|
Center for Food Safety & Applied Nutrition Office of Food Additive Safety July 2001 |
DRAFT GUIDANCE
This guidance document is being distributed for comment purposes only.
Draft released for comment July 2001
Comments and suggestions regarding this draft document should be submitted within 60 days of publication in the Federal Register of the notice announcing the availability of the draft guidance. Submit Comments to the Dockets Management Branch (HFA-305), Food and Drug Administration, 5630 Fishers Lane, rm. 1061, Rockville, MD, 20852. All comments should be identified with the docket number listed in the notice of availability that publishes in the Federal Register.
For questions on the content of the draft document contact the Electronic Submissions Coordinator, Office of Food Additive Safety, Center for Food Safety and Applied Nutrition, E-Mail: elecsub@cfsan.fda.gov.
Additional contact information.
U.S. Department of Health and Human Services
Food and Drug Adminstration
Center for Food Safety and Applied
Nutrition
Office of Food Additive Safety
July 2001
This draft guidance represents FDA's current thinking on regulatory submissions in electronic format. It does not create or confer any rights for or on any person and does not operate to bind FDA or the public. An alternative approach may be used if such approach satisfies the requirement of applicable statutes and regulations. The draft guidance is being distributed for comment purposes in accordance with FDA's Good Guidance Practices (65 FR 56468; September 19, 2000).
TABLE OF CONTENTS
- INTRODUCTION
- ORGANIZING THE MAIN FOLDER
- Appendices
- Appendix A - Instructions for completing Application Forms
- Appendix B - Food Additive Petition Submission Application (available in Word Template and PDF)
- Appendix C - Color Additive Petition Submission Application (available in Word Template and PDF)
This is one in a series of guidance documents intended to assist applicants when making regulatory submissions in electronic format to the Office of Food Additive Safety (OFAS), Center for Food Safety and Applied Nutrition (CFSAN) of the Food and Drug Administration (FDA). OFAS will update guidance documents on electronic submissions periodically to reflect evolving technology and the experience of those using this technology.
This guidance discusses issues related to the electronic submission to OFAS of Food Additive Petitions (FAPs) and Color Additive Petitions (CAPs), and any supplements and amendments to those petitions. Guidance for Generally Recognized as Safe Notifications (GRNs), Biotechnology Notifications (BNFs), and Food Contact Substances Notifications (FCNs) will be published separately. For guidance on general issues relevant to electronic submissions for GRNs, FCNs, and BNFs, as well as FAPs and CAPs, refer to the companion guidance document, Providing Regulatory Submissions in Electronic Format - General Considerations.
If you have any questions regarding electronic submissions or requirements for submitting petitions, send an e-mail: elecsub@cfsan.fda.gov.
Title 21, Code of Federal Regulations (CFR), Section 171.1 for food additives and Section 71.1 for color additives provide general requirements for submitting petitions to the Office of Food Additive Safety (OFAS), CFSAN of FDA. The additional information prepared by OFAS to facilitate the preparation and submission of petitions is located at the CFSAN Web site: http://www.cfsan.fda.gov/~dms/opa-toc.html.
A. Refuse to File
OFAS may refuse to file a submission, amendment or supplement under 21 CFR 71.1 and 171.1, if either paper or electronic portions are incomplete. See 21 CFR 71.1(d), 171.1(g). Following this guidance document will help to ensure that your electronic submission meets the general requirements in 21 CFR 71.1 and 171.1 and can be easily archived, entered on our network drives, and reviewed within the specified time frame using our software tools.
B. Paper Copies
Under 21 CFR 71.1(c) and 171.1(c), petitioners must submit FAPs and CAPs in triplicate. However in the Federal Register of March 20, 1997 (62 FR 13467), the Agency announced the establishment of a docket, number 92S-0251, listing the type of submissions the Agency can accept in electronic format. When certain criteria are met, 21 CFR part 11 provides for both the submission of electronic records in lieu of paper records and the substitution of electronic signatures for "handwritten signatures…or general signings" in connection with such submissions. However, OFAS has not developed procedures sufficient to permit acceptance of electronic signatures at this time. Under 21 CFR 71.1(e) and 171.1(e), FAPs and CAPs must be signed. Therefore, after OFAS has indicated in the docket number 92S-0251, that it is accepting FAPs and CAPs in electronic format, a complete version of the petition in paper format ("paper copy"), including the signed application, must accompany any petition submitted in electronic format. When OFAS is prepared to accept electronic signatures, the docket number 92S-0251 will so indicate.
