
 1663
1663
|
|

Studying West Nile Virus in BirdsWhat humans can learn from the immune response of our feathered friends.Abstract: Some bird species fall prey to West Nile virus while others are immune. Los Alamos researchers are the first to analyze how a bird’s immune system responds to the disease.

Birds delight us with their songs, colorful plumage, and aerial artistry. But they also harbor diseases that can be transmitted to humans.
Late afternoon at the Los Alamos landfill—that’s the best time and place to catch ravens. When the facility closes, the ravens show up to pick through the day’s refuse. Wednesdays are ideal. That’s when the restaurant garbage arrives. Jeanne Fair knows all about the ravens’ scrounging habits. Fair, an ornithologist (a scientist who studies birds) from Los Alamos National Laboratory’s Earth and Environmental Sciences Division, makes regular trips to the county landfill in search of the big black birds. She’s working on a study of the immune systems of several northern New Mexico bird species. Fair and Babetta (Babs) Marrone, a molecular biologist from the Bioscience Division, are principal investigators for the Laboratory project. The study combines Fair’s work in the field and Marrone’s expertise with an advanced analytical technique called flow cytometry, with which she examines blood samples taken from the captured birds. Fair and Marrone are seeking evidence of the birds’ response to West Nile virus, a mosquito-borne pathogen that appeared in the United States in 1999. It was first seen around New York City but has now traveled to all 48 contiguous states. It has also been found in Canada and Mexico. West Nile infects mostly birds but is a zoonotic (pronounced zo-oh-not-ic) disease, one that can move from species to species and, in particular, from animals to humans. It has already affected small mammals and horses. In 2003, the Centers for Disease Control documented 2,500 human cases in New Mexico. In severe cases the virus causes meningitis and encephalitis, diseases characterized by inflammation of the brain and surrounding tissues. 
Lucas Bare, an experienced bird handler, holds a raven captured at the Los Alamos landfill. Ravens are susceptible to West Nile virus and often succumb to the disease.
Among the birds, those in the family Corvidae (the corvids), which includes magpies, ravens, crows, and jays, are the most susceptible and have the highest mortality rate. The virus has killed 95 percent of the magpies around the northern New Mexico towns of Española, Pojoaque, Nambé, and Chimayo, making the birds’ once-familiar flashes of black and white quite scarce. It has also caused a significant die-off of crows and ravens across the country. Strangely, while the corvids are susceptible to the virus, other bird species are resistant, meaning they may harbor the virus without getting sick. Domestic chickens, for example, are resistant to the virus, much to the relief of the poultry industry. In the wild, pigeons and the western bluebird are resistant as well. Fair and Marrone are trying to understand how the immune system of one bird species can resist the virus while another succumbs to it. If that difference can be understood, that knowledge may lead to intervention methods that could halt West Nile’s spread through bird populations. But there’s a larger picture in the recognition that birds are a reservoir for diseases that can affect humans. Says Fair, “West Nile virus is a model system for understanding zoonotic diseases in general. The big fear is that something like the avian flu will become zoonotic, and we need to prepare for that. If we focus only on humans, we’ll never get at the root cause.” Back to BasicsFair and Marrone wanted to investigate the susceptibility versus resistance issue by studying how a portion of the avian immune system, specifically a class of white blood cells known as lymphocytes, responds to the virus. Such studies would be a common strategy for understanding the issue in humans, but they had never before been attempted for birds. Lymphocytes play crucial roles in the human immune system, and likely in all animals. Cells known as B lymphocytes produce antibodies, specialized proteins that stick to bodily invaders such as viruses and help neutralize them. Another lymphocyte, the helper T cell, activates and directs other cells of the immune system, while yet another, the cytotoxic, or “killer,” T cell, attacks cells that have been infected by viruses.  Jeanne Fair, ornithologist at Los Alamos National Laboratory’s Earth and Environmental Sciences Division.
