| October 1, 2008

[NIST Tech Beat Search] [Credits] [NIST Tech Beat Archives] [Media Contacts] [Subscription Information]

New Building Code Revisions Adopt NIST Recommendations from WTC Study
Future buildings—especially tall structures—should be increasingly resistant to fire, more easily evacuated in emergencies, and safer overall thanks to 23 major and far-reaching building and fire code changes approved recently by the International Code Council (ICC) based on recommendations from the National Institute of Standards and Technology (NIST). The recommendations were part of NIST’s investigation of the collapses of New York City’s World Trade Center (WTC) towers on Sept. 11, 2001.
The changes, adopted at the ICC hearings held Sept. 15-21, 2008, in Minneapolis, Minn., will be incorporated into the 2009 edition of the ICC’s I-Codes (specifically the International Building Code, or IBC, and the International Fire Code, or IFC), a state-of-the-art model code used as the basis for building and fire regulations promulgated and enforced by U.S. state and local jurisdictions. Those jurisdictions have the option of incorporating some or all of the code’s provisions but generally adopt most provisions.
“We applaud this historic action by the ICC—and the tremendous effort by NIST and its WTC investigation team that led to it,” said Commerce Secretary Carlos Gutierrez. “The lessons learned from the tragic events of 9/11 have yielded stronger building and fire codes for a new generation of safer, more robust buildings across the nation.”
The new codes address areas such as increasing structural resistance to building collapse from fire and other incidents; requiring a third exit stairway for tall buildings; increasing the width of all stairways by 50 percent in new high-rises; strengthening criteria for the bonding, proper installation and inspection of sprayed fire-resistive materials (commonly known as “fireproofing”); improving the reliability of active fire protection systems (i.e., automatic sprinklers); requiring a new class of robust elevators for access by emergency responders in lieu of an additional stairway; making exit path markings more prevalent and more visible, and ensuring effective coverage throughout a building for emergency responder radio communications.
Nine additional code change proposals based on the NIST WTC recommendations were not approved for the 2009 edition of the I-Codes.
These proposals address areas such as designing structures to mitigate disproportionate progressive collapse, mandating the use of a nationally accepted standard for conducting wind tunnel tests (routinely used for determining wind loads in the design of tall buildings), limiting the length of horizontal transfer corridors in stairways, installing stairway communication and monitoring systems on specific floors of tall buildings, and requiring risk assessments for buildings with substantial hazard (such as buildings more than 420 feet high with occupant loads exceeding 5,000 persons).
For more information and details on the code revisions, see “Safer Buildings are Goal of New Code Changes Based on Recommendations from NIST World Trade Center Investigation” A chart tracking the progress toward implementing all of the NIST WTC recommendations, may be found at http://wtc.nist.gov.
Media Contact: Michael E. Newman, michael.newman@nist.gov, (301) 975-3025 

