 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
Laboratory Surveillance for Wild and Vaccine-Derived Polioviruses, January 2003--June 2004In 1988, the World Health Assembly resolved to eradicate poliomyelitis globally by 2000. Progress toward achieving this goal has been reported from countries where polio is endemic, and three World Health Organization (WHO) regions (Americas, Europe, and Western Pacific) appear to be free of indigenous wild poliovirus (WPV) transmission. One key strategy for eradicating polio is establishing sensitive polio surveillance systems by investigating acute flaccid paralysis (AFP) cases. To ensure that specimens from persons with AFP undergo appropriate processing for viral isolation, WHO established a global polio laboratory network in 1988. This report updates previous publications (1--4), summarizes the laboratory network's performance, and describes the location and characterization of WPV and vaccine-derived poliovirus (VDPV) during January 2003--June 2004. Laboratory Network PerformanceThe global polio laboratory network, which operates in all six WHO regions, comprises 123 national facilities, 15 regional reference laboratories, and seven global specialized laboratories. High-quality performance is ensured through a WHO-administered laboratory accreditation program with a comprehensive annual review of criteria related to timely and accurate laboratory results. Of the 145 network laboratories, 139 (96%) were fully accredited by WHO in 2003. Three laboratories that passed annual proficiency tests but were deficient in some other aspect of performance were provisionally accredited. Three laboratories were not accredited because they failed the annual proficiency test. Nonaccredited laboratories split samples for parallel testing in accredited laboratories while implementing measures to improve performance. During January 2003--June 2004, the laboratory network tested 104,946 stool samples from persons with AFP. For more than 90% of the samples, virus isolation results were available within 28 days of receipt by laboratories (program target: >80% within 28 days). For 79% of persons with AFP with poliovirus isolates, the results of intratypic differentiation (ITD) tests confirmed the wild or vaccine-like nature of isolates within 60 days of paralysis onset (program target: >80% within 60 days) (Table 1). During the first 6 months of 2004, a total of 38,432 AFP samples were processed by network laboratories, compared with 29,232 samples during the same period in 2003, a 31% increase. Workload increased 23% and 40% in the Africa and Southeast Asia regions, respectively. WPV Serotypes and GenotypesDuring January 2003--June 2004, WPVs were confirmed in 19 countries (Table 2). The polio laboratory network routinely performs genetic characterization of all WPVs and all isolates with inconclusive results on ITD tests. Analysis of genetic sequence data from WPVs identifies circulating virus genotypes as well as the genetic links among viruses from diverse locations. Six WPV genotypes were detected during January 2003--June 2004, including three type 1 genotypes (NEAF, WEAF-B, and SOAS)* and three type 3 genotypes (WEAF-B, SOAS, and EAAF)*. The NEAF genotype was identified in Egypt. The SOAS genotypes (types 1 and 3) were detected in Afghanistan, India, and Pakistan. The type 1 WEAF-B genotype was identified in Botswana, Sudan, and 11 countries in western and central Africa. The type 3 WEAF-B genotype was detected only in Niger and Nigeria. The type 3 EAAF genotype reemerged in Sudan in 2004. Wild type 2 poliovirus has not been detected anywhere in the world since October 1999 (5). Indigenous WPVs were detected in Afghanistan, Egypt, India, Niger, Nigeria, and Pakistan in 2003 and 2004. Indigenous type 3 virus from central Africa/Horn of Africa, which was thought to have been eliminated 3 years earlier, was detected in Sudan in 2004. Type 1 virus detected in Lebanon in 2003 had been imported from northern India. VDPVsVaccine-derived polioviruses, defined as viruses with >1% sequence differences compared with Sabin vaccine virus of the same serotype, are also detected by the laboratory network (Table 3). Although VDPVs previously have been shown to circulate in Egypt, Hispaniola, Madagascar, and Philippines (6--9), no VDPV outbreaks were detected in 2003. Type 1 VDPVs detected in two persons with AFP (June and July 2004) and in two contacts of persons with AFP (August 2004) in Guizhou Province, China, are the subject of an ongoing investigation. Type 2 VDPVs were isolated from single AFP cases in Kazakhstan, Peru, and Thailand in 2003. VDPVs from non-AFP sources also have been reported. In 2003, a type 1 VDPV was isolated from a healthy child in Mongolia, and a type 2 VDPV was isolated from a healthy