Any portion of the submission that is not provided in electronic format and submitted on paper only must be submitted in triplicate. See 21 CFR 71.1(c), 171.1(c). However, for any portion that is submitted in both paper and electronic format, the Agency intends to accept one copy in each format. Further, if a submission contains both electronic and paper sections, the index (commonly referred to as the table of contents) for the submission should include the location of information submitted as a paper copy. It should also identify the location of the electronic files by file and folder name. The location of information provided on paper only should be noted within the electronic submission through the use of a placeholder. The placeholder should direct the reviewer to the paper copy and provide the volume and page number of the submission.Whenever possible, the electronic copy of the petition should include all elements of the submission.
The following steps will help you assemble a paper copy:
The paper copy should be assembled in the same order as it appears in Table 1, Section III Part B.
If the documents are small, you may combine many in one volume, separating each document by a tab identifying the document.
Include a copy of the section's table of contents at the beginning of the technical section so that each document can be located by volume number. The table of contents should include the document name and the location of copy by volume(s). If the copy is electronic, you should provide the folder(s) and file name.
C. Supplements and Amendments
This guidance applies equally to the original submissions, supplements, and amendments to all of the Food Additive and Color Additive Petitions except the cover letter. If the submission is a supplement or amendment, the cover letter should be placed in the Administrative/Correspondence/Incoming Folder and the cover letter for the supplement or the amendment should include hypertext links to the data in the appropriate folder (e.g. Chemistry Folder, Safety Folder, etc.).
D. Electronic Signatures
OFAS is developing procedures to allow for the submission of electronic signatures. Until OFAS is prepared to accept electronic signatures, a complete paper copy, including the signed application, must accompany all FAPs and CAPs submitted in electronic format. OFAS will post a notice at docket 92S-0251 when it is prepared to accept electronic signatures.
Currently, the Food Additive Submission Application Form (Appendix B) and the Color Additive Petition Application Form (Appendix C) outline the components required for the submission of food additive or color additive petitions.
All electronic documents and datasets that are part of the electronic petition should be placed in a Main Folder entitled MAIN. After assigning a reference number to the petition, the reference number will be used as the title of the Main Folder. When information is submitted to a preexisting electronic submission, the assigned petition type and number will be used as the title of the Main Folder (e.g. FAP0B1234).
A. Submission Structure
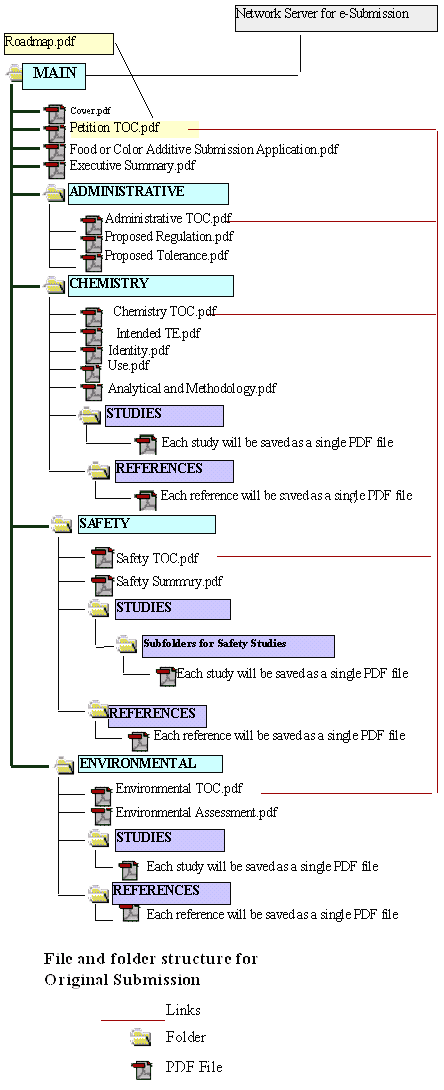
The structure and content of both electronic and paper submissions to OFAS should be based on the Application Form. Subsequent to the delivery of the original submission or supplement, any additional electronic and/or paper information will be added to the existing network based copy of the submission and distributed to appropriate reviewers. The root directory of an electronic petition should contain a roadmap.pdf file to direct the review team to the original submission and to all subsequent information added to the petition.
OFAS recommends that a roadmap.pdf file be used to establish hypertext links to the petition's main table of contents as well as associated folders and files of the submission. This "roadmap" should be updated and resubmitted as additional information is added to the application. An example of a roadmap format is shown in Figure 1.
The roadmap file will not contribute in any way to the content of the petition. It is merely a map, intended to facilitate navigation through the contents of the submission. The submission's roadmap.pdf file should be easily updated or modified using the "Replace file" command under the "Document" menu option in Adobe Exchange or Adobe Acrobat 4.0 or 5.0. This function automatically replaces the old hypertext links to previously submitted sections of the petition while still allowing creation of the new links corresponding to newly submitted information.