Models of Eel Cells Suggest Electrifying Possibilities
 |
Electric eel anatomy: The first detail shows stacks of electrocytes, cells linked in series (to build up voltage) and parallel (to build up current). Second detail shows an individual cell with ion channels and pumps penetratimng the membrance, The Yale/NIST model represents the behavior of several such cells. Final detail shows an individual ion channel, one of the building blocks of the model.
Credit: Daniel Zukowski, Yale University
View hi-resolution image |
Engineers long have known that great ideas can be lifted from Mother Nature, but a new paper* by researchers at Yale University and the National Institute of Standards and Technology (NIST) takes it to a cellular level. Applying modern engineering design tools to one of the basic units of life, they argue that artificial cells could be built that not only replicate the electrical behavior of electric eel cells but in fact improve on them. Artificial versions of the eel’s electricity generating cells could be developed as a power source for medical implants and other tiny devices, they say.
The paper, according to NIST engineer David LaVan, is an example of the relatively new field of systems biology. “Do we understand how a cell produces electricity well enough to design one—and to optimize that design?” he asks.
Electric eels channel the output of thousands of specialized cells called electrocytes to generate electric potentials of up to 600 volts, according to biologists. The mechanism is similar to nerve cells. The arrival of a chemical signal triggers the opening of highly selective channels in a cell membrane causing sodium ions to flow in and potassium ions to flow out. The ion swap increases the voltage across the membrane, which causes even more channels to open. Past a certain point the process becomes self-perpetuating, resulting in an electric pulse traveling through the cell. The channels then close and alternate paths open to “pump” the ions back to their initial concentrations during a “resting” state.
In all, according LaVan, there are at least seven different types of channels, each with several possible variables to tweak, such as their density in the membrane. Nerve cells, which move information rather than energy, can fire rapidly but with relatively little power. Electrocytes have a slower cycle, but deliver more power for longer periods. LaVan and partner Jian Xu developed a complex numerical model to represent the conversion of ion concentrations to electrical impulses and tested it against previously published data on electrocytes and nerve cells to verify its accuracy. Then they considered how to optimize the system to maximize power output by changing the overall mix of channel types.
Their calculations show that substantial improvements are possible. One design for an artificial cell generates more than 40 percent more energy in a single pulse than a natural electrocyte. Another would produce peak power outputs over 28 percent higher. In principle, say the authors, stacked layers of artificial cells in a cube slightly over 4 mm on a side are capable of producing continuous power output of about 300 microwatts to drive small implant devices. The individual components of such artificial cells—including a pair of artificial membranes separated by an insulated partition and ion channels that could be created by engineering proteins—already have been demonstrated by other researchers. Like the natural counterpart, the cell’s energy source would be adenosine triphosphate (ATP), synthesized from the body’s sugars and fats using tailored bacteria or mitochondria.
* J. Xu and D.A. LaVan. Designing artificial cells to harness the biological ion concentration gradient. Nature Nanotechnology, published online: September 21, 2008.
Media Contact: Michael Baum, michael.baum@nist.gov, (301) 975-2763 

Moths with a Nose for Learning
 |
Researchers conditioned a moth to extend its proboscis in anticipation of a dollop of sucrose after being given a scent cue to study how the insect learned. Understanding how associations are built between stimuli and behavior can offer significant insights into how to build artificial systems to discriminate odors.
Credit: I. Ito and R.C.Y. Ong, NIH
View hi-resolution image |
Much like Pavlov conditioned his dog to salivate in anticipation of food when a bell rang, insects can be trained to perform certain behaviors when enticed with different smells. Researchers at the National Institutes of Health (NIH), the Chinese University of Hong Kong and the National Institute of Standards and Technology (NIST) have discovered that when training insects, the interval between the signal, or odor, and the reward—delicious sugar water—is everything. They also found that this process of building odor-sucrose associations would involve a mechanism that allows integration of neural activities (mental representations) that are not nearly coincident. Understanding how associations are built between stimuli and behavior gives insight into the nature of learning. Their findings were published online in Nature Neuroscience.*
Associations or meanings are formed when a connection is perceived among mental representations. In Pavlov’s experiments the dog was taught to understand that the ringing of the bell meant food. In this case, the researchers conditioned a particular species of moth, Manduca sexta, to extend its proboscis in anticipation of a dollop of sucrose after being given a scent cue. The researchers attached electrodes to the insects’ mushroom bodies, a structure in their brains known to be integral to learning and memory, in order to observe the mental representation of the scent through spikes in activity across groups of neurons called Kenyon cells. By observing this process, the group hoped to characterize how odors are represented by this neural population and how this representation gets associated with the reward.
The group found that most of the odor-elicited spiking in the Kenyon cells occurred during the beginning and end of an odor pulse, with little to no spiking in between. NIST/NIH researcher Baranidharan Raman says that this is can be understood by considering the analogy of going into a coffee shop. The onset signal occurs upon entering and smelling the coffee for the first time. That smell remains intense and noticeable for a few moments before it fades into the background. Again, when you the leave the coffee shop, you notice that change, the offset signal represents that change of state, and it does not elicit as much activity as is caused by the onset.
The group found that the interval between the odor stimulus onset and sucrose reward was crucial to whether or not the insect learned to link the representations. If the sucrose was presented during the onset of spiking, the insect did not learn as well. Moreover, if the researchers used a long odor stimulus and administered the sucrose just after the offset signal (long after the onset), the insect wouldn’t learn to expect it.
Learning, or the capacity to associate the cue and its reinforcement, occurs when reward was presented a few seconds after the onset of neural activity. Since most Kenyon cells have finished producing action potentials (or spikes) by the time the reward is presented, the odor-sucrose association cannot be achieved through a well-known model of learning called spike-timing dependent plasticity where nearly coincident spiking on a millisecond timescale results in the association of two events.
Raman says that the study of biological olfactory systems can offer significant insights into how to build artificial systems to discriminate odors with sensor arrays.
* I. Ito, R. Chik-ying Ong, B. Raman and Mark Stopfer. Sparse odor representation and olfactory learning. Nature Neuroscience. Published online Sept. 14, 2008.
Edited Oct. 2, 2008 to correct image caption. Edited Oct. 3, 2008 to correct URL for paper.
Media Contact: Mark Esser, mark.esser@nist.gov, (301) 975-8735 

