software systems. Workshop on building certifiably dependable systems, The National Academies, Washington, DC, April 19-21, 2004.
Jones PL . RTP guidance document announcement - safety cases & essential performance, NEMA workshop, Pittsburgh , PA , July 26, 2004 .
Kim CS, Luu HM, Johnson W, Hutter JC, Ross IA , Sapienza PP. Biodistribution of Bisphenol A in the neuroendocrine organs of female rats. Society of Toxicology, 43 rd Annual Meeting, Baltimore , MD , March 21-25, 2004 .
Krauthamer V . Safety of electrical stimulation in excitable tissues. George Washington University , Department of Biology Seminar, Washington , DC , January 31, 2004 .
Krauthamer V . Non-synaptic modulation in the receptive field size of a sensory neuron. Sarvey Symposium on Synaptic Plasticity, Bethesda , MD , April 2-3, 2004 .
Kuster N, Kainz W. Advances in numerical dosimetry for magnetic reso-nance imaging. ICNIRP-NIR (International Commission on Non-Ionizing Radia-tion Protection - Non-Ionizing Radiation) Workshop & Symposium, Seville , Spain , May 20-22 2004 .
McGuiggan PM, Deng Y, Simon Jr. CG, Wiederhorn SM, Lawn BR, Vegella T, Kaplan DS. Dynamical adhesion force of PSA film and cells. Materials Research Society (MRS) Fall Meetings, Boston , MA , December 1-5, 2003 .
Midgette W . Device risk management: The FDA/regulatory perspective, AAMI 3-day course. Risk Management for Medical Device Manufacturers, Washington , DC , November 12-14, 2003 .
Midgette W . Device risk management and premarket submissions, ISO TC 210/IEC SC 62A JWG1, 3 rd Round Table Forum: ISO 14971- Risk Management for Medical Devices, Sanibel Island , FL , December 2, 2003 .
Midgette W . FDA device regulation and medical device risk management, ADVAMED 2-day course, risk management: ISO 14971: How Medical Device Firms Can Utilize This Standard for the Product Life Cycle, Fort Lauderdale , FL , January 23-24, 2004 .
MidgetteW . Device risk management: The FDA/regulatory perspective, AAMI 3-day course. Risk Management for Medical Device Manufacturers, Phoenix , AZ , February 22-24, 2004 .
Midgette W . Device risk management: The FDA/regulatory perspective, AAMI 3-day course. Risk Management for Medical Device Manufacturers, Dallas , TX , April 17-19, 2004 .
Midgette W . Medical device risk management. 31 st Annual Meeting of the Association of Medical Diagnostic Manufacturers (AMDM), Washington , DC , April 20-21, 2004 .
Midgette W . Device risk management: The FDA/regulatory perspective, AAMI 3-day course. Risk Management for Medical Device Manufacturers, Washington , DC , September 13-15, 2004 .
Myers KJ . Recent developments in medical imaging. GSF-- German National Research Center for Environment and Health, Munich , Germany , October 2004.
Myers KJ . Image perception and its impact on image quality. NCI Young Investigator's Workshop, Rockville , MD , September 2004.
Myers KJ . State of the art in task-based assessment of image quality. Keynote presentation, SPIE Conference on Medical Imaging Physics, San Diego , CA , February 2004.
Pastel MS . FDA/CDRH perspective on regulatory requirements for array bioinformatics. American Association for Clinical Chemistry Lab 2007: Your Eye to the Future, Chicago , IL , October 21-22, 2004 .
Petrick N , Wagner RF. Training and testing CAD algorithms: observer performance studies. NIH Research Festival (Invited Talk), Bethesda , MD , 2004.
Pfefer TJ , Agrawal A, Drezek RA. Computational analysis of fluorescence spectroscopy devices with angled delivery and collection. FDA Science Forum, Washington , DC , May 2004. (conference presentation)
Pritchard WF , Karanian JW. Regulation of medical devices: FDA perspective for university researchers. Institute for Medicine & Engineering, University of Pennsylvania , Philadelphia , PA , December 2003.
Renukananda R, Krauthamer V. Effect of defibrillator-drug interactions on cytosolic calcium in cultured cardiac myocytes. FDA Science Forum, Washington , DC , May 18-19, 2004 .
