Coordinating Office for Global Health
International Health Regulations
Revised for Today′s World
Scott J.N. McNabb, PhD, MS
Director, Division of Integrated Surveillance Systems and Services
National Center for Public Health Informatics
Katrin S. Kohl, MD, PhD, MPH
Deputy Director, Division of Global Migration and Quarantine
National Center for Preparedness, Detection, and Control of Infectious Diseases
IHR e-mail: IHRQuestions@cdc.gov
IHR in Context
International Health Regulations (IHR)
slide —2—
What? – formal code of conduct for public health emergencies ofinternational concern
Why? – a matter of responsible citizenship and collective protection
How? – the U.S. national, tribal, territorial, state, and local roles
Who? – all World Health Organization (WHO) Member Countries
When? – July 18, 2007
Revised IHR
What
slide —3—
- International agreement giving rise to international obligations
- Focuses on serious public health threats with potential to spread beyond a country′s borders, to other parts of the world
- Such events are defined as a Public Health Emergency of International Concern (PHEIC)
- Outlines assessment, management, and information sharing for PHEICs
IHR Serves a Common Interest
why
slide —4—
- Serious and unusual disease events are inevitable.
- A health threat in one part of the world can threaten health anywhere or everywhere.
- A formal code of conduct:
- helps contain or prevent serious risks to public health
- discourages unnecessary or excessive traffic or trade restrictions, for "public health purposes"
Revised IHR
key changes from old (1969) IHR
slide —5—
Member Countries must:
- Notify WHO of events meeting defined criteria – beyond prescribed list
- Enhance their events management – especially alert and response actions
- Meet minimum core capacities – notably in surveillance, response, and at points of entry
International Health Regulations
in brief
slide —6—
Are:
- Written in legal language
- Supported by guidelines to aid compliance
- Intended to contain public health threats and minimize economic disruption
Are not:
- Self—explanatory
- Recommendations for safe travel
- A scientific consensus on everything possible to prevent disease spread
United States Accepts IHR
how
slide —7—
- The United States accepted the IHR with a reservation and three understandings.
- The deadline for registering an objection to the Reservation and Understandings was July 17, 2007.
- United States is encouraging local and state governments to aid compliance.
- Sec. Leavitt′s letter to Governors
- CSTE′s position statement in support
United States Accepts IHR
how
slide —8—
- Reservation
The US will implement the IHR under the principles of federalism. - Federalism
The system of government in which power is divided between a central authority (U.S. federal government) and constituent political units (local and state governments).
United States Accepts IHR
how
slide —9—
Understandings
- Under the IHR, incidents that involve the natural, accidental or deliberate release of chemical, biological, or radiological materials must be reported.
- Countries that accept the IHR are obligated to report, to the extent possible, potential public health emergencies that occur outside their borders.
- The IHR do not create any separate private right to legal action against the Federal government.
United States Accepts IHR
how
slide —10—
- HHS Secretary′s Operations Center is the U.S. National Focal Point to the WHO.
- WHO access to IHR information will be "24 / 7".
- CDC assumes a lead role in IHR implementation as it relates to human disease.
- Detection, prevention, and control
- One major role for CDC is to support existing health monitoring systems that identify and report.
- Local, state, and federal public health authorities need to collaborate to improve the ability of national health monitoring systems to report possible PHEICs under IHR provisions.
IHR in a Small World
why
slide —11—

Public health threats with the potential to spread around the world are a fact of life. CDC is ready to deploy domestically or internationally to assist in identification, containment, and response.
IHR: Practically Correct
why
slide —12—
"As we have seen recently with SARS and H5N1 avian influenza, diseases respect no boundaries. In today′s world, a threat anywhere means danger everywhere."
December 13, 2006
HHS Secretary Michael O. Leavitt, on occasion of official United States acceptance of revised IHRAssessing the Threat under IHR
PHEIC
slide -13-
Always Notifiable
- Smallpox
- Poliomyelitis, wild—type
- Human influenza, new sub—type
- SARS
Other Events Potentially Notifiable
- Examples: cholera, pneumonic plague, yellow fever, viral hemorrhagic fever, and West Nile fever
- Other biological, radiological, or chemical events may fit the decision algorithm and be reportable
Making the Determination
PHEIC
slide —14—
Criteria from Annex 2
- Is the public health impact of the event serious?
- Is the event unusual or unexpected?
- Is there a significant risk of international spread?
- Is there a significant risk of international travel or trade restrictions?
WHO makes the final determination that a PHEIC exists
Serious Impact on Public Health?
how
slide —15—
- There is potentially high morbidity and/or mortality
- The geographic scope is large or spreading over a large area (e.g. multi—state or regional); is in area of high population density
- The agent is highly transmissible/pathogenic
- The event has compromised containment or control efforts
- Therapeutic/prophylactic agents are unavailable, absent, or ineffective
- Cases occurring among health care staff
- Assistance for investigation & response required
Unusual or Unexpected?
slide —16—
- The disease—causing agent is yet unknown or a new (emergent) pathogen
- The population affected is highly susceptible
- United States is encouraging local and state governments to aid compliance.
- The event is unusual for the season, locality or host
- There is a suspicion that this may have been an intentional act
- Agent had been eliminated or never reported in U.S.