NIST/CSM Sensor Could Help Avert Pipeline Failures
Researchers at the National Institute of Standards and Technology (NIST) and Colorado School of Mines (CSM) have developed a prototype sensor that quickly detects very small amounts of hydrogen accumulation in coated pipeline steel. The new sensor could provide early warning of pipes that have accumulated excessive amounts of hydrogen—a notorious source of embrittlement—and avert potentially disastrous failures of pipelines carrying hydrogen fuel.
Hydrogen is attractive as a fuel because it burns cleanly without carbon emissions and can be derived from domestic sources. However one long-recognized challenge is that hydrogen can cause gradual embrittlement in conventional pipelines by slowly diffusing into the metal. The NIST/CSM sensor, described today at the 7th International Pipeline Conference,* could monitor for hydrogen buildup before a pipeline actually fails in service, or during testing or production.
The nondestructive, non-contact hydrogen sensor is approximately 4 square inches and is designed to be a portable sensor to make measurements on excavated or unexcavated pipeline steels. The sensor sends a current through the pipe and measures changes in impedance (resistance with a function of depth) as an indicator of hydrogen content within the steel and the overall steel pipe integrity. The hydrogen sensor is based on electromagnetic concepts to generate alternating currents into the pipeline steel, which in turn induces an opposing magnetic field. Any change in the hydrogen content in the steel modifies the current, resistivity, and thus the impedance.
NIST laboratory and field test results show that a pipe’s impedance, and thus resistivity, increases with increasing hydrogen content. The measurement sensitivity is exceptional: the sensor can measure hydrogen content levels in pipeline steel well below 1 part per million (ppm). High strength pipeline steels can tolerate only a few parts per million of hydrogen before significant problems arise. By contrast, conventional analytical techniques do not have sensitivity or accuracy below 1 ppm. The new hydrogen sensor also acts as a forewarning or preventative monitoring system to detect the agents that actually cause the flaws, cracks and defects before they arise. Most traditional nondestructive tools used in the pipeline industry (such as ultrasonics and magnetic flux leakage) are used for determination of cracks, corrosion or other flaws that have already occurred.
The sensor development was supported in part by the Minerals Management Service and Department of Transportation.
* A. Lasseigne, K. Koenig, J. Jackson, D. Olson, B. Mishra, T.A. Siewert and J.D. McColskey. Advanced non-destructive hydrogen sensors to prevent material degradation from hydrogen damage. Paper presented Oct. 1 at IPC2008, 7th International Pipeline Conference, Sept. 29 – Oct. 3, 2008, Calgary, Alberta, Canada.
Media Contact: Laura Ost, laura.ost@nist.gov, (303) 497-4880 