Ross IA, Sapienza PP, Johnson WW, Luu HM, Hutter JC, Kim CS. Partitioning of Bisphenol a in rat tissue for a physiologically based pharmacokinetic model. Society of Toxicology, 43 rd Annual Meeting, Baltimore , MD , March 21-25, 2004 .
Sergeev N . Microarray detection and typing. Food Microbiology Research Conference XIX, Rosemont , IL , November 9-12, 2003 .
Sergeev N . DNA-microarray-based detection of microbial pathogens. Advanced Topics in Mechanical Engineering: Sensors and MEMS Packaging, University of Maryland , College Park , MD , April 28, 2004 .
Taylor AR . Cybersecurity in healthcare networks. The Information Technology Committee of the Greater New York Hospital Association (monthly meeting), New York , NY , May 12, 2004 .
Taylor AR . Quality by design: whole product life cycle. 2004 FDA Science Forum, Washington , DC , May 18-19, 2004 .
Taylor AR , Dolan A. Risk management for medical devices. American Society of Quality 58 th Annual Quality Congress, Toronto , Ontario , Canada , May 23-26, 2004 .
Tomazic-Jezic VJ , et al. Performance of methods for the measurement of natural rubber latex (NRL) protein, antigen and allergen. Journal of Allergy Clinical Immunology113:s78, 2004.
Wear KA , Stiles TA, Frank GR, Madsen EL, Cheng F, Chérin E, Feleppa EJ, Foster S, Garra BS, Kim BS, Lee P, Miller HL, O’Brien Jr. WD, Oelze ML, Shung KK, Teo TJ, Wilson TA, Yaun JR. Interlaboratory comparison of ultrasonic backscatter coefficient measurements from 2 to 14 MHz. Annual Conference of American Institute of Ultrasound in Medicine, Phoenix , AZ , June 20-22, J. Ultrasound. Med.,23(6) (suppl.), p. S:75-76, June 2004.
Wear KA . Ultrasonic wave propagation and scattering in cancellous bone. 148 th Meeting of the Acoustical Society of America , San Diego , CA , November 15, 2004 . Journal of the Acoustical Society of America116:2477, 2004. (Invited talk)
Weininger , Shang, Goldman, Kopotic, Pennello. Using the Infrared (IR) plethysmogram to assess the effects of motion on the performance of pulse oximeters. Society for Technology in Anesthesia, New Mexico , January 2004.
Weininger S . Plan for quality, BIOMED Seminar. Drexel University , Philadelphia , PA , February 24, 2004 .
Weininger , Shang, Goldman, Kopotic, Pennello. Using the Infrared (IR) plethysmogram to assess the effects of motion on the performance of pulse oximeters. World Congress on Anesthesia, Paris , France , April 2004.
Weininger S . Plan for quality, BEACON Seminar. Springfield , MA , May 20, 2004 .
Weininger S . Medical systems validation - plan for quality. CIMIT Seminar. Cambridge , MA , May 25, 2004 .
Weininger , Osborn. Basic safety and essential performance in medical electrical equipment. 1st IEEE Product Safety Symposium, Santa Clara , CA , August, 2004.
Woods TO . Standards for compatibility of medical devices in the MR environment. Special Lecture, Transcatheter Cardiovascular Therapeutics 2004 Scientific Symposium on Cardiovascular MRI & CT: State of the Art, Washington , DC , October 1, 2004 .
Woods TO . FDA’s critical path research initiative. National Research Council Roundtable 11 th BEMA (Biomedical Engineering Materials and Applications) Meeting, Chips and Arrays, Washington , DC , April 7, 2004
Yamaguchi Y, Tadokoro T, Zmudzka BZ, Miller SA, Beer JZ, Hearing VJ. Physiological regulation of melanocyte proliferation and differentiation in human skin following ultraviolet radiation. PanAmerican Society for Pigment Cell Research Annual Meeting, Newport Beach , CA , June 24-27, 2004 .
October 1, 2003 – December 31, 2004
Badano, Aldo, Ph.D.
University of Michigan
College of Engineering
Department of Electrical Engineering
and Computer Science
Visiting Research Scientist
Bassen, Howard I.
