
|
 |
Dispatch
Age and Variant Creutzfeldt-Jakob
Disease
Peter Bacchetti*
*University of California, San Francisco, California, USA
Suggested citation
for this article:
Bacchetti P. Age and variant Creutzfeldt-Jakob disease. Emerg Infect
Dis [serial online] 2003 Dec [date cited]. Available from: URL:
http://www.cdc.gov/ncidod/EID/vol9no12/03-0361.htm
The young and stable
median age of those who die of variant Creutzfeldt-Jakob disease has
been attributed to age-dependent infection rates. This analysis shows
that an influence of age on risk for death after infection better explains
age patterns, suggesting that biologic factors peaking in the third
decade of life may hasten disease.
The epidemic of variant Creutzfeldt-Jakob disease (vCJD) in Great Britain
is now thought to be caused by the same prion responsible for bovine spongiform
encephalopathy (BSE) in cattle (1). A striking feature
of the human epidemic has been the young age of most patients and the
lack of any trend toward older ages in patients infected later in the
epidemic. Investigations of this stability have agreed that age must influence
risk for infection (2,3), and studies projecting future
numbers of cases have assumed that age only influences infection risk
and does not influence risk for disease after infection (3–5).
By extending previous methods to model age as a time-dependent covariate,
I show here that the stable age distribution over time is in fact better
explained by an influence of age on risk for disease after infection.
As was done in previous studies (2–5), I used methods
to exploit the relation among date of infection, incubation time, and
date of disease. The incubation period was defined as the time from infection
to disease, which can be onset, diagnosis, or death. I focused on death
from vCJD because no measurement error exists in the date of death, and
ascertainment delay is less of an issue for this than for the date of
disease onset. These methods were developed extensively for analyzing
the HIV epidemic, both for estimating past infection rates, assuming a
known incubation period distribution (6,7), and for estimating
incubation, assuming a known infection pattern (8,9).
To match the last approach, I assumed that the shape over time of the
infection hazard (the risk for infection among uninfected persons) is
determined by what is known about the BSE epidemic (10)
and that the scale of the infection hazard is large enough so that the
total number of infections (approximately 1.2 million) is much larger.
(This large number of infections implies that risk of developing disease
after infection must be very low, so most of those infected will die of
other causes, and disease will never develop.) I assumed that age has
a multiplicative effect on the risk for infection or on the risk for disease
after infection, corresponding to the proportional hazards assumption
(11) frequently used in survival analysis. Detailed statistical
methods are described in the Appendix.
|
Figure |
|
|
 |
|
|
Click to view
enlarged image
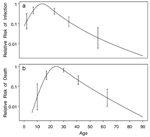
Figure. Estimated influence of age on a)
risk for infection with the variant Creutzfeldt-Jakob disease (vCJD)
agent and b) risk for death from vCJD after infection...
|
|
Using the 121 reported deaths from 1995 to the end of 2002, I obtained
similar shapes for the estimated influence of age on risk for infection
(Figure a) or on risk for death (Figure
b), with both showing strong peaks. The model of Figure
b, where age influenced only risk for death, had a log likelihood
that was better by 1.91 than the model of Figure a,
where age influenced only risk for infection. A simulation test using
data generated under the model of Figure a found
differences this large 75 times in 2,000 runs (p = 0.038), indicating
that the data are more compatible with an influence of age on risk for
death. This age model appears to better explain the observed stability
of ages at death over time. A simple regression of age at death on quarter
of death estimates that the mean age has remained nearly constant at around
29 years, with an average increase of 26 days for each year of the epidemic.
(A robust regression [12] of age at death on quarter
of death found an increase of 20 days per year, nearly identical to the
26 days found by ordinary least squares regression.) The model in Figure
b matched this finding with an estimated overall increase in mean
age of 34 days per year, but the model in Figure a
predicts an increase of 214 days per year.
The shapes in the Figure contrast with those assumed
by previous studies. One study assumed constant infection risk up to age
15, which is ruled out by the confidence intervals shown on the left in
Figure a (3). Another study modeled
the influence of age on both risk for infection and risk for death from
vCJD after infection, but it imposed a mathematical form for the influence
on risk for death that can only be monotonic, an assumption incompatible
with the peaked shape in Figure b (2).