Baked Slug: New Method to Test Fireproofing Material
 |
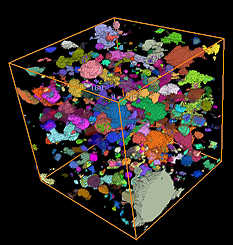 |
(l) Experimental setup of Slug Calorimetry Method: Fireproofing material of interest sandwiches a platelike steel slug. Measuring heat flow through the slug can provide information on the thermal conductivity of the material.
(r) Microtomography image of the pore structure of a fireproofing material. The different colors identify different individual pores within the material, whose size and shape have a large influence on the thermal conductivity of the material at high temperatures.
Credit: Talbott, NIST
View hi-resolution images left and right. |
In a high-temperature blaze, how well does a fireproofing material shield a building’s important steel structures from heat? Answering this question has been surprisingly difficult, but it is important information for builders selecting high-performance fire-resistive materials and for scientists conducting computer simulations that investigate fires. Now, researchers at the National Institute of Standards and Technology (NIST) and their colleagues have developed a technique for measuring a key thermal property of fire-resistive materials at high temperatures. The measurement technique has already been adopted commercially and incorporated into a national standard.*
In creating computer simulations to study the collapses of the World Trade Center buildings on Sept. 11, 2001, NIST researchers needed to know important properties of the fireproofing materials that protected structural steel columns. One key property was the thermal conductivity of the material: How quickly does heat transfer through it? Thermal insulation has a low thermal conductivity and metals have a high thermal conductivity. There are long-established methods for measuring thermal conductivity under ambient conditions, but a material’s thermal conductivity can change markedly when it is subjected to extremely high temperatures that cause important chemical and structural changes. Traditional methods for measuring thermal conductivity at high temperatures have not been adequate. They have relied on “hot wire” techniques, which use wire probes to measure heat flow through a wire surrounded by the material of interest. At sufficiently elevated temperatures, the material can separate from the wire preventing the measurement of the thermal conductivity in a highly heated material.
NIST’s Dale Bentz and his colleagues developed a “slug calorimeter” technique for obtaining the thermal conductivity information at elevated temperatures. In this technique, they use a thin square slab of steel material known as a slug and sandwich it between slabs of the fireproofing material of interest. Guard insulation surrounds the sides of the sample so that heat flows preferentially through the sandwich when it is placed in a high-temperature furnace. Three temperature probes inserted into the steel slug measure the heat flowing to the steel. Combining this data with the known heat capacities and densities of the steel slug and the fire-resistive material, the researchers can determine the material’s thermal conductivity at various temperatures.
Following the successful demonstration of this method at NIST, two large U.S. testing labs have worked with NIST to develop their own in-house slug calorimeters as a testing service to their clients, and a third U.S. company recently introduced a commercial version of a slug calorimeter. ASTM International (formerly the American Society for Testing and Materials) has published a standard (ASTM E 2584) detailing how to conduct thermal conductivity measurements with the new method. Possible applications beyond steel fireproofing material, Bentz says, involve measuring the thermal conductivity of wood-based materials, as well as the insulating materials used to protect spacecraft such as the Space Shuttle.
* D.P. Bentz, D. Flynn, J.H. Kim and R.R. Zarr. Fire Materials, 2006; 30:257-270; and ASTM Standard E 2584-07, “Standard Practice for Thermal Conductivity of Materials.”
Edited on Oct. 2, 2008 to correct caption for microtomography image.
Media Contact: Ben Stein, bstein@nist.gov, 301-975-3097 