Figure 1: Example of Roadmap.pdf for Original Food Additive Petition Submission
B. Folders
Inside the Main Folder, all documents and datasets should be organized in accordance with the items described on page 2 of the Application Form (Appendices B and C). Each item should have an assigned subfolder where documents and datasets that belong to that item are placed. Table 1 lists these items and file/folder names.
| Item | Description | Folder/File Name |
|---|---|---|
| 1 | Cover Letter (Box 16 in Application Form) | Cover.pdf |
| 2 | Petition Table of Contents (Box 16 in Application Form) | Petition TOC.pdf |
| 3 | Application Form | Application.pdf |
| 4 | Executive Summary (Box 16 in Application Form) | Executive Summary.pdf |
| 5 | Administrative Section (Box 19 in Application Form) | Administrative Folder |
| 6 | Chemistry Information (Box 17 in Application Form) | Chemistry Folder |
| 7 | Safety Information (Box 18 in Application Form) | Safety Folder |
| 8 | Environmental Information (Box 20 in Application Form) | Environmental Folder |
Figure 2 is an example of the contents included in the Main Folder. Each item from Table 1 appears as a separate folder and a file.
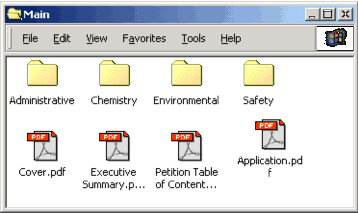
Figure 2: Example of the contents of the Main folder
C. Item 1: Cover Letter
Inside the MAIN folder, you should provide a cover letter as a PDF file named cover.pdf. This cover letter should be included with any paper only portion of the archival copy. The cover letter should include the following items:
A description of the submission including applicable regulatory information.
A listing of the submitted sections filed as paper, filed only in electronic format, or in both paper and electronic formats.
A description of the electronic submission including the type and number of electronic media used (e.g., two sets of three CD-ROMs). The approximate size of the submission (e.g., 2 GB), including the format used for DLT tapes.
A statement that the submission is virus free with a description of the software (name, version and company) used to check the files for viruses.
The regulatory and information technology points of contact for the application to include names, telephone numbers, and electronic mail addresses if available.
D. Item 2: Petition Table of Contents
Each electronic submission should provide a comprehensive table of contents that will include the appropriate hypertext links. The Petition Table of Contents is simply a list of the items as shown on page 2 (Boxes 16 through 20) of the Application Forms. It should be a single PDF file. The file containing the table of contents of an original submission for the petition should be named Petition TOC.pdf (See Table 2, below). It should be placed inside of Main Folder.
When an item is in paper format only, the volume numbers that hold the item should be listed in the table of contents. When items in a petition are in both paper and electronic format, both the volume number and the folder name should be listed in the table of contents.
| Item | Description | Paper copy volume number | Electronic copy folder |
|---|---|---|---|
| 1 | Cover Letter | 1 | Main Folder |
| 2 | Petition Table of Contents | 1 | Main Folder |
| 3 | Application | 1 | Main Folder |
| 4 | Executive Summary | 1 | Main Folder |
| 5 | Administrative | N/A | Administrative Folder |
| 6 | Chemistry | N/A | Chemistry Folder |
| 7 | Safety | N/A | Safety Folder |
| 8 | Environmental | N/A | Environmental Folder |
A hypertext link should be provided from the petition table of contents to the corresponding tables of contents for each item (e.g., Chemistry, Safety, Administrative, and Environmental). The table of contents for a particular item should appear within that item's folder. Within the item's table of contents, hypertext links should be included for the Petition TOC.pdf.
Items 1, 2, 3, and 4 in Table 2 are single documents and do not have their own table of contents. In such cases, the hypertext link from the first level table of contents should go directly to the document.
E. Item 3: Food or Color Additive Submission Application
You should provide the Application as a PDF file named application.pdf inside the Main Folder. See Appendix B for a Food Additive Petition Submission Application or Appendix C for a Color Additive Petition Submission Application. On page 2 of both Application Forms, you should note, next to each item, in what format the documents reside, paper format, or both paper and electronic format. The applicant should follow the format of the electronic submission on page 2 of the Application Form and indicate with a check mark next to each item if the documents are included in the submission. The paper copy must also include a signed application.
F. Item 4: Executive Summary
This is not required by 21 CFR 71.1 or 171.1. However, if an executive summary is included in your submission, it should be placed inside the Main Folder. This is a single PDF file and named as Executive Summary.pdf. Executive summaries help the agency to understand the petition request and, therefore, help to facilitate review of the petition.
An electronic copy of the application should contain the documents and datasets corresponding to the items listed on page 2 (Boxes 16 through 20 of the Food or Color Application Form).