University of Maryland
Department of Biological
Resources Engineering
Lecturer
George Washington University
Department of Electrical
and Computer Engineering
Adjunct Professor
Brown, Stanley A.
University of Maryland
Baltimore County (UMBC)
Mechanical Engineering
Adjunct Professor
Das, Srilekha S., Ph.D.
Henry M. Jackson Foundation for
the Advancement of Military Medicine
Guest Scientist
Goering, Peter L., Ph.D.
University of Maryland School of Medicine
Graduate Program in Toxicology
Adjunct Professor
George Washington University
Department of Biological Sciences
Adjunct Associate Professor
Harris, Gerald R., Ph.D.
Drexel University
Department of Electrical
and Computer Engineering
Member, Doctoral Dissertation Committee
Hilbert, Stephen L., M.D., Ph.D.
Brown University School of Medicine
Department of Surgery
Division of Cardiothoracic Surgery
Adjunct Professor of Surgery (Research)
Karanian, John W., Ph.D.
Georgetown University Medical Center
Department of Physiology
Adjunct Professor
Krauthamer, Victor, Ph.D.
Uniformed Services University
of the Health Sciences
Department of Anatomy, Physiology
and Genetics
Adjunct Assistant Professor
American University
Department of Biology
Adjunct Associate Professor
George Washington University
Department of Biology
Adjunct Associate Professor
Myers, Kyle J., Ph.D.
Georgetown University Medical Center
Department of Radiology
Adjunct Associate Professor
University of Arizona
Optical Sciences Center
Adjunct Associate Professor
O'Hara, Michael D., Ph.D.
Thomas Jefferson University
Department of Radiation Oncology
Adjunct Assistant Professor
Petrick, Nicholas, Ph.D.
University of Michigan
Department of Radiology
Assistant Research Scientist
Pfefer TJ, Ph.D.
Rice University
Department of Bioengineering
Doctoral thesis committee
Valentine, Karen D.
Montgomery College
Department of Continuing Education
Instructor/lecturer
Waynant, Ronald W., Ph.D.
Catholic University of America
Electrical Engineering Department
Adjunct Associate Professor
Uniformed Services University
of the Health Sciences
Adjunct Professor
Wear, Keith A., Ph. D.
Georgetown University
Department of Radiology
Adjunct Professor
Henry M. Jackson Foundation for the
Advancement of Military Medicine
Guest Scientist
October 1, 2003 – September 30, 2004
O'Hara MD and McDaniels. Method and assembly for containing radioactive materials.
Patent # 6,669,621, December 30, 2003.
Amols, Kiorpes, McDaniel, O'Hara. Low attenuating radioactive seeds.
Patent # 6,800,055, October 5, 2004.
October 1, 2003 – December 31, 2004
Calcagnini G. (National Institute of Health, Rome, Italy), Science, engineering and the regulation of medical devices in Italy. CDRH Science Seminar, Rockville, MD, February 6, 2004.
Coburn Z, Agrawal A, Miller S, Pfefer J. Diffuse reflectance spectroscopy for measuring sunscreen effectiveness in the UVA/UVB. OSEL Student Poster Session, 2004.
Cohen AH. ( University of Maryland). Controlling the central pattern generator for locomotion after spinal cord injury: problems and solutions. CDRH Meet the Experts Seminar, Rockville, MD, May 12, 2004.
Dabos P. Improving the safety and effectiveness of software. Presentation to CDRH, September 17, 2004.
Ediger W, Ph.D. (InLight Solutions, Inc.) Prospects for noninvasive screening for diabetes: results from clinical and in vitro studies. CDRH Science Seminar, April 28, 2004.
Eloff BC. ( Case Western Reserve University). Role of intracellular coupling in the formation and prevention of arrhythmogenic substrates in the heart. CDRH Science Seminar, Rockville, MD, June 10, 2004.
Fernando A. Impact of environmental stressors on safety-critical embedded systems. Presentation to CDRH, June 17, 2004 .
Goering PL. The toxicology of mercury. Lecture to graduate students. Program in Toxicology, University of Maryland School of Medicine, Baltimore MD, November 1, 2003.
Godar DE. A melanoma hypothesis: the paradox of outdoor and indoor solar UV contributions toward skin cancer. Pay Day Club seminar, Rockville, MD, December 7, 2004.