The T cells are the primary regulatory cells within the human immune system, and characterizing how their numbers change in response to an infection is a natural diagnostic for all kinds of diseases. For example, the aids virus resides within and eventually kills helper T cells, and that particular lymphocyte is often used to monitor disease progression in aids patients. Procedures for learning about a bird’s T cells have traditionally been crudely quantitative. One technique involves a researcher injecting a protein called phytohemagglutin (blessedly known as PHA for short) into the flap of skin under a bird’s wing (the wing web). PHA activates the T cells, causing them to divide. The researcher measures the wing web before the injection and again the day after. The increased thickness relates to the amount of T-cell multiplication and so reveals T-cell vigor, which relates to the strength of the bird’s immune system. 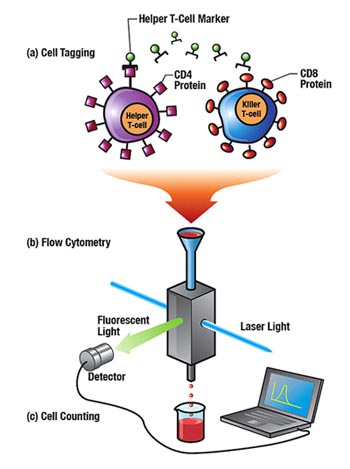 Developed at Los Alamos, flow cytometry is a method of counting thousands of cells per second. (a) Cells are tagged with a marker that lights up, or fluoresces, when it and its host cell pass through the brilliant light of a laser beam. The markers are often artificial antibodies that bind to proteins found only on the cells of interest. (b) The tagged cells are suspended in fluid and run through the cytometer, which sends them single file through the laser beam, where they light up. (c) A detector sees the fluorescent light and tells the computer, which tallies the number of tagged cells.
A much more accurate tool used for counting cells is flow cytometry, Marrone’s field of expertise. Marrone is working at the Bioscience Division’s flow cytometry center, the National Flow Cytometry Resource, which is supported by the National Institutes of Health. The idea is to tag each type of lymphocyte with its own marker (see illustration), the marker often being an antibody. For example, the surface of all helper T cells is studded with a protein known as CD4, which the cell uses to recognize molecules presented to it by other immune system cells. Killer T cells are studded with the protein CD8, while other lymphocytes have their own unique proteins. An “anti-CD4” antibody will stick only to CD4 and uniquely tag the helper T cell. The challenge Marrone faced in applying flow cytometry to avian studies was that proven markers were unavailable. A set of markers had been developed from chicken antibodies, but they had not been used for cell counting. It needed to be rigorously demonstrated that the marker set could tag the different lymphocytes reliably. Using chickens as her test species, Marrone—along with co-workers Kirsten McCabe and Yulin Shou—developed and demonstrated the first-ever lymphocyte subpopulation measurement in birds. Elated, the researchers trained their sights on pigeons, a potentially critical reservoir for the virus because of the bird’s omnipresence among people. It was not clear that the antibody markers, developed to stick to chicken proteins, would bind to the corresponding pigeon proteins. But the markers seemed to work, in that measurements of pigeon lymphocytes were in the expected proportions.  Birds Not of a Feather “Ravens are the challenge of this project,” says Jeanne Fair. “They’re smart. We use a compressed-air cannon to fire a net at the birds to catch them. The ravens don’t like it, so they stay out of sight. They even remember what vehicle we were driving the last time we came to the landfill, so we have to change cars for each trip. “Pigeons are much easier. We simply put a baited trap—a cage with an entrance but no exit—on the roof of a business plagued by too many of the birds: the car wash in Pojoaque, New Mexico, for example. By nightfall the trap is crowded with pigeons.” All of the birds are released after the Los Alamos researchers take their samples for analysis. The ravens are returned to their original location. Happily for businesses like the car wash, the pigeons are released far from their point of capture. But then came a surprise. There seemed to be few differences in the lymphocyte subpopulations between infected and healthy pigeons. The researchers were again surprised when they tried to examine raven lymphocytes. The markers didn’t seem to tag anything, so the researchers have yet to acquire any raven data. “It appeared that the immune cells were more distinct between the different bird species than we had anticipated,” explains Marrone. "That’s making our job much harder. It’s the nature of research." Marrone and Fair are in the process of developing the means to culture avian lymphocytes in the laboratory, thereby making them readily available for further studies. The two have already established procedures to directly detect West Nile virus from a bird’s blood sample and to determine whether a bird’s immune system is making antibodies to the virus. They are also looking at developing ways to measure immune response that are less species specific than their previous efforts were. The Los Alamos researchers hope that what they learn about birds’ immune systems will someday lead to vaccines against the new diseases. In the meantime, their procedures that allow them to reliably test for immune responses to West Nile virus can be used to help predict where and how quickly the disease may spread through host bird populations and, by extension, through human populations. So their work isn’t just for the birds. It’s for all of us.
|