A. Item 5: Administrative Subfolder
All letters to OFAS for the submission should be placed in a single folder named Administrative except for the cover letter for the original submission. If the submission is a supplement or amendment, the cover letter should be placed in a subfolder named Incoming within the subfolder named Correspondence. Examples of the organization of the Administrative folder for original submission and supplement are pictured below (Figure 3).
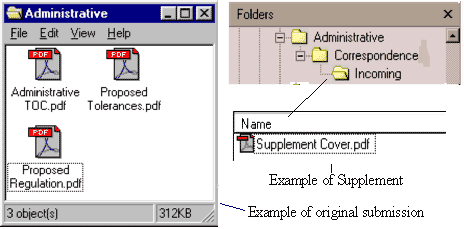
Figure 3: Example of Administrative Folder for Food Additive Petition
1. Administrative Table of Contents (Administrative TOC.PDF)
A table of contents listing all files provided in the Administrative item should be included as a PDF file named Administrative TOC.pdf. You should use the example headings below to organize the files in the table of contents. You should provide the location of the paper copy by volume number and the location of the electronic copy by folder and file name. As part of the table of contents for the submission, a hypertext link should be created between the documents listed in the table of contents and the corresponding PDF file and bookmarks for each item in the table of contents. You should place the Administrative TOC.pdf file in the Administrative folder.
All files in the Administrative Folder should have hypertext links from Administrative TOC.pdf. If a submission is a supplement or amendment, hypertext links to appropriate data should be created.
1a. Bookmarks and Hypertext links
For all documents with a table of contents, you should provide bookmarks for each item in the document's table of contents including all tables, figures, references, and appendices including datasets, if applicable. These bookmarks and hypertext links serve as part of the comprehensive table of contents for submission.
Hypertext links provided throughout the body of the document to supporting annotations, related sections, publications, appendices, tables, or figures that are not located on the same page help the user to navigate more efficiently through a document. For a reference list at the end of the document, a hypertext link should be provided at the end of the document to the appropriate PDF files in References Folder.
1b. Dataset Format
Each dataset should be provided as an SAS transport file as described in the companion guidance, "Providing Regulatory Submissions to OFAS in Electronic Format - General Considerations." Data is imported using various software tools. The most commonly used tools include: database programs, spreadsheet programs, and statistical analysis programs.
Many of the software tools used by the reviewers require datasets to be loaded into random access memory (RAM) prior to opening the file. Therefore, dataset files should be organized so the size is less than 50 MB per file. The files should not be compressed. Each dataset should be saved as an individual file and the name of the file will not exceed 8 characters in length.
| File/Folder Name | Description | Petition Type |
|---|---|---|
| Administrative TOC.pdf (2) | Administrative Table of Contents | CAP, FAP |
| Proposed Tolerances.pdf | 21 CFR 171.1(c) F; 71.1(c) F | CAP, FAP |
| Proposed Regulation.pdf | 21 CFR 171.1(c) G; 71.1(c) H | CAP, FAP |
| Batch Certification.pdf
Or Information to Exempt.pdf |
21 CFR 71.1(c) G | CAP |
| Prescribed Fee.pdf | 21 CFR 71.1(c) I | CAP |
2. Proposed Tolerances.PDF
A Proposed Tolerance.pdf must be provided if tolerances are required in order to ensure its safety.(3) (21 CFR 171.1(c) F; 71.1(c) F)
3. Proposed Regulation.PDF
A proposed regulation may be included (Proposed Regulation.pdf). If a petition is submitted to modify an existing regulation issued pursuant to section 409(c)(1)(A) of the Federal Food, Drug and Cosmetic Act, full information on each proposed change that is to be made in the original regulation must be submitted. (21 CFR 171.1(c) G; 71.1(c) H)
4. Batch Certification.PDF / Information to Exempt.PDF
If exemption from batch certification is requested, you must include the reasons such certification is not necessary, including supporting data to establish the safety of the intended use. (21 CFR 71.1(c) G)
5. Prescribed Fee.PDF
You must state either that the prescribed fee of $_______ for admitting the color additive to listing is enclosed or that there is an advance deposit adequate to cover the fee. (21 CFR 71.1(c) I)
B. Item 6: Chemistry Subfolder
OFAS has published several recommendations to assist petitioners in the preparation of the chemistry portion of petitions. They are available at the CFSAN web site, Chemistry Guidance Documents, Chemistry Reference Documents and Guidance for Industry - Preparation of Premarket Notification for Food Contact Substances: Chemistry Recommendation. These recommendations will assist applicants to organize studies in the Studies folder.
Two folders should be created to organize files in the Chemistry folder: Studies and References. The folder structure is pictured in the Figure 4.
