ISSN: 1080-6059
Volume 12, Number 10–October 2006
Dispatch
Fourth Human Parechovirus Serotype
Kimberley S.M. Benschop,*![]() Janke Schinkel,* Manon E. Luken,† Peter J.M. van den Broek,†
Matthias F.C. Beersma,‡ Negassi Menelik,§ Hetty W.M. van Eijk,* Hans L.
Zaaijer,* Christina M.J.E. VandenBroucke-Grauls,* Marcel G.H.M. Beld,* and
Katja C. Wolthers*
Janke Schinkel,* Manon E. Luken,† Peter J.M. van den Broek,†
Matthias F.C. Beersma,‡ Negassi Menelik,§ Hetty W.M. van Eijk,* Hans L.
Zaaijer,* Christina M.J.E. VandenBroucke-Grauls,* Marcel G.H.M. Beld,* and
Katja C. Wolthers*
*Academic Medical Center, Amsterdam, the Netherlands; †Primagen, Amsterdam, the Netherlands;
‡Leiden University Medical Center, Leiden, the Netherlands; and §BovenIJ
Ziekenhuis, Amsterdam, the Netherlands
Suggested citation for this article
We identified a novel human parechovirus (HPeV) type (K251176-02) from a neonate with fever. Analysis of the complete genome showed K251176-02 to be a new HPeV genotype. Since K251176-02 could not be neutralized with antibodies against known HPeV serotypes 1–3, it should be classified as a fourth HPeV serotype.
Infections with human parechoviruses (HPeVs) are commonly associated with mild gastrointestinal and respiratory symptoms in young children (1–3), but more severe conditions, such as flaccid paralysis (4) and encephalitis (5), have also been described. Recently, a new serotype (HPeV3) has been isolated, which has been associated with transient paralysis (6) and neonatal sepsis (7).
HPeV1 and HPeV2 were previously known as the enteroviruses echovirus 22 and 23 but were reclassified into a new genus within the family Picornaviridae after phylogenetic analysis showed that parechoviruses were distinct from other picornaviruses (1–3,8–11). HPeVs have predominantly been isolated from young children, and increasing evidence shows that HPeV can cause serious illness in these patients.
We recently showed that infection with HPeV3 is associated with younger age and more severe disease than is infection with HPeV1 (12). During the screening of patient samples, we identified 1 aberrant HPeV type. Phylogenetic analysis of the full-length sequence and viral neutralization assays showed that the isolate designated K251176-02 is a new HPeV genotype and serotype.
The Study
Viral culture of the stool of a 6-day-old patient with a 2-day history of high fever and poor feeding and no history of gastrointestinal or respiratory symptoms showed enterovirus cytopathic effects. However, PCR targeted at the 5´ untranslated region (UTR) of enterovirus (13) was negative, whereas a 5´ UTR PCR specific for HPeV (12) was positive.
Results of sequencing the VP1 region (12) suggested that K251176-02 was a novel HPeV genotype. Therefore, the full-length sequence was determined. Combinations of consensus primers were used to generate partially overlapping amplicons that covered the complete genome. Amplicons were sequenced according to a primer walking strategy. The 5´ UTR was amplified by using the 5´ RACE System (Invitrogen, Carlsbad, CA, USA). Because a primer composed of the first 22 nucleotides (nt) of published consensus parechovirus sequences was used to amplify the 5´ UTR proximal end, these 22 nt could not be determined with absolute certainty (8). The 3´ UTR end was amplified with a tagged oligo-dT primer.
The complete genome of K251176-02 was 7,348 nt long, containing a 5´ UTR of 708 nt, a large single open reading frame (ORF) of 6,549 nt, and a 3´ UTR of 91 nt followed by a poly(A) tract. The full-length sequence of K251176-02 has been deposited in GenBank under accession no. DQ315670.
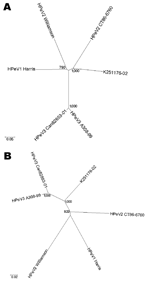 |
Figure 1. Unrooted phylogenetic trees showing the relationship between K251176-02 (DQ315670) and the prototype strains human parechovirus serotype 1... |
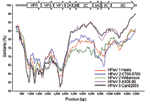 |
Figure 2. Similarity plot of human parechovirus serotype 1 (HPeV1) Harris (S45208), HPeV2 Williamson (AJ005695), HPeV2 CT86-6760 (AF055846)... |
We found a best-match nucleotide identity (14) of 72.2% in the VP1 gene with HPeV2 CT86-6760, which suggests that K251176-02 is most closely related to HPeV2 CT86-6760. Indeed, phylogenetic analysis of the capsid nucleotide sequence based on Jukes and Cantor distances showed K251176-02 to cluster with HPeV2 CT86-6760 (Figure 1A). However, the genetic distance was considerable (0.327) and comparable to the genetic distance between HPeV1 Harris and HPeV2 Williamson (0.332). Phylogenetic analysis of the nonstructural region showed that K251176-02 clustered with the HPeV3 prototypes A308-99 and Can82853-01 (Figure 1B).