JILA Scientists Create First Dense Gas of Ultracold ‘Polar’ Molecules
Scientists at JILA, a joint institute of the National Institute of Standards and Technology (NIST) and the University of Colorado at Boulder (CU-Boulder), NIST and Temple University in Philadelphia have produced the first high-density gas of ultracold “polar” molecules—molecules with a positive electric charge at one end and a negative charge at the other—that are both stable and capable of strong interactions. The long-sought milestone in physics has potential applications in quantum computing, precision measurement and designer chemistry.
Described in a recent paper,* the ultracold polar molecules could be used to study controlled interactions of molecules separated by relatively long distances, offering a richer selection of features than is possible with individual atoms and potentially leading to new states of matter.
The JILA group combined potassium and rubidium to create molecules that have a stable and measurable separation of electric charge. That, along with the ultracold temperatures and high density —the gas has a density of 10 quadrillion molecules per cubic centimeter and a temperature of 350 nanoKelvin above absolute zero (about minus 273 degrees Celsius)—allows the molecules to exert strong forces on each other. They also have what is considered a long lifespan for the quantum world, lasting about 30 thousandths of a second.
After creating an initial large, weakly bound potassium-rubidium molecules using a magnetic field technique pioneered by NIST/JILA Fellow Deborah Jin, one of the co-authors, the scientists faced the considerable challenge of efficiently converting atoms that are far apart into tightly bound molecules without allowing the released binding energy to heat the gas. In a process that Jin describes as “chemistry without explosions,” the team used two lasers, each tuned to a different energy jump in the molecules, to convert the binding energy into light instead of heat. The molecules absorb near-infrared laser light and release red light. In the process, more than 80 percent of the molecules are converted to the lowest and most stable energy level.
The research, which was supported by the National Science Foundation, NIST, the Air Force Office of Scientific Research and the W.M. Keck Foundation, is part of a larger NIST/JILA effort to develop techniques to understand and control the complex features of molecules and their interactions. Practical benefits could include new chemical reactions and processes for making designer materials and improving energy production, new methods for quantum computing using charged molecules as quantum bits, new tools for precision measurement such as optical molecular clocks or molecular systems that enable searches for new theories of physics beyond the Standard Model, and improved understanding of condensed matter phenomena such as colossal magnetoresistance and superconductivity.
For more details and illustrations, see “JILA scientists create first dense gas of ultracold ’polar’ molecules.”
* K.K. Ni, S. Ospelkaus, M.H.G. de Miranda, A. Pe’er, B. Neyenhuis, J.J. Zirbel, S. Kotochigova, P.S. Julienne, D.S. Jin, J. Ye. A high phase-space-density gas of polar molecules. Science Express. Sept. 18, 2008.
Media Contact: Laura Ost, laura.ost@nist.gov, (303) 497-4880 

Updated Standards Site Offers New Support for Exports to Asia
The National Institute of Standards and Technology (NIST) is supporting the re-launch of www.StandardsPortal.org, an online resource that facilitates international trade globally, with in-depth information on China and India, by helping companies and policy officials understand the standards, regulatory and conformance related technical requirements they face around the world. The portal is maintained by the American National Standards Institute (ANSI); NIST is supplying the funding for redevelopment and maintenance for the portal.
Originally released in 2006 to support trade between the United States and the People’s Republic of China, the newly redesigned StandardsPortal helps exporters navigate the standards, regulatory and conformance systems in foreign markets. Specifically, it provides answers to such questions as what requirements need to be met to compete in a given target market, how a company can get early warning about requirement changes, and how a company can ensure that its perspectives are heard and considered in the development of voluntary consensus standards, requirements and policies that could affect that company’s business.
StandardsPortal includes a searchable directory of U.S.-based standards developing organizations and trade associations, including each organization’s contact information, scope of standards development and conformity assessment work, and international activities organized by geographic region.
The portal also includes an International Trade and Investment Toolbox, which provides guidelines, archives and information specifically for the U.S. stakeholders engaged in global trade activities. It includes a speaker’s package for performing international outreach and information packages on initiating business development in China and Europe.
StandardsPortal was created through a partnership between the American National Standards Institute, the Standardization Administration of the People’s Republic of China, the Bureau of Indian Standards and the Confederation of Indian Industry.
Media Contact: Evelyn Brown, evelyn.brown@nist.gov, (301) 975-5661 

|