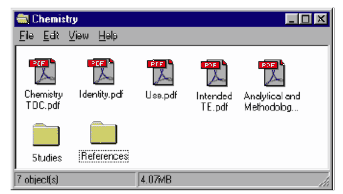
Figure 4: Example of Chemistry Folder
1. Chemistry Table of Contents (Chemistry TOC.PDF)
A table of contents listing all files should be provided in the Chemistry folder as a PDF file named Chemistry TOC.pdf. You should use the example headings below (Table 4) to organize the files in the table of contents. Also, you should provide the location of the paper copy by volume number and the location of the electronic copy by folder and file name. As part of the comprehensive table of contents for the submission, you should provide a hypertext link between the documents listed in the table of contents and the corresponding PDF file and bookmarks for the each item in the table of contents. The Chemistry TOC.pdf file should be placed in the Chemistry folder.
1a. Bookmarks and Hypertext links
For all documents with a table of contents, you should provide bookmarks for each item in the document's table of contents including all tables, figures, references, and appendices including datasets, if applicable. These bookmarks and hypertext links serve as part of the comprehensive table of contents for submission.
Hypertext links provided throughout the body of the document to supporting annotations, related sections, publications, appendices, tables, or figures that are not located on the same page help the user to navigate more efficiently through a document. For a reference list at the end of the document, a hypertext link should be provided at the end of the document to the appropriate PDF files in References Folder.
1b. Dataset Format
Each dataset should be provided as an SAS transport file as described in the companion guidance, "Providing Regulatory Submissions to OFAS in Electronic Format - General Considerations." Data is imported using various software tools. The most commonly used tools include: database programs, spreadsheet programs, and statistical analysis programs.
Many of the software tools used by the reviewers require datasets to be loaded into random access memory (RAM) prior to opening the file. Therefore, dataset files should be organized so the size is less than 50 MB per file. The files should not be compressed. Each dataset should be saved as an individual file and the name of the file will not exceed 8 characters in length.
| File/Folder Name | Description | Petition Type |
|---|---|---|
| Chemistry TOC.pdf | Chemistry Table of Contents | CAP, FAP |
| Identity.pdf | Chemical Identity, 21 CFR 171.1 (c) A; 71.1(c) A | CAP, FAP |
| Use.pdf | Chemical Use, 21 CFR 171.1 (c) B; 71.1 (c) B | CAP, FAP |
| Intended TE.pdf | Intended Technical Effect, 21 CFR 171.1 (c) C; 71.1 (c) B | CAP, FAP |
| Analytical and Methodology.pdf | Analytical and Methodology, 21 CFR 171.1(c) D; 71.1 (c) C | CAP, FAP |
| Studies | Folder | CAP, FAP |
| References | Folder | CAP, FAP |
2. Identity.PDF
This file must contain information concerning the food or color additive including:
The name and all pertinent information concerning the additive, including chemical identity and composition of the food or color additive.
The additive's physical, chemical, and biological properties.
Specifications prescribing the minimum content of the desired component(s) and specifications that identify and limit the reaction byproducts and other impurities.
Stability data, and, if the data indicate that it is needed to insure the identity, strength, quality, or purity of the color or food additive, the expiration period that will be employed as well as any packaging and labeling precautions needed to preserve stability (21 CFR 171.1(c) A; 71.1 (c) A).
3. Use.PDF
You must include information regarding the chemical use and detail the amount of the food or color additive proposed for use, the type of food or product in which the additive is to be used, and the purposes for which it is proposed. The Use.pdf must also include all directions, recommendations, and suggestions regarding the proposed use, as well as specimens of the labeling proposed for the food additive and any labeling that will be required by applicable provisions of the Federal Food, Drug, and Cosmetic Act on the finished food by reason of the use of the food or color additive (21 CFR 171.1(c) B; 71.1(c) B).
4. Intended TE.PDF
You must include information regarding data establishing that the food additive will have the intended physical or other technical effect or that it may reasonably be expected to become a component, or to affect the characteristics, directly or indirectly, of food and the amount necessary to accomplish this or the amount of the color additive proposed for use and the color effect intended to be achieved. These data should include information in sufficient detail to permit evaluation with control data. This information should be a single PDF file and placed in the Chemistry folder (21 CFR 171.1(c) C; 71.1 (c) B).
5. Analytical and Methodology.PDF
You must include a description of practicable methods to determine the amount of the food or color additive in the raw, processed, and/or finished food or product and, for food additives, any additional description of any substance formed in or on such food or product because of its use. The test proposed must be one that can be used for control purposes and that can be applied with consistent results by any properly equipped and trained laboratory personnel. (21 CFR 171.1(c) D; 71.1(c) C)
6. Studies
Each study report should be provided as a separate PDF file. All studies should be placed into a single folder named Studies. The Studies Folder should be placed in the Chemistry Folder. The PDF file name will be the same as the title of the study. A file name can contain up to 80 characters, including spaces. It cannot contain any of the following characters: \ / : * ? " < > | " % # +.