To identify recombination events between the different HPeV prototypes, a SimPlot analysis was performed on the known full-length nucleotide HPeV genomes against K251176-02. The SimPlot analysis (Figure 2) showed the differential similarity of K251176-02 with HPeV2 CT86-6760 in the highly variable P1 region and with HPeV3 in the more conserved P2–P3 region. This finding may be the result of a recombination event.
The secondary structure of the 5´ UTR of K251176-02, determined by the Mfold program of Zuker and Turner (http://mfold2.wustl.edu), was predicted to be highly structured and was characterized by a stable hairpin at the proximal end that was also found in known HPeV prototypes (8,11, data not shown). The predicted secondary structure of the 3´ UTR of K251176-02 contained the same 1-stem loop organization as the HPeV prototypes and was similar to the secondary structure of HPeV1 Harris and HPeV2 Williamson and CT86-6760 (15).
A comparison of the complete ORF of K251176-02 with the HPeV prototypes showed an amino acid identity of 86.9% to 90.1% (Table 1). This amount is in the same range of amino acid identity as observed between known HPeV protoypes. For the VP1 gene, the greatest amino acid identity was observed with HPeV2 CT86-6760 (80.4%). In the nonstructural region, identity was greater to HPeV3, with 98.1% identity in the polymerase gene (3Dpol).
Comparison of the deduced amino acid sequence in the capsid region of K251176-02 with the HPeV prototypes showed that the sequences that are predicted to be part of the β-barrel structure (6,10,11) are well conserved in K251176-02. Like HPeV1 and HPeV2, K251176-02 also contained an RGD motif at the C-terminal end of the VP1 gene, which was absent in HPeV3 (6,7,15). K251176-02 also contained the common motifs X2GXGK(S/T) and DDLXQ (2C gene), which are predicted to have a helicase function. The active-site cysteine of the protease 3C is in the context of GXCG, and the active site of polymerase 3Dpol contains the conserved sequence YGDD. The well-conserved motifs within the 3Dpol gene (KDELR, PSG, and FLKR) were also found in K251176-02 (6,9,11).
In summary, K251176-02 represents a new genotype in the genus Parechovirus. To confirm that K251176-02 is also a new serotype, a neutralization assay was performed. Table 2 shows that K251176-02 could not be neutralized by antisera directed against HPeV1 Harris, HPeV2 Williamson, and HPeV3 A308-99, which confirms that K251176-02 is a new genotype that can be classified as a fourth HPeV serotype.
Conclusions
HPeVs are classified in the genus Parechovirus in the family Picornaviridae. The recently identified HPeV3 has been associated with severe illness in young children in several studies (6,7,12). This association has increased the awareness of HPeVs as relevant pathogens in young children.
We identified a new HPeV genotype in a stool specimen from a neonate with high fever. Since classification criteria based on genotyping have not been defined for HPeVs, we used the criteria proposed by Oberste et al. (14) for the classification of new enteroviral genotypes. According to these criteria, a new genotype is defined when a best-match nucleotide identity of <70% is found in the VP1 gene. A 70%–75% best-match nucleotide identity indicates further characterization is needed. Therefore, neutralization assays were conducted; these assays showed that K251176-02 did not neutralize with antisera directed against the 3 known HPeV serotypes. This finding indicates that K251176-02 is a new genotype that can be classified as a fourth HPeV serotype.
The patient from whom K251176-02 was isolated had high fever but no signs of neonatal sepsis, as has been found in infections with HPeV3 (6,7,12). Previous data suggest differences in severity of disease between the different HPeV serotypes (12); however, more data are needed to elucidate epidemiologic and pathogenic features of the different HPeV serotypes, including K251176-02.
HPeV2 CT86–6760 was genotypically as distinct from HPeV2 Williamson as from other HPeV types (Table 1). The existence of 2 genotypically divergent HPeV serotypes 2 is surprising and needs to be elucidated further. This finding, however, argues in favor of a universal typing method that is based on molecular characteristics (genotyping) instead of serotyping, provided classification criteria are well defined.
Acknowledgments
We thank Georgios Pollakis for his assistance in the phylogenetic analysis and for technical support in sequencing the full-length genome, Rene Minnaar for further technical support, and Hiroyuki Shimizu for antisera used in the neutralization assay.
This study was supported by the Department of Medical Microbiology at the Academic Medical Center, Amsterdam.
