 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
Detection of West Nile Virus in Blood Donations --- Puerto Rico, 2007In the United States, West Nile virus (WNV) was first detected in humans in 1999; it subsequently spread to countries of Central and South America and the Caribbean. WNV is a mosquito-borne virus that produces potentially serious clinical disease, particularly among persons aged >50 years. Transmission by routes other than mosquito bites, including blood transfusion, transplacental infection, organ transplant, and possibly breast milk, also have been reported.* On July 19, 2007, the American Red Cross in Puerto Rico notified the Puerto Rico Department of Health (PRDH) of three persons whose blood donations were positive for WNV by nucleic acid-amplification test (NAT) screening. These three donors had the first confirmed human WNV infections detected in Puerto Rico. In response, PRDH and CDC conducted in-depth interviews of the blood donors. This report describes these human infections and other recent surveillance for transmission of WNV in Puerto Rico. Detection of WNV infections in human blood donors indicates that heightened clinician awareness, ongoing surveillance, and educational activities are needed to monitor and assess the public health threat posed by WNV in Puerto Rico. Universal blood donor screening for WNV began in July 2003 at all blood collection agencies (BCAs) in the United States and Puerto Rico. Accepted donors must be healthy and afebrile at the time of donation. Numerous health conditions result in deferral or ineligibility to donate blood. NAT screening for WNV uses pooling of blood donations from multiple donors. Testing of individual samples from positive pools is then used to identify positive donors so their blood can be quarantined and removed from the blood supply. Three donors positive for WNV were reported to PRDH on July 19, 2007. The next day, PRDH notified BCAs islandwide by letter that WNV-positive blood donors had been identified in Puerto Rico and emphasized the importance of appropriate blood screening in protecting the integrity of the blood supply. The first donor was a woman aged 40 years who donated blood on June 22, 2007. She reported no illness in the 2 weeks before donation. The second donor was a woman aged 33 years who donated blood on July 5, 2007. She reported a headache on the day of donation, but was not febrile and reported no other symptoms. In addition to detection of WNV nucleic acid by NAT, WNV was isolated from this patient's serum. The third WNV-infected donor was a man aged 22 years who donated blood on July 12, 2007. He reported no illness in the 2 weeks before or after donation. None of the three donors reported travel outside of Puerto Rico within 2 weeks before donation. All three lived near San Juan and had not traveled to areas where WNV transmission previously was detected in animals. All three were notified of their positive screening tests. The WNV NAT-positive blood products donated by them were quarantined and not released for transfusion. Islandwide physician-based passive surveillance for neuroinvasive WNV disease in humans began in 2002. This system has relied on voluntary reporting, specimen collection, and submission to CDC laboratories by clinicians who suspect neuroinvasive illness consistent with possible WNV infection. No human WNV disease has been detected through this passive surveillance system. WNV transmission among animals in Puerto Rico was reported first in 2004, when a specific antibody was detected in a free-ranging native bird (1) and three asymptomatic, unvaccinated horses (CDC, unpublished data), all in the northeastern area of the island. During 2006--2007, CDC maintained a sentinel chicken surveillance system in northeastern Puerto Rico. In June 2007, specific anti-WNV neutralizing antibodies were detected in these birds, indicating active WNV transmission (2). WNV nucleic acid was detected by polymerase chain reaction (PCR) in mosquitoes in the same area (2). As a result, PRDH and CDC began enhanced surveillance for human WNV disease in the neighboring municipios† of Ceiba (where the sentinel chicken seroconversions and WNV-positive mosquitoes were detected), Humacao, Naguabo, and Fajardo. Enhanced surveillance included asking hospitals and clinics in the four