Hammer D, Ph.D. (PSI, Inc.) Retinal tracking for optical coherence tomography. CDRH Science Seminar, May 12, 2004.
Huang S, Agrawal A, Pfefer J. Characterization of an optical coherence tomography system for high-resolution imaging. OSEL Student Poster Session, 2004.
Leeper DB. Acidification and oxygenation induced by inhibitors of respiration plus hyperglycemia enhance tumor therapeutic response. White Oak MD, November 4, 2004.
Optical Diagnostics Laboratory Tour for HHS Secretary, FDA Commissioner and Center Director, April 23, 2004.
Rasooly A. DNA-microarray-based detection of microbial pathogens. Advanced course on Sensors and MEMS. University of Maryland, College Park MD, 2004.
Sharma D, Agrawal A, Matchette S, Pfefer J. Novel techniques for reflectance-based determination of tissue optical properties. OSEL Student Poster Session, 2004.
FY 2004 Reimbursable IAG’s
Air Force Office of Scientific Research (AFOSR) (IAG #224-98-6005). Infrared fiber and wave guide testing.
Federal Aviation Administration (FAA) (IAG #224-00-6061). Electromagnetic interference research and testing with medical devices.
National Institute for Biomedical Imaging and Bioengineering (NIBIB) (IAG # 224-04-6055). Joint NIBIB/CDRH Laboratory for the assessment of medical imaging system.
National Institutes of Health (NIH) (IAG #224-04-6070). Image-guided international therapeutics.
National Cancer Institute, NIH (NCI) (IAG# 224-04-6058). Assessment of computer-aided diagnostics.
FY 2004 Service IAG’s
National Institutes of Health (NIH) (IAG #224-04-6053). Professional services of visiting scientist.
Uniformed ServicesUniversity of the Health Sciences (USUHS) (IAG #224-04-6064). Ethical and psychiatric aspects of guidance documents for manufacturer’s submissions for electroconvulsive therapy device applications.
Uniformed ServicesUniversity of the Health Sciences (USUHS) (IAG #224-98-6015). Maintenance of an animal model of the pathophysiology of diabetes for end organ studies.
Department of the Interior (IAG #224-04-6063). Cardiovascular effects of ultrasound contrast agents.
Proposal # |
Title/Investigators |
|---|---|
6 |
Detection, Identification and Evaluation of the Virulence Potential of FDA Relevant Pathogens Using: 1) Combined Real-time PCR plus Microarray Approaches, and 2) a Novel High Speed Nano-scale PCR Procedure Larry E. Bockstahler, Ph.D. , CDRH/OST-OSEL Daya Ranamukhaarachchi, Ph.D., CDRH/OST-OSEL |
17 |
Semiconductor Nanocrystal Field Deployable Biosensor for Simultaneous Detection of Biological Warfare Agents including Clostridium botulinum Neurotoxins A, B, E, and F Kun-Ho Seo, Ph.D., CFSAN |
23 |
Decontamination of surgical and other instruments exposed to the infectious agents of transmissible spongiform encephalopathies (TSE agents or proions) David M. Asher, M.D., CBER |
29 |
Prioritizing sources of variability in genomic profiling data for standards and guidance development Rosalie Elespuru, Ph.D., CDRH/OST-OSEL |
33 |
Mass Spectrometric Screening for Protein Biomarkers to Indicate Food Animal Origin of Bacterial Pathogens Tracie L. Williams, CFSAN |
|
TOTAL |
| AAMI | - American Association for Medical Instrumentation | |
| AAPM | - American Association of Physicists in Medicine | |
| ACCA | - Associate Commissioner for Consumer Affairs, OC, FDA, DHHS | |
| ACF | - Administration for Children and Families, DHHS | |
| ACCME | - Accreditation Council for Continuing Medical Education | |
| ACHA | - Associate Commissioner for Health Affairs, OC, FDA, DHHS | |