Ms Benschop is a PhD candidate who works at the Academic Medical Center, Amsterdam. Her primary research interests are the clinical and molecular epidemiology and pathogenesis of enteroviruses and human parechoviruses.
References
- Stanway G, Joki-Korpela P, Hyypia T. Human parechoviruses—biology and clinical significance. Rev Med Virol. 2000;10:57–69.
- Joki-Korpela P, Hyypia T. Parechoviruses, a novel group of human picornaviruses. Ann Med. 2001;33:466–71.
- Stanway G, Hyypia T. Parechoviruses. J Virol. 1999;73:5249–54.
- Figueroa JP, Ashley D, King D, Hull B. An outbreak of acute flaccid paralysis in Jamaica associated with echovirus type 22. J Med Virol. 1989;29:315–9.
- Koskiniemi M, Paetau R, Linnavuori K. Severe encephalitis associated with disseminated echovirus 22 infection. Scand J Infect Dis. 1989;21:463–6.
- Ito M, Yamashita T, Tsuzuki H, Takeda N, Sakae K. Isolation and identification of a novel human parechovirus. J Gen Virol. 2004;85:391–8.
- Boivin G. Human parechovirus 3 and neonatal infections. Emerg Infect Dis. 2005;11:103–5.
- Oberste MS, Maher K, Pallansch MA. Complete sequence of echovirus 23 and its relationship to echovirus 22 and other human enteroviruses. Virus Res. 1998;56:217–23.
- Hyypia T, Horsnell C, Maaronen M, Khan M, Kalkkinen N, Auvinen P, et al. A distinct picornavirus group identified by sequence analysis. Proc Natl Acad Sci U S A. 1992;89:8847–51.
- Stanway G, Kalkkinen N, Roivainen M, Ghazi F, Khan M, Smyth M, et al. Molecular and biological characteristics of echovirus 22, a representative of a new picornavirus group. J Virol. 1994;68:8232–8.
- Ghazi F, Hughes PJ, Hyypia T, Stanway G. Molecular analysis of human parechovirus type 2 (formerly echovirus 23). J Gen Virol. 1998;79:2641–50.
- Benschop KSM, Schinkel J, Minnaar RP, Pajkrt D, Spanjerberg L, Kraakman HC, et al. Human parechovirus infections in Dutch children and the association between serotype and disease severity. Clin Infect Dis. 2006;42:204–10.
- Beld M, Minnaar R, Weel J, Sol C, damen M, van der Avoort H, et al. Highly sensitive assay for detection of enterovirus in clinical specimens by reverse transcription–PCR with an armored RNA internal control. J Clin Microbiol. 2004;42:3059–64.
- Oberste MS, Michele SM, Maher K, Schnurr D, Cisterna D, Junttila N, et al. Molecular identification and characterization of two proposed new enterovirus serotypes, EV74 and EV75. J Gen Virol. 2004;85:3205–12.
- Abed Y, Boivin G. Molecular characterization of a Canadian human parechovirus (HPeV)-3 isolate and its relationship to other HPeVs. J Med Virol. 2005;77:566–70.
Figures
Figure 1. Unrooted phylogenetic trees showing the relationship between K251176-02
(DQ315670) and the prototype strains human parechovirus serotype 1...
Figure 2. Similarity plot of human parechovirus serotype 1 (HPeV1) Harris (S45208),
HPeV2 Williamson (AJ005695), HPeV2 CT86-6760 (AF055846)...
Tables
Table 1. Amino acid identity matrix of all known
human parechovirus (HPeV) full-length sequences
Table 2. Neutralization assay with LLcMk2 cells
Suggested Citation for this Article
Benschop KSM, Schinkel J, Luken ME, van den Broek PJM, Beersma MFC, Menelik N, et al. Fourth human parechovirus serotype. Emerg Infect Dis [serial on the Internet]. 2006 Oct [date cited]. Available from http://www.cdc.gov/ncidod/EID/vol12no10/05-1647.htm
Please use the form below to submit correspondence to the authors or contact them at the following address:
Kimberley S.M. Benschop, Department of Clinical Virology, Meibergdreef 15, 1105 AZ Amsterdam, the Netherlands; email: k.s.benschop@amc.uva.nl
Please note: To prevent email errors, please use no web addresses, email addresses, HTML code, or the characters <, >, and @ in the body of your message.
Please contact the EID Editors at eideditor@cdc.gov
This page posted September 5, 2006
This page last reviewed October 19, 2006
Centers for Disease Control and Prevention, 1600 Clifton Rd, Atlanta, GA 30333, U.S.A
Tel: (404) 639-3311 / Public Inquiries: (404) 639-3534 / (800) 311-3435