Because files sized 50 Megabytes (MB) or larger are technically more difficult to handle, study reports that are larger than 50 MB should be divided into two PDF files. The first file will include the body of the study report, the reference list, and the tables and figures that follow the study report. The second file should include all of the protocol, all amendments, and all remaining appendices from the main file. Add the number one (1) to the end of the file name to distinguish the file with the appendices from the main file.
Figure 5: Example of the Studies Folder
7. References
You should provide each reference as a separate PDF file. You should place all references into a single folder named References. The References Folder should be placed in the Chemistry Folder. The PDF file name should be the document's title. A filename can contain up to 80 characters, including spaces. It cannot contain any of the following characters: \ / : * ? " < > | " % # +. A hyperlink should be established between the reference list in reports and the references in the References Folder. All pages in each reference should be legible and complete.
Figure 6: Example of References Folder
C. Item 7: Safety Subfolder
Full reports of investigations on the safety of the food additive or color additive should be placed in a folder named Safety.
Two folders should be created, References and Studies to organize the files in this section. Place these folders in a single folder named Safety. The file structure is pictured in the figure below (Figure 7)
Figure 7: Example of Safety Folder
1. Safety Table of Contents (Safety TOC.PDF)
A table of contents is required listing all files provided in the Safety item as a PDF file named Safety TOC.pdf. You should use the example headings below to organize the files in the table of contents. The location of the paper review copy by volume number and the location of the electronic copy by folder and file name is required (See Guidance for Industry, Providing Submissions to OFAS in Electronic Format - General Considerations - VIII). As part of the comprehensive table of contents for the submission, a hypertext link between the documents listed in the table of contents and the corresponding PDF file and bookmarks for the each item in the table of contents should be created. You should place the Safety TOC.pdf file in the Safety Folder.
1a. Bookmarks and Hypertext links
For all documents with a table of contents, you should provide bookmarks for each item in the document's table of contents including all tables, figures, references, and appendices including datasets, if applicable. These bookmarks and hypertext links serve as part of the comprehensive table of contents for submission.
Hypertext links provided throughout the body of the document to supporting annotations, related sections, publications, appendices, tables, or figures that are not located on the same page help the user to navigate more efficiently through a document. For a reference list at the end of the document, a hypertext link should be provided at the end of the document to the appropriate PDF files in References Folder.
1b. Dataset Format
Each dataset should be provided as an SAS transport file as described in the companion guidance, "Providing Regulatory Submissions to OFAS in Electronic Format - General Considerations." Data is imported using various software tools. The most commonly used tools include: database programs, spreadsheet programs, and statistical analysis programs.
Many of the software tools used by the reviewers require datasets to be loaded into random access memory (RAM) prior to opening the file. Therefore, dataset files should be organized so the size is less than 50 MB per file. The files should not be compressed. Each dataset should be saved as an individual file and the name of the file will not exceed 8 characters in length.
| File/Folder Name | Description | Petition Type |
|---|---|---|
| Safety TOC.pdf | Safety Table of Contents | CAP, FAP |
| Safety Summary.pdf | Summary of studies placed in Sudies folder (or Vol. X of paper copy) | CAP, FAP |
| Studies
(4)
may include subfolders |
Folder, 21 CFR 171.1 (c) E; 71.1 (c) D | CAP, FAP |
| References | Folder | CAP, FAP |
2. Safety Summary.PDF
A summary document named Safety Summary.pdf for studies should be included in the Studies Folder.
3. Studies (21 CFR 171.1(c) E; 71.1 (c) D)
Information regarding this file will cover a full report of food or color additive safety for its intended use as described in 21 CFR 71.1 and 171.1. This information will be included in a single PDF file and placed in the appropriate Folders of the Studies Folder.
All of the studies regarding Safety of the chemical should be placed inside the Studies' Subfolders. These subfolders are created based on Redbook 2000 and OFAS recommends the list of studies below for Safety Data:
Inside the Studies Folder, the studies should be placed in the above categories. These study categories are subfolders inside the Studies Folder (See Figure 8). The categories should include the subfolders' name and placed in the Studies Folder inside the Safety Folder.
Each study report is a single PDF file and placed into the appropriate subfolder (See Figure 9).
Figure 8: Example of Studies Folder
Figure 9: Example of Metabolism and Pharmacokinetic
Studies Folder of Food Additive Submission
Because files 50 MB or larger are technically more difficult to handle, study reports that are larger than 50 MB should be divided into two PDF files. The first file will include the body of the study report, the reference list, the tables and the figures that follow the study report. The second file will include all of the protocol, all amendments, and remaining appendices from the main file. The number one (1) will be added to the end of the file name to distinguish the file with the appendices from the main file.