That study’s finding that age must influence risk for infection rather
than only influencing risk for death is therefore suspect. In addition,
a later analysis (13) claiming to confirm the impact
of age on risk for infection did so only by assuming that age had no influence
on subsequent risk for death.
In the Figure b model, younger persons’ risks for
death increase as they age (moving up the left slope), while older persons’
risks decrease (moving down the right slope). Therefore, the highest risk
moves toward younger and younger age cohorts over time, counteracting
the aging of the entire infected population and thereby matching the observed
stability in ages at death. Nothing counteracts the aging of the infected
population if age is assumed to influence only risk for infection. This
finding and reasoning contrast with an argument (3) that
an influence of age on risk for death would result in a shift toward older
cases later in the epidemic because younger persons would have died earlier.
This argument relies on the unstated assumption that the total number
of infections is small enough that cases to date constitute a substantial
fraction of the total infected in younger age cohorts. I have considered
that many more were infected. Even if the unstated assumption were true,
this finding would not explain the observed stability because aging of
the entire infected cohort should still produce an upward trend.
The estimates in the Figure assume that total infections
were much greater than deaths reported to date but not so large that the
pool of >20 million persons with the susceptible genotype became noticeably
depleted before measures were implementedat the end of 1989 to keep contaminated
beef out of the human food supply. I evaluated such a scenario, with most
susceptible persons infected by 1989, and found even stronger evidence
in favor of an influence of age on risk for death rather than risk for
infection. The log likelihood was better by 3.13, and only 12 of 2,000
iterations in a simulation test produced a difference this large (p =
0.006).
The reasons for the age distribution of vCJD cases and its stability
over time remain unclear, and epidemiologic analyses can provide limited
insight. Previous assertions that age must influence risk for infection
and that age does not influence development of disease may have been incorrect.
Our findings suggest that the possibility should not be discounted that
biologic factors peaking in the third decade of life may promote vCJD
prion replication and consequent development of disease.
Acknowledgment
I thank the United
Kingdom’s Creutzfeldt-Jakob Disease Surveillance Unit for providing
the data on variant Creutzfeldt-Jakob disease cases.
Dr. Bacchetti is
a professor of biostatistics at the University of California at San
Francisco. His research interests include analysis of incomplete data,
with particular emphasis on infectious diseases.
References
- Haywood AM. Transmissible
spongiform encephalopathies. N Engl J Med 1997;337:1821–8.
- Ghani AC, Ferguson NM, Donnelly CA, Anderson RM. Predicted
vCJD mortality in Great Britain. Nature 2000;406:583–4.
- Valleron A-J, Boelle P-Y, Will R, Cesbron J-Y. Estimation
of epidemic size and incubation time based on age characteristics of
vCJD in the United Kingdom. Science 2001;294:1726–8.
- Ferguson NM, Ghani AC, Donnelly CA, Hagenaars TJ, Anderson RM. Estimating
the human health risk from possible BSE infection of the British sheep
flock. Nature 2002;415:420–4.
- Huillard d’Aignaux JN, Cousens SN, Smith PG. Predictability
of the UK variant Creutzfeldt-Jakob disease epidemic. Science 2001;294:1729–31.
- Brookmeyer R, Gail MH. Minimum size of the acquired immunodeficiency
syndrome (AIDS) epidemic in the United States. Lancet 1986;ii:1320–2.
- Rosenberg PS. Scope
of the AIDS epidemic in the United States. Science 1995;270:1372–5.
- Bacchetti P, Moss AR. Incubation
period of AIDS in San Francisco. Nature 1989;338:251–3.
- Bacchetti P. Estimating the incubation period of AIDS by comparing
population infection and diagnosis patterns. J Am Stat Assoc 1990;85:1002–8.
- Donnelly CA, Ferguson NM. Statistical aspects of BSE and vCJD. London:
Chapman & Hall/CRC; 2000. p. 170.
- Cox DR, Oakes D. Analysis of survival data. London:
Chapman & Hall; 1984. p. 23–4.
- Heiberger R, Becker RA. Design of an S function for robust regression
using iteratively reweighted least squares. Journal of Computational
and Graphical Statistics 1992;1:181–96.
- Ghani AC, Ferguson NM, Donnelly CA, Anderson RM. Factors
determining the pattern of the variant Creutzfeldt-Jakob disease (vCJD)
epidemic in the UK. Proc R Soc Lond B 2003;270:689–98.
|