municipios to obtain blood samples from local residents with acute febrile disease, with or without neurologic manifestations. Specimens were submitted to CDC's Dengue Branch for WNV and dengue testing. During July 1--December 31, 2007, enhanced surveillance generated submission of serum specimens from 1,250 persons for WNV and dengue testing. None of the specimens were positive for WNV by PCR. Reporting of human WNV disease was urged through a physician advisory letter sent to all licensed physicians in Puerto Rico by PRDH. Vector control efforts and advisories for use of repellents and protective clothing already were in effect because of high levels of dengue on the island. In September 2007, WNV infection detected by PCR in postmortem brain tissue taken from an encephalitic horse and by virus isolation from a dead bird confirmed WNV transmission in southwest Puerto Rico. Reported by: J Torres Aponte, MS, E García Rivera, MD, Puerto Rico Dept of Health. S Stramer, PhD, R Casanova, MD, G Foster, MS, American Red Cross. R Luce, DVM, K Tomashek, MD, H Mohammed, PhD, W Sun, MD, E Hunsperger, PhD, JL Muñoz-Jordán, PhD, C Colon, MS, E Vergne, M Verduin, Dengue Branch, Div of Vector-Borne Infectious Diseases, National Center for Zoonotic, Vector-borne, and Enteric Diseases, CDC. Editorial Note:In the United States, WNV transmission to humans was detected first in 1999 during an outbreak of encephalitis in New York City (3) and has since been reported in all states except Alaska, Hawaii, and Maine§, likely spreading through bird migration (4). WNV was detected first among blood donors in the United States in 2002 (5). In 2001, the first human case of locally acquired WNV disease in the Americas south of the continental United States was reported in the Cayman Islands (6). Since 2001, WNV has been reported in 16 countries in Latin America and the Caribbean¶ (Table). Despite the spread of WNV to Latin America and the Caribbean, few cases of human WNV disease have been reported (7), and reports of animal deaths and illness from WNV in those regions have been rare compared with reports from North America (7). Several factors might contribute to this difference. First, the capacity of surveillance systems in Latin America and the Caribbean region to identify WNV disease might differ from those in North America. Second, dengue, caused by a related flavivirus, is endemic in most countries south of the United States, which can make the diagnosis of WNV infections more difficult. Dengue and WNF can have similar signs and symptoms, and the tests for specific antibody to dengue virus and WNV often cross-react (8). Third, previous dengue or other flavivirus infection might confer some degree of immunologic cross-protection that could modulate infection with WNV.** Finally, circulation of attenuated viral strains might result in less disease. However, in one study, the only isolate reported to date from the Caribbean had no genetic evidence of attenuation; in another study, only one of nine WNV isolates from Mexico had evidence of attenuation (2,9). Identification of WNV in animals and subsequently in human blood donations in Puerto Rico suggests that human WNV disease is likely to occur in Puerto Rico. Serosurveys and studies of blood donors in North America and Europe indicate that 70%--80% of people infected with WNV are asymptomatic (10). This proportion might be higher in Latin American and Caribbean populations if other circulating flaviviruses, such as dengue, modify the clinical presentation of WNV illness. WNV should be considered in the differential diagnosis of acute febrile or neurologic illness in residents of and visitors to Puerto Rico. Accurate laboratory diagnosis of WNV infection in Puerto Rico and other areas where flaviviruses are endemic requires careful evaluation of serologic antibody assays for cross-reactivity, or direct detection of WNV in diagnostic samples using specific nucleic acid detection tests, viral antigen detection, or viral isolation. PRDH and CDC will continue WNV surveillance activities in the 2008 WNV transmission season. References
Table 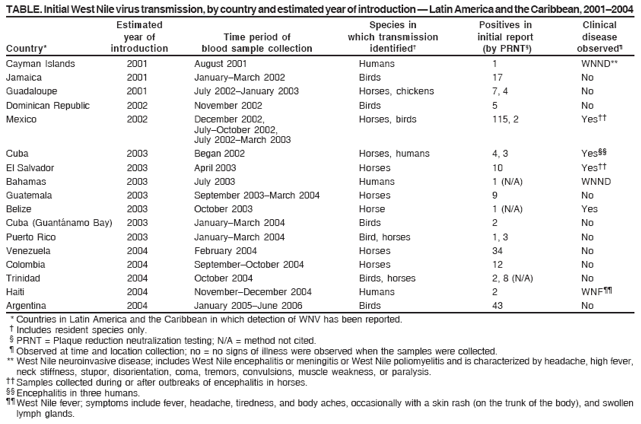 Return to top.
All MMWR HTML versions of articles are electronic conversions from typeset documents. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 5/28/2008 |
|||||||||
|