4. References
Each reference should be provided as a separate PDF file. You should place all references into a single folder named References. The References Folder should be placed in the Safety Folder. The PDF file name should be the document's title. A file name can contain up to 80 characters including spaces. It cannot contain any of the following characters: \ / : * ? " < > | " % # +. Establish a hyperlink between the reference list in reports and the references in the References Folder. All pages in each reference should be legible and complete.
D. Item 8: Environmental Subfolder
Two folders should be created, References and Studies to organize the files in this section. These folders should be placed in a single folder called named Environmental. An example file structure is pictured in the figure below (Figure 10).
Figure 10: Example of Environmental Folder
1. Environmental Table of Contents (Environmental TOC.PDF)
A table of contents should be provided listing all files provided in the Environmental item as a PDF file named Environmental TOC.pdf. You should use the example headings below to organize the files in the table of contents.
1a. Bookmarks and Hypertext links
For all documents with a table of contents, you should provide bookmarks for each item in the document's table of contents including all tables, figures, references, and appendices including datasets, if applicable. These bookmarks and hypertext links serve as part of the comprehensive table of contents for submission.
Hypertext links provided throughout the body of the document to supporting annotations, related sections, publications, appendices, tables, or figures that are not located on the same page help the user to navigate more efficiently through a document. For a reference list at the end of the document, a hypertext link should be provided at the end of the document to the appropriate PDF files in References Folder.
1b. Dataset Format
Each dataset should be provided as an SAS transport file as described in the companion guidance, "Providing Regulatory Submissions to OFAS in Electronic Format - General Considerations." Data is imported using various software tools. The most commonly used tools include: database programs, spreadsheet programs, and statistical analysis programs.
Many of the software tools used by the reviewers require datasets to be loaded into random access memory (RAM) prior to opening the file. Therefore, dataset files should be organized so the size is less than 50 MB per file. The files should not be compressed. Each dataset should be saved as an individual file and the name of the file will not exceed 8 characters in length.
| File/Folder Name | Description | Petition Type |
|---|---|---|
| Environmental TOC.pdf | Environmental Table of Contents | CAP, FAP |
| Environmental Assessment.pdf
Or Categorical Exclusion.pdf |
21 CFR 171.1 (c) H; 71.1 (c) J | CAP, FAP |
| Studies | Folder | CAP, FAP |
| References | Folder | CAP, FAP |
2. Environment Assessment.PDF/ Categorical Exclusion.PDF
A claim can be submitted for categorical exclusion under 21 CFR 25.30 or 25.32 or an environmental assessment under 25.40. (21 CFR 171.1 (c) H; 71.1 (c) J) This should be a single PDF file and hypertext linked from Environmental TOC.pdf.
3. Studies
Each study report should be provided as a separate PDF file. You should place all studies into a single folder named Studies. The Studies Folder will be placed in the Environmental Folder. The PDF file name will be the same as the study's title. A filename can contain up to 80 characters, including spaces; however, it cannot contain any of the following characters: \ / : * ? " < > | " % # +.
Because files 50 MB or larger are technically more difficult to handle, study reports that are larger than 50 MB should be divided into two PDF files. The first file should include the body of the study report, the reference list, the tables and figures that follow the study report. The second file should include all of the protocol, all amendments, and remaining appendices from the main file. Add the number one (1) to the end of the file name to distinguish the file with the appendices from the main file.
Each file should be hypertext linked from the Environmental TOC.pdf and contain bookmarks for each section. These bookmarks should replace the table of contents in each study.
4. References
Each reference should be provided as a separate PDF file. You should place all references into a single folder named References. The References Folder should be placed in the Environmental Folder. The PDF file name should be document's title. A filename can contain up to 80 characters, including spaces; however, it cannot contain any of the following characters: \ / : * ? " < > | " % # +. Establish a hyperlink between the reference list in reports and the references in the References Folder. All pages in each reference should be legible and complete.
BOXES 1 - 6: APPLICANT INFORMATION
This section should include the name, street address, telephone and facsimile numbers of the legal person or entity submitting the application in the appropriate areas. The name, street address and telephone number of the legal person or entity authorized to represent a non-U.S. Applicant should be entered in the indicated section.
BOXES 7 - 11: SUBMISSION DESCRIPTION
This section should include all of the information necessary to identify the product that is the subject of this submission.
ADDITIVE FUNCTION: Check the appropriate box.
PRODUCT: Any product categories in which the additive should be used, e.g. human food.
TECHNICAL EFFECT: Technical effect of the additive, e.g. emulsifier.
CHEMICAL IDENTITIES: Enter up to 10 chemicals used in the additive.
Chemical Type: Enter P for primary chemical or C for components.
CAS Number: Insert nine (9) digit CAS number, if the CAS number is 111-11-1 then put 000111111.
Chemical Name: Insert chemical name. If the space in the application is not enough space to put chemical name, insert the page number of the chemical name listed and create a hypertext link to the page.