| ACLA | - Associate Commissioner for Legislative Affairs, OC, FDA, DHHS | |
| ACMP | - American College of Medical Physicists | |
| ACOM | - Associate Commissioner for Office of Management, OC, FDA | |
| ACPA | - Associate Commissioner for Public Affairs, OC, FDA, DHHS (Press) | |
| ACPE | - Associate Commissioner for Planning and Evaluation, OC, FDA, DHHS | |
| ACPE | - American Council on Pharmaceutical Education | |
| ACR | - American College of Radiology | |
| ACRA | - Associate Commissioner for Regulatory Affairs, OC, FDA, DHHS | |
| ADA | - American Dental Association | |
| ADAMHA | - Alcohol, Drug Abuse, and Mental Health Administration, PHS, DHHS | |
| AFGE | - American Federation of Government Employees (Union) | |
| AFIP | - Armed Forces Institute of Pathology (located at WRAMC), DOD | |
| AHA | - American Hospital Association | |
| AHCPR | - Agency for Health Care Policy and Research, PHS, DHHS | |
| AIMBE | - American Institute of Medical and Biological Engineering | |
| AMA | - American Medical Association | |
| ANSI | - American National Standards Institute | |
| ARCRT | - American Registry of Clinical Radiography Technologists (MQSA) | |
| ARPA | - Advanced Research Projects Agency | |
| ARRT | - American Registry of Radiologic Technologists (MQSA) | |
| ASH | - Assistant Secretary for Health, DHHS | |
| ASPE | - Assistant Secretary for Planning and Evaluation, DHHS | |
| ASPER | - Assistant Secretary for Personnel Administration, DHHS | |
| ASTM | - American Society for Testing and Materials | |
| BRMD | - Bureau of Radiation and Medical Devices, CANADA | |
| CBER | - Center for Biologics Evaluation and Research, FDA, DHHS | |
| CC | - Clinical Center (Warren Magnuson Clinical Center), NIH, DHHS | |
| CEU | - Continuing Education Unit | |
| CDC/CDCP | - Centers for Disease Control/Centers for Disease Control and Prevention | |
| CENELEC | - European Committee for Electrotechnical Standardization (French term, English translation) | |
| CDER | - Center for Drug Evaluation and Research, FDA, DHHS | |
| CDRH | - Center for Devices and Radiological Health, FDA, DHHS | |
| CFSAN | - Center for Food Safety and Applied Nutrition, FDA, DHHS | |
| CIA | - U.S. Central Intelligence Agency (Headquarters: Arlington, VA) | |
| CIRMS | - Council on Ionizing Radiation Measurements and Standards, NIST | |
| CLIA | - Clinical Laboratory Improvement Amendments of 1988 | |
| CME | - Continuing Medical Education | |
| CRADA | - Cooperative Research and Development Agreement | |
| CRCPD | - Conference of Radiation Control Program Directors | |
| CTIA | - Cellular Telephone Industry Association | |
| CVM | - Center for Veterinary Medicine, FDA, DHHS | |
| DARPA | Defense Advanced Research Projects Agency | |
| DASH | - Deputy Assistant Secretary for Health, OASH, DHHS | |
| DCP | - Division of Commissioned Personnel, OASH, OSG (Parklawn Building) | |
| DHHS | - U.S. Department of Health and Human Services | |
| DHSS | - Department of Health and Social Security, ENGLAND | |
| DOC | - U.S. Department of Commerce | |
| DOD | - U.S. Department of Defense | |
| DOL | - U.S. Department of Labor | |
| DOE | - U.S. Department of Energy | |
| DOT | - U.S. Department of Transportation | |
| ECRI | - Emergency Care Research Institute (no longer uses name—initials only) | |
| EEO | - Equal Employment Opportunity Act | |
| EMBS | - Engineering in Medicine and Biology Society, IEEE | |
| ERIM | - Environmental Research Institute of Michigan | |
| FAA | - Federal Aeronautics Administration | |
| FBI | - Federal Bureau of Investigation, Department of Justice | |
