Trends in Spina Bifida by Race/Ethnicity, United States
Women of childbearing age: consume at least 400 mcg of folic acid daily to prevent neural tube defects like spina bifida.
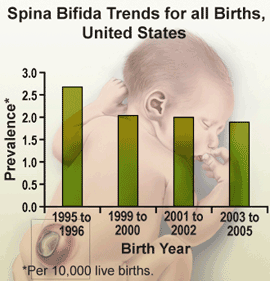
In 1992, the U.S. Public Health Service recommended that all women of childbearing age consume 400µg of folic acid daily to help prevent pregnancies affected by neural tube defects (NTDs) such as spina bifida (1). Four years later, the United States Food and Drug Administration ordered the addition of folic acid to all enriched cereal grain products by January of 1998 (2). During October 1998 – December 1999, the birth prevalence (number of existing disease cases in a defined group of people during a specific time period) of spina bifida in the United States declined 23% compared with 1995-1996 (3); however, by 2003-2004, no further decrease had been observed (4). The prevalence of NTD-affected pregnancies has been higher among Hispanic women than women in other racial/ethnic populations before and after fortification (4,5).
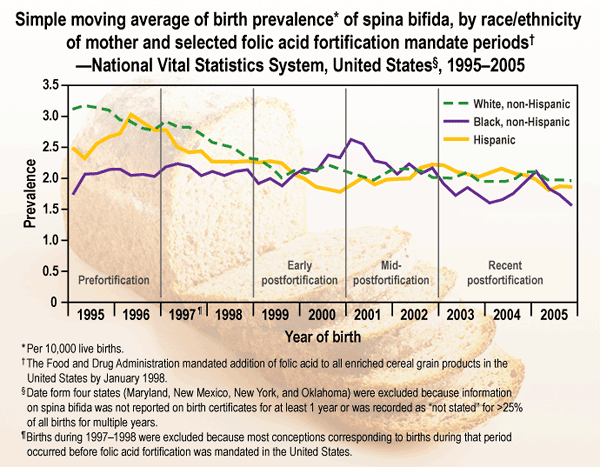
To update previously reported data and assess racial/ethnic differences, CDC analyzed birth certificate data for four periods during 1995-2005. These analyses indicated that from the early post-fortification period (1999-2000) to the most recent period (2003-2005), the prevalence of spina bifida declined 6.9%, from 2.04 to 1.90 per 10,000 live births. Among infants with non-Hispanic black mothers, prevalence fell 19.8%, from 2.17 to 1.74 per 10,000 live births while prevalence among infants with non-Hispanic white and Hispanic mothers remained nearly constant. Additional public health efforts targeting women with known risk factors (e.g., obesity and certain genetic factors) likely are needed to further reduce the prevalence of spina bifida in the United States.
References
- Centers for Disease Control and Prevention. Recommendations for the use of folic acid to reduce the number of cases of spina bifida and other neural tube defects. MMWR 1992;11;41:1-6.
- Food and Drug Administration. Food standards: amendment of standards of identity for enriched grain products to require addition of folic acid. Federal Register 1996;61:8781-97.
- Honein MA, Paulozzi LJ, Mathews TJ, Erickson JD, Wong LY. Impact of folic acid fortification of the US food supply on the occurrence of neural tube defects. JAMA 2001;285(23):2981-6.
- Boulet SL, Yang Q, Mai C, Kirby RS, Collins JS, Robbins JM, Meyer R, Canfield MA, Mulinare J. Trends in the postfortification prevalence of spina bifida and anencephaly in the United States. Birth Defects Res.A Clin.Mol.Teratol. 2008;82(7):527-32.
- Williams LJ, Rasmussen SA, Flores A, Kirby RS, Edmonds LD. Decline in the prevalence of spina bifida and anencephaly by race/ethnicity: 1995-2002. Pediatrics 2005;116(3):580-6.