Trade Name: Insert trade name if the chemical has one.
Structure: Insert page number of the chemical structure and create a hypertext link to the page.
BOXES 12 - 23: APPLICATION INFORMATION
TYPE OF SUBMISSION Indicate by checking the appropriate box:
New Additive Petition: A complete new petition that has never before been submitted.
Amendment: All submissions to pending original petition, or pending supplements to approved petition, including responses to information Request Letters.
Supplement: All supplements.
Other: Any submission that does not fit in one of the other categories. If this box is checked the type of the submission can be explained in the REASON FOR SUBMISSION block.
REASON FOR SUBMISSION This section should contain a brief explanation of the submission.
NUMBER OF VOLUMES SUBMITTED Please enter the number of volumes, contained in the paper copy of this submission.
THIS SUBMISSION IS Please check the appropriate box to indicate whether this submission contains only paper or both paper and electronic media.
- 20. THIS APPLICATION CONTAINS THE FOLLOWING ITEMS This constitutes a check list that should be used to indicate the types of information contained within a particular submission. Please check all that apply.
-23. SIGNATURE The paper copy of the form should be signed and dated by an agent or official authorized by the applicant to represent the applicant to the Agency. The agent's typed name, and title should be provided in the areas indicated.
BOXES 1 - 6: APPLICANT INFORMATION
This section should include the name, street address, telephone and facsimile numbers of the legal person or entity submitting the application in the appropriate areas. The name, street address and telephone number of the legal person or entity authorized to represent a non-U.S. Applicant should be entered in the indicated section.
BOXES 7 - 11: SUBMISSION DESCRIPTION
This section should include all of the information necessary to identify the product that is the subject of this submission.
ADDITIVE FUNCTION: Check the appropriate box.
PRODUCT: Any product categories in which the additive should be used, e.g. human food.
FEE ENCLOSED: Check the appropriate box.
CHEMICAL IDENTITIES: Enter up to 10 chemicals used in the additive.
Chemical Type:Enter P for primary chemical or C for components.
CAS Number: Insert nine (9) digit CAS number, if the CAS number is 111-11-1 then put 000111111.
Chemical Name: Insert chemical name. If the space in the application is not enough space to put chemical name, insert the page number of the chemical name listed and create a hypertext link to the page.
Trade Name: Insert trade name if the chemical has one.
Structure: Insert page number of the chemical structure and create a hypertext link to the page.
BOXES 12 - 23: APPLICATION INFORMATION
TYPE OF SUBMISSION Indicate by checking the appropriate box:
New Additive Petition: A complete new petition that has never before been submitted.
Amendment: All submissions to pending original petition, or pending supplements to approved petition, including responses to information Request Letters.
Supplement: All supplements.
Other: Any submission that does not fit in one of the other categories. If this box is checked the type of the submission can be explained in the REASON FOR SUBMISSION block.
REASON FOR SUBMISSION This section should contain a brief explanation of the submission.
NUMBER OF VOLUMES SUBMITTED Please enter the number of volumes, contained in the paper copy of this submission.
THIS SUBMISSION IS Please check the appropriate box to indicate whether this submission contains only paper or both paper and electronic media.
- 20. THIS APPLICATION CONTAINS THE FOLLOWING ITEMS This constitutes a check list that should be used to indicate the types of information contained within a particular submission. Please check all that apply.
-23. SIGNATURE The paper copy of the form should be signed and dated by an agent or official authorized by the applicant to represent the applicant to the Agency. The agent's typed name, and title should be provided in the areas indicated.
This guidance was prepared by the Office of Food Additive Safety (OFAS), Center for Food Safety and Applied Nutrition (CFSAN) at the Food and Drug Administration (FDA). This guidance document represents the Agency's current thinking on regulatory submissions in electronic format. The current document is being published by the OFAS. It does not create or confer any rights for or on any person and does not operate to bind FDA or the public. An alternative approach may be used if such approach satisfies the requirements of the applicable statute and regulation.
If there are additional correspondences, a subfolder should be created under the Administrative folder named Correspondence. The cover letter for a supplement or amendment should be placed in the Administrative folder under the Correspondence subfolder. Subfolders should then be created under Correspondence for upcoming and outgoing letters, etc.
As used in this section of the guidance, "must" refers to a regulatory requirement that may be fulfilled in electronic format, in addition to paper format.
Office of Food Additive Safety
HFS-200
200 C Street, SW
Washington, DC 20004
(Tel) 202-418-3100**
(Internet) http://www.cfsan.fda.gov/~dms/opa-toc.html
** New address and telephone number:
5100 Paint Branch Parkway
College Park, MD 20740-3835
301-436-1200
Hypertext updated by bap/emw/cjm 2006-FEB-21