| FCC | - Federal Communications Commission | |
| FCCSET | - Federal Coordinating Council for Science, Engineering and Technology, | |
| FIC | - Fogarty International Center, NIH, DHHS | |
| FDA | - U.S. Food and Drug Administration, PHS, DHHS | |
| FDAMA | - Food and Drug Administration Modernization Act of 1997 | |
| FDLI | - Food and Drug Law Institute | |
| FOIA | - Freedom of Information Act | |
| FTC | - U.S. Federal Trade Commission | |
| GAO | - General Accounting Office | |
| GC | - General Counsel, FDA (now Office of Chief Counsel, FDA) | |
| GGP | - Good Guidance Practices | |
| GPRA | - Government Performance and Results Act | |
| GPRE | - Government Program Review and Evaluation | |
| GSA | - General Services Administration | |
| HCFA | - Health Care Financing Administration | |
| HIMA | - Health Industry Manufacturers Association | |
| HRG | - Health Research Group (Public Citizen: Ralph Nader-Dr. Sidney Wolfe) (Consumers Health Political Action Committee - PAC) |
|
| HRSA | - Health Resources and Services Administration, PHS, DHHS | |
| ICRP | - International Commission on Radiological Protection | |
| ICRU | - International Commission on Radiation Units and Measurements | |
| IEC | - International Electrotechnical Commission | |
| IEEE | - Institute of Electrical and Electronic Engineers, Inc. | |
| IFIP | - International Federation for Information Processing | |
| IG | - Inspector General, OIG, DHHS | |
| IHS | - Indian Health Service, DHHS | |
| INNS | - International Neural Networks Society | |
| INS | - U.S. Immigration and Naturalization Service | |
| IOM | - Institute of Medicine, NAS | |
| IRB | - Institutional Review Board | |
| IRS | - U.S. Internal Revenue Service | |
| ISO | - International Standards Organization | |
| JCAHCA | - Joint Commission on Accreditation of Health Care Organizations | |
| MDUFMA | - Medical Device User Fee and Modernization Act of 2002 | |
| NAAP | - National Association of Apnea Professionals | |
| NAS | - National Academy of Sciences | |
| NBS | - National Bureau of Standards, DOC (No longer exists: See NIST), | |
| NCCLS | - National Committee for Clinical Laboratory Science | |
| NCHS | - National Center for Health Statistics, CDCP, DHHS | |
| NCHGR | - National Center for Human Genome Research, NIH, DHHS | |
| NCI | - National Cancer Institute, NIH, DHHS | |
| NCNR | - National Center for Nursing Research, NIH, DHHS | |
| NCRP | - National Council on Radiation Protection | |
| NCTR | - National Center for Toxicological Research, FDA, DHHS | |
| NEI | - National Eye Institute, NIH, DHHS | |
| NEMA | - National Electrical Manufacturers Association | |
| NHLBI | - National Heart, Lung, and Blood Institute, NIH, DHHS | |
| NIA | - National Institute on Aging, NIH, DHHS | |
| NIAAA | - National Institute on Alcohol Abuse and Alcoholism, NIH, DHHS | |
| NIAID | - National Institute of Allergy and Infectious Diseases, NIH, DHHS | |
| NIAMSK | - National Institute of Arthritis and Musculoskeletal and Skin Diseases, NIH, DHHS | |
| NIBIB | - National Institute for Biomedical Imaging and Bioengineering | |
| NICHHD | - National Institute of Child Health and Human Development, NIH, | |
| NIDCD | - National Institute on Deafness and Other Communication Disorders, NIH, DHHS NIDA | |
| NIDA | - National Institute on Drug Abuse, NIH, DHHS | |
| NIDDKD | - National Institute of Diabetes and Digestive and Kidney Diseases, NIH NIDR - National Institute of Dental Research, NIH, DHHS | |
| NIEHS | - National Institute of Environmental Health Sciences, NIH, DHHS | |