child in Latvia. A type 3 VDPV was isolated from a single sewage sample collected in Estonia in 2003 (10). Type 2 VDPVs were isolated intermittently from sewage in Slovakia during October 2003--June 2004 and from a single sewage sample collected in Israel in April 2004. Reported by: Immunization, Vaccines, and Biologicals Dept, WHO, Geneva, Switzerland. Div of Viral and Rickettsial Diseases, National Center for Infectious Diseases; Global Immunization Div, National Immunization Program, CDC. Editorial Note:Data from the global polio laboratory network confirm the continuing polio-free status of the American, European, and Western Pacific regions. Timely confirmation of WPV transmission in the remaining countries where polio is endemic has been essential for planning and targeting of supplemental immunization activities. Characterization of WPV isolates through analysis of VP1 genetic sequences allows for tracing of transmission pathways and investigation of linkages among isolates. Sequence data indicate that WPVs detected in the majority of countries in the African region during 2003 and 2004 do not represent a resurgence of indigenous viruses in these locations but resulted from importations from a major WPV reservoir in northern Nigeria. The laboratory network has achieved a high quality of performance and accuracy, achieving the program standard of providing virology results for more than 80% of persons with AFP within 60 days of paralysis onset. To minimize reporting delays, the network routinely monitors and analyzes the timeliness of all stages of AFP case investigation, including sample collection, shipment, and testing. These analyses reveal that the logistics of sample and isolate shipment remain the biggest challenge to providing timely results. Shipping isolates between laboratories usually takes 5--7 days but can take substantially longer in certain locations. To improve the timeliness of isolate shipment, the network plans to make ITD testing available in laboratories in Côte d'Ivoire, Ibadan-Nigeria, and Senegal, which serve 14 African countries. As a result of enhanced surveillance efforts to identify the last remaining WPV transmission chains, several laboratories in regions where WPV is endemic have experienced substantial workload increases, necessitating additional resources to meet demands for culture supplies, equipment, and trained personnel. Policies for eventual cessation of oral poliovirus-vaccine (OPV) use depend on an assessment of VDPV risk. The laboratory network has a critical role in generating data to estimate the frequency of VDPVs and monitoring their ability to cause paralysis or to circulate. Cumulative data since 1999 suggest that approximately 0.5% of all Sabin-related isolates are classified as VDPVs. All VDPV isolates from any source should be investigated to identify either unrecognized circulation or the presence of a chronically infected immunodeficient person in the community. Investigation of reported VDPV isolates revealed immunodeficient persons with AFP from Thailand and Peru in 2003. These persons did not excrete VDPVs for prolonged periods; no VDPVs were isolated from their follow-up stool samples. Investigation of VDPVs in Slovakia has not revealed gaps in vaccination coverage nor identified paralyzed persons in the communities in which VDPVs were detected. Health officials are continuing efforts to identify the source of these viruses. Poliovirus surveillance should continue for >3 years after OPV cessation, implying that laboratory support might be needed through 2011. WHO has initiated discussions with national governments and partner agencies regarding the future of network laboratories. WHO is also pursuing greater government support of laboratories to facilitate the transition to other high-priority public health activities and to maximize the investments made in developing high-quality laboratory services. Continued involvement of national governments and partner agencies† is essential to sustain high-quality laboratory performance. References
* Genotype abbreviations: NEAF = Northeast Africa; WEAF-B = West Africa-B; SOAS = South Asia; EAAF = East Africa. † Rotary International, CDC, U.S. Agency for International Development, United Nations Foundation, Wyeth Lederle American Association for World Health, Japan International Cooperation Agency, Canadian International Development Agency (CIDA), Australian Agency for International Development, and various national governments, including Finland, Italy, and the Netherlands. Table 1  Return to top. Table 2 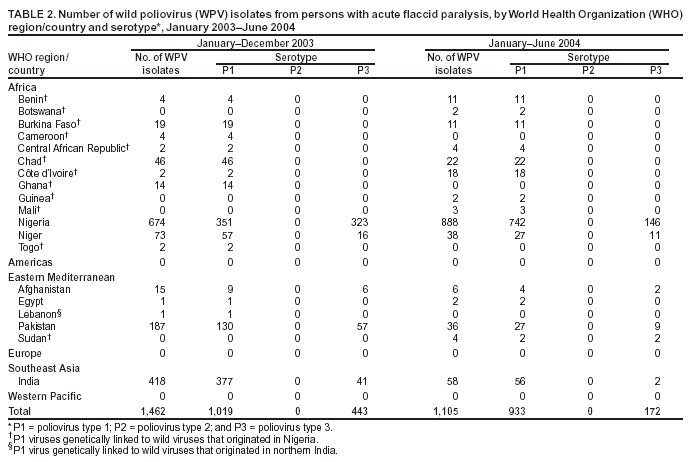 Return to top. Table 3 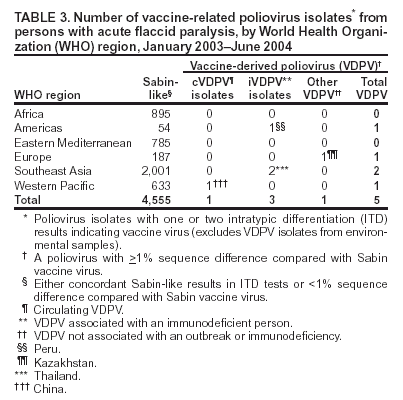 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 10/28/2004 |
|||||||||
This page last reviewed 10/28/2004
|