
Recommendations Focus on Imaging to Predict Treatment Response
 NCI recently published new clinical recommendations on the use of the imaging technique known as FDG-PET in NCI-sponsored clinical trials. The recommendations, developed in collaboration with some of the nation's top researchers and clinicians in nuclear medicine and radiology following an NCI-sponsored workshop in 2005, apply to clinical trials where FDG-PET results are being studied as early indicators, or biomarkers, of response to treatment. NCI recently published new clinical recommendations on the use of the imaging technique known as FDG-PET in NCI-sponsored clinical trials. The recommendations, developed in collaboration with some of the nation's top researchers and clinicians in nuclear medicine and radiology following an NCI-sponsored workshop in 2005, apply to clinical trials where FDG-PET results are being studied as early indicators, or biomarkers, of response to treatment.
The premise behind the use of FDG-PET relies on the inner workings of tumor cells, which need a lot of energy to survive and proliferate. This need is so great that tumor cells will simultaneously use two different methods of consuming glucose to provide enough energy to fuel their activity.
FDG is a glucose analog with a radioactive tracer (F18) attached to it. Tumor cells, particularly those from especially aggressive tumors, will consume significantly larger amounts of it than normal surrounding tissue. The result is that FDG's presence can be detected by PET imaging in tumors as small as 1 cm.
Currently, FDG-PET is used in everyday clinical practice to help detect the presence of a variety of tumor types and to stage them (at initial diagnosis as well as for restaging after treatment), explained Dr. Lalitha K. Shankar, of the NCI Cancer Imaging Program and the lead author of the clinical recommendations.
More recently, though, researchers have been investigating whether FDG-PET results can predict treatment response early (in the first 2 to 6 weeks) after treatment. In these studies, the levels of "tumor uptake" of FDG are measured before treatment and then again a short time later to see if that uptake has decreased, signaling a decline in the tumor cell population.
For instance, a small clinical trial published last year, of patients with advanced ovarian cancer undergoing presurgical chemotherapy, showed that changes in FDG-PET, even after the first chemotherapy cycle, accurately predicted outcomes and had superior accuracy compared with standard response criteria.
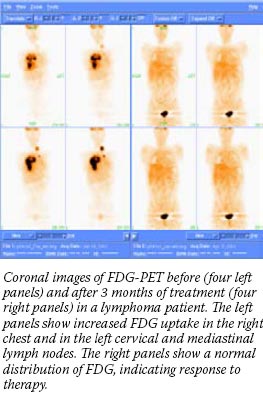
Similar trials involving other tumor types - lymphoma, esophageal and lung cancer - also have had good results, said Dr. Anthony Shields, a medical oncologist at the Karmanos Cancer Institute at Wayne State University, who also develops radiopharmaceuticals for use with PET imaging.
"Now we need to know in detail the sensitivity and specificity of that measurement," Dr. Shields said. "We need to figure out how good it is at predicting response and whether you can change treatment based on the PET results."
What's been missing from the studies and small clinical trials done to date, Dr. Shankar said, is consistency in the technical aspects of how the imaging procedures are performed. This includes measures such as preparing patients for the imaging procedures (varying blood glucose levels, for example, can skew the results); the timing of when the procedures are performed after treatment; and analyzing the resulting images, which can be done in different ways according to a number of different variables.
Ensuring consistency in the clinical trials testing FDG-PET in this fashion will be critical to moving the field ahead, says Dr. Steven M. Larson, of Memorial Sloan-Kettering Cancer Center, who directs the Laurent and Alberta Gerschel Positron Emission Tomography Center there.
"FDG-PET is likely to be the best candidate for a qualified biomarker," Dr. Larson argues. "But it will need to be assessed in large, multicenter trials. That's why standardization of the procedures from site to site is so important - to improve the reproducibility of the test."
Implementing the clinical recommendations, published in the June 2006 issue of The Journal of Nuclear Medicine, in NCI-sponsored trials "gives us a good place to start the next series of evaluations of FDG-PET as a marker of therapeutic response," Dr. Shankar explains. "This way, we can better evaluate, in a more uniform fashion, whether FDG-PET can help as a biomarker for assessing treatment response in a variety of tumors, doing it as a correlative study within the context of a larger therapeutic trial."
Approximately 10 clinical trials approved by the NCI Cancer Therapy Evaluation Program (CTEP) are already working under the auspices of these recommendations, and Dr. Shankar is working with CTEP to ensure that other trials follow suit.
"The imaging and oncology communities have really welcomed this activity," Dr. Shankar says. "And our colleagues in the pharmaceutical industry have as well. They participated in the workshop last year, and they are also using these recommendations in their trials."
Because of the rapid changes in imaging technologies, including PET, the recommendations will be revisited every few years, Dr. Shankar notes. NCI is also interested in consulting with cancer centers and other institutions who wish to work the recommendations into their own single- or multi-institution trials.
By Carmen Phillips |