| NIGMS | - National Institute of General Medical Sciences, NIH, DHHS | |
| NIMH | - National Institute of Mental Health, NIH, DHHS | |
| NINDS | - National Institute of Neurological Disorders and Stroke, NIH, DHHS | |
| NIH | - National Institutes of Health | |
| NIOSH | - National Institute for Occupational Safety and Health, CDCP, DHHS | |
| NIST | - National Institute of Standards and Technology, DOC (formerly NBS) | |
| NLM | - National Library of Medicine, NIH, DHHS | |
| NMQAAC | - National Mammography Quality Assurance Advisory Committee, FDA | |
| NRC | - National Research Council | |
| NRC | - U.S. Nuclear Regulatory Commission | |
| NSA | - U.S. National Security Agency (Headquarters: Fort Meade, MD) | |
| NSF | - National Science Foundation | |
| NOAA | - National Oceanographic and Atmospheric Administration | |
| NVLAP | - National Association of Voluntary Laboratory Accreditation Practices | |
| OC | - Office of the Commissioner, FDA | |
| OCA | - U.S. Office of Consumer Affairs | |
| OCC | - Office of the Chief Counsel, FDA (formerly OGC) | |
| OCR | - Office for Civil Rights, DHHS | |
| OHA | - Office of Health Affairs, FDA, DHHS | |
| OIG | - Office of the Inspector General | |
| OLA | - Office of Legislative Affairs, OC, FDA, DHHS | |
| OMB | - Office of Management and Budget | |
| OPA | - Office of Public Affairs, OC, FDA, DHHS (Press Office/Relations) | |
| OPE | - Office of Planning and Evaluation, FDA, DHHS | |
| ORA | - Office of Regulatory Affairs, FDA, DHHS | |
| OPM | - Office of Personnel Management | |
| OS | - Office of Secretary, DHHS | |
| OSG | - Office of the Surgeon General, PHS, DHHS (Commissioned Corps) | |
| OSHA | - Occupational Safety and Health Administration | |
| PAC | - Political Action Committee | |
| PAHO | - Pan-American Health Organization, WHO, UN | |
| PHS | - U.S. Public Health Service | |
| RESNA | - Rehabilitation Engineering Society of North America, ANSI | |
| RSNA | - Radiological Society of North America | |
| SAMHSA | - Substance Abuse and Mental Health Services Administration, DHHS | |
| SCVIR | - Society for Cardiovascular and Interventional Radiology | |
| SMDA | - Safe Medical Devices Act of 1990 | |
| SNL | - Sandia National Laboratories | |
| SPIE | - Society of Photo-Optical Instrumentation Engineers | |
| SSA | - Social Security Administration (formerly part of DHHS) | |
| SSRCR | - Suggested State Regulations for Control of Radiation | |
| UL | - Underwriters Laboratories | |
| UN | - United Nations | |
| USDA | - U.S. Department of Agriculture | |
| WCNN | - World Congress of Neural Networks | |
| WEAC | - Winchester Engineering and Analytical Center, FDA, DHHS | |
| WHO | - World Health Organization, UN | |
| WRAIR | - Walter Reed Army Institute of Research, WRAMC, U.S. Army | |
| WRAMC | - Walter Reed Army Medical Center, U.S. Army | |
CDRH ABBREVIATIONS AND ACRONYMS |
||
DB |
- Division of Biology |
|
| DCLD | - DivisonDivision of Clinical Laboratory Devices, ODE | |
| DCMS | - Division of Chemistry and Materials Sciences | |
| DCRND | - Division of Cardiovascular, Respiratory and Neurological Devices, ODE | |
| DDL | - Devices and Diagnostics Letter (also known as The Orange Sheet) (Weekly Trade Magazine) | |
| DECS | - Division of Electronics and Computer Science, OST | |
| DESE | - Division of Electronics and Software Engineering | |
| DGRD | - Division of General and Restorative Devices, ODE | |
| DIAM | - Division of Imaging and Applied Mathematics | |
| DLS | - Division of Life Sciences, OST | |
