

Guideline No. 45
Guideline for Uniform Labeling of Drugs for Dairy and Beef Cattle
Revised August 1993
U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES
PUBLIC HEALTH SERVICE
FOOD AND DRUG ADMINISTRATION
CENTER FOR VETERINARY MEDICINE
This Guideline represents the agency's position on a
procedure or practice at the time of its issuance. This
Guideline is not a legal requirement. A person may follow the
Guideline or may choose to follow alternative procedures or
practices. If a person chooses to use alternate procedures or
practices that person may wish to discuss the matter further
with the FDA/CVM to prevent an expenditure of money and
effort on activities that may later be determined to be
unacceptable. This Guideline does not bind the agency, and it
does not create or confer any rights, privileges, immunities,
or benefits for or on any person. When a Guideline states a
requirement imposed by statute or regulation, however, the
requirement is law and its force and effect are not changed
in any way by virtue of its inclusion in the guideline.
FORWARD
INTRODUCTION
The objective of this uniform labeling initiative is to
promote standard labeling that will be uniformly understood
and interpreted by the user of dairy and beef cattle
veterinary pharmaceuticals. Informational symbols are the
tools that will be used to meet this objective. Symbolic
representation of the following informational categories are
presented in this document:
1. Prescription versus Over-the-Counter marketing status
2. Indications for Use by Class of Cattle
3. Milk discard times
4. Slaughter Withholding Times
This document is intended for the cooperative use of both
the regulatory agencies and the animal pharmaceutical
industry. The intent of this guideline, through its voluntary
use, is to further control and reduce milk and meat residue
violations and further promote the labeled use of dairy and
beef cattle drugs. Labeling changes to incorporate the
uniform labeling set out in this guideline are voluntary. Any
revisions to the currently approved labeling, however, must
be the subject of a supplemental new animal drug application
and approved prior to use.
GENERAL REGULATIONS AND CONSIDERATIONS
The labeling requirements for both over-the-counter (OTC) and
prescription (Rx) drugs are found in the Code of Federal
Regulations Title 21. These requirements may differ,
depending on the specific drug. As an aid to the user of this
guideline, a summary of these regulations follows.
Section 21 CFR Summary
§ 1.24(b) Exempts veterinary injectable OTC drugs from the
requirement of labeling contents in terms of the U.S.
gallon, quart, pint and ounce, but rather,
permits the net contents to be expressed in terms of
liters, milliliters, or cubic centimeters. While
this regulation provides for use of "cubic
centimeters," the Center prefers use of milliliter"
to express liquid quantities.
§ 201.1 Provides for name and place of business of
manufacturer, packer or distributor.
§ 201.2 Provides for NDC number.
§ 201.5 Discusses adequate directions for use on the label
of an OTC drug.
§ 201.17 Provides for the location of the Expiration Date.
§ 201.18 Explains the significance of the Lot Number.
§ 201.51 Net contents labeling requirements for a
prescription drug.
§ 201.62 Net contents labeling requirements for an OTC drug.
§ 201.105 Veterinary prescription drug labeling requirements.
NOTE: The prescription veterinary product must bear
the legend: "Caution: Federal law restricts this
drug to use by or on the order of a licensed
veterinarian".
§ 211.137 Provides for the product expiration date.
§ 211.130(b) Provides for the Lot Number.
§ 329.10(d)(2) Labeling for chlorobutanol used as a preservative.
§ 369.9 Warnings to keep the drug from children.
§ 510.105 Milk discard provisions for drugs used in dairy
animals.
§ 510.106 Milk discard provisions for antibiotic-containing
drugs intended for the use in milk producing dairy
cattle.
These are the regulations which contain the primary labeling
rules enforced by the FDA. There are additional regulations
issued by other agencies and individual states which may
apply to drugs used in dairy cattle. Examples include the
container disposal statement required by EPA, and the special
statements required for registration by the State of
California. Where warning statements are required because of
state laws such as, "Restricted Drug-Use Only As Directed",
it is recommended that the name of the state requiring such a
restriction, be placed in parenthesis, e.g., "Restricted Drug
(California)-Use Only As Directed."
CVM POLICY GUIDANCE
The Center for Veterinary Medicine (CVM) has additional
guidance pertaining to labeling criteria.
1. Policy and Procedure Manual Guide 1240.4000 entitled
"Identification/Promotion of Product Approval" provides for
the label and package labeling to bear the statement,
"NADA ..... #, Approved by FDA."
2. Statements such as "For Veterinary Use Only", "Sales to
Graduate Veterinarians Only" and "Sold only to Veterinarians"
are often confused with the veterinary prescription legend.
These statements should not appear on labels or package
labeling. Alternatively, statements such as "For Animal Use
Only" or "Not for Human Use" are acceptable.
3. Any sulfamethazine - containing product intended for use
in dairy cattle should bear the label statement "Not for use
in female dairy cattle over 20 months of age."
SYMBOLIC REPRESENTATION AND INFORMATION LOCATION
A. Geometric Forms
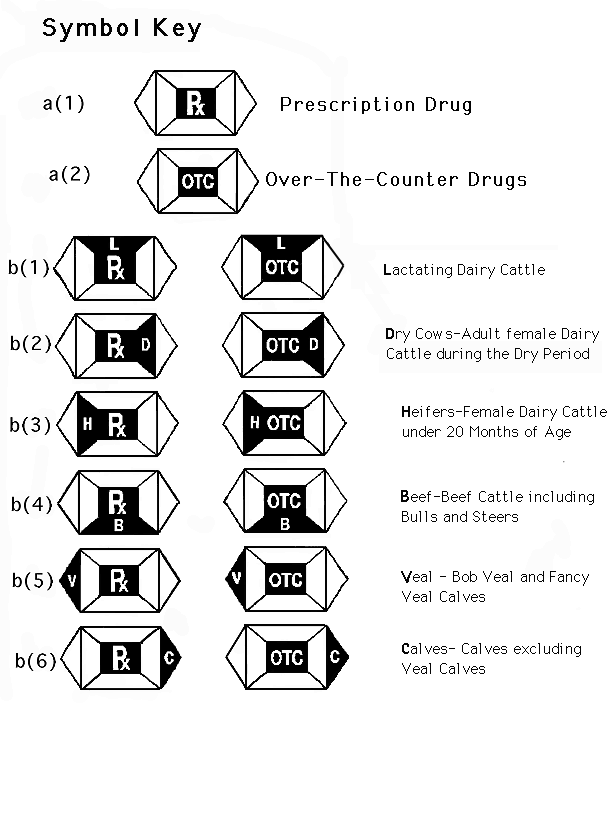