Significant Risk for International Spread?
slide —17—
- Epidemiologic link to a similar event outside the United States
- International travel or gathering
- Contact with traveler or mobile population
- Potential cross—border movement of pathogen/agent/host
- Conducive transmission vehicles: air, water, food or environmental
Risk for Trade or Travel Restrictions?
how
slide —18—
- There is a history of similar events in the past that have resulted in restrictions
- The event is associated with an international gathering or a tourist area
- The event is or has gained significant government or media attention
- There is a zoonotic disease or the potential for an epizootic event, or exported/imported food/water—related
PHEIC Decision Instrument
annex 2*
slide —19—
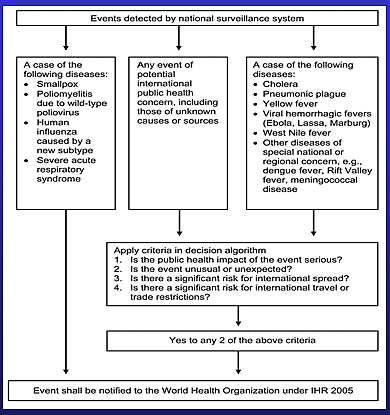
This diagram is a description from the July 2006 Emerging Infectious Disease Article by Michael G. Baker and David P. Fidler titled “Global Public Health Surveillance Under New International Health Regulations.” This diagram is in an easier to read format than the diagram in Annex 2 of the IHR (2005).
- This diagram represents an overview of the risk assessment algorithm for determining if an event is notifiable to WHO.
- It includes the list of conditions that are always notifiable to WHO – smallpox, polio, SARS, human influenza caused by a new subtype.
- It includes a list of conditions that are notifiable if the decision criteria are met, such as such as cholera, pneumonic plague, yellow fever, West Nile fever, and meningococcal disease.
- It includes the provision for any other event of potential international concern, including those of unknown causes or sources, to be notifiable, if the decision criteria are met.
- The criteria for assessment include the 4 major questions mentioned previously. If any 2 criteria are met, the condition is notifiable.
- Assessment using this instrument is intended to take place at the national level.
* Baker MG, Fidler DP. Global public health surveillance under the new International Health Regulations. EID; July 2006, Vol. 12. http://www.cdc.gov/ncidod/eid/vol12no07/05-1497.htm
Making the Determination
PHEIC
slide —20—
In summary …
- Local situational assessment required
- Decision instrument available
- WHO will also assess before any publication or formal response
Criteria from Annex 2
IHR in Practice
reporting timeline
slide —21—
48—hour Time Requirement
- After a U.S. Governmental Agency (USGA) learns of a potential PHEIC in a U.S. state or territory, it must assess the event within 48 hours.
24—hour Time Requirement
- The USGA has 24 hours to notify WHO after it believes that a potential PHEIC may exist.
Global Health and IHR
IHR mandate
slide —22—
Shared responsibility – to establish core capacities:
- Surveillance and response
- Points of entry
- Country—specific procedures—key element of WHO′s strategy for global health security
Global Health and IHR
IHR mandate
slide —23—
Robust National Response Effort is Expected
- Context—specific
- Flexible
- Interventional health measures permitted
Entrance Screening Permissible
- Medical exams and interviews
- Vaccination and other measures by consent
- Quarantine/isolation – respect for human rights
The IHR Timeline
when
slide —24—
- May 2005: World Health Assembly approved revised IHR
- December 2006: United States accepted the revised IHR (with reservation and understandings)
- June 15, 2007: Initial start—date for revised IHR
- July 18, 2007: United States starts adherence to revised IHR
- June 2009: Within 2 years after IHR enters into force, Member Countries complete assessment of the ability of their national structures and resources to meet minimum core capacities*
- 2012: Within 5 years after IHR enters into force, Member Countries achieve the required minimum level of core capacities, unless WHO grants an extension
- 2014: End of 2—year extensions on achieving core capacity, unless an exceptional circumstance exists and a further extension is granted by WHO
- 2016: End of final 2—year extensions (for exceptional circumstances) on achieving core capacities
*Core capacities as listed in Annex 1 of the IHR
United States and IHR
federal government partners
slide —25—
- Central Intelligence Agency
- Department of Agriculture
- Department of Commerce
- Department of Defense
- Department of Energy
- Department of Health and Human Services
- Department of Homeland Security
- Department of Justice
- Department of State
- Department of the Treasury
- Department of Transportation
- Department of Veterans Affairs
- Environmental Protection Agency
- Joint Chiefs of Staff
- Nuclear Regulatory Commission
- Office of Management and Budget
- Office of Science and Technology Policy
- U.S. Agency for International Development
- U.S. Trade Representative
- United States Postal Service
IHR References
how
slide —26—
- WHO IHR website: http://www.who.int/csr/ihr/en/
- HHS Global Health website: http://www.globalhealth.gov/ihr/
- HHS Announcement the U.S. accepted the IHR (2005): http://www.hhs.gov/news/press/2006pres/20061213.html
- CDC IHR website: http://www.cdc.gov/cogh/ihregulations.htm
- Baker MG, Fidler DP. Global public health surveillance under the new International Health Regulations. EID; July 2006, Vol. 12. http://www.cdc.gov/ncidod/eid/vol12no07/05-1497.htm
- CSTE Position statement: http://www.cste.org/ps/2007ps/2007psfinal/id/07-id-06.pdf
- The NNDSS notifiable diseases website: http://www.cdc.gov/epo/dphsi/nndsshis.htm
International Health Regulations
Revised for Today′s World
slide —27—
Thank you
Scott J.N. McNabb, PhD, MS
Director, Division of Integrated Surveillance Systems and Services
National Center for Public Health Informatics
Katrin S. Kohl, MD, PhD, MPH
Deputy Director, Division of Global Migration and Quarantine
National Center for Preparedness, Detection, and Control of Infectious Diseases
IHR e—mail: IHRQuestions@cdc.gov
Page last modified: July 18, 2006