| DMISS | - Division of Management, Information and Support Services, OST | |
| DMMS | - Division of Mechanics and Materials Science, OST | |
| DMQRP | - Division of Mammography Quality and Radiation Programs, OHIP | |
| DOD | - Division of Ophthalmic Devices, ODE | |
| DP | - Division of Physics | |
| DPS | - Division of Physical Sciences, OST | |
| DRAERD | - Division of Reproductive, Abdominal, ENT, & Radiological Devices, ODE | |
| DSFM | - Division of Solid and Fluid Mechanics | |
| EIR | - Establishment Inspection Report | |
| EMC | - Electromagnetic Capability | |
| EMI | - Electromagnetic Interference | |
| ERC | - NSF Engineering Research Center, Duke University (National Science Foundation) | |
| 510(k) | - Five-Ten K: Pre-market Notification of New Medical Device (Clearance Based on a Similar, Previously Cleared Device) |
|
| HL | - High Level or High-Level Control | |
| IDE | - Investigational Device Exemption | |
| IND | - Investigational New Device (or Drug) (application for transitional devices) | |
| IAG | - Interagency Agreement | |
| kVp | - Measurement of Meters (as in kVp Meters) | |
| MDDI | - Medical Devices, Diagnostics & Instrumentation (also known as The Gray Sheet) (Weekly Trade Magazine)) | |
| MDH | - X-ray radiation instrument used by FDA in its inspections (originally marketed by a company called MDH) | |
| MDR | - Mandatory Device Reporting Program | |
| MON | - Memorandum (Memoranda) of Need | |
| MQC | - Mammography Quality Control (as in MQC Manual) | |
| MQSA | - Mammography Quality Standards Act of 1992 | |
| MRI | - Magnetic Resonance Imaging (formerly nuclear magnetic resonance) | |
| MRS | - Magnetic Resonance Spectroscopy | |
| NEXT | - Nationwide Evaluation X-ray Trends (Data Bank) | |
| NSWL | - Naval Surface Warfare Laboratory (in White Oak, Silver Spring) | |
| NVLAP | - National Voluntary Laboratory Accredited Program, (NIST, DOC) (MQSA) | |
| OC | - Office of Compliance, CDRH, FDA | |
| OCD | - Office of the Center Director, CDRH, FDA, DHHS | |
| ODE | - Office of Device Evaluation, CDRH, FDA | |
| OHIP | - Office of Health and Industry Programs, CDRH, FDA | |
| OSM | - Office of Systems and Management, CDRH, FDA | |
| OPA | - Office of Public Affairs, FDA, DHHS (Press Office) | |
| ORA | - Office of Regulatory Affairs, FDA, DHHS (field offices) | |
| OSB | - Office of Surveillance and Biometrics, CDRH, FDA | |
| OST | - Office of Science and Technology, CDRH, FDA | |
| PDP | - Product Development Protocol | |
| PMA/PMAA | - Pre-Market Approval Application | |
| PMS | - Post-Market Surveillance | |
| QA | - Quality Assurance | |
| QC | - Quality Control | |
| RIHSC | - Research Involving Human Subjects Committee, FDA | |
| ROC | - Receiver Operating Characteristic Curve | |
| RRHR | - Regional Radiological Health Representative, FDA | |
| SCLIR | - Secondary Calibration Laboratories for Ionizing Radiation | |
| SIDS | - Sudden Infant Death Syndrome | |
| TEPRSSC | - Technical Electronic Product Radiation Safety Standards Committee, CDRH, FDA, DHHS | |
| TMJ | - Temporomandibular Joint | |
| TQM | - Total Quality Management | |
OFFICE OF SCIENCE AND ENGINEERING LABORATORIES
As of 11/29/04
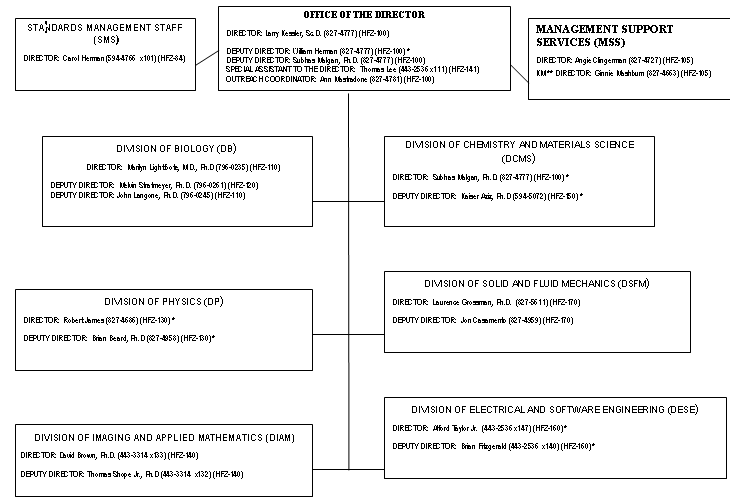
*Acting
** Knowledge Management
Chart created by Jennifer Lubin 9/1/04; Updated 12/29/04
Updated June 8, 2005
![]()
CDRH Home Page | CDRH A-Z Index | Contact CDRH | Accessibility | Disclaimer
FDA Home Page | Search FDA Site | FDA A-Z Index | Contact FDA | HHS Home Page
Center for Devices and Radiological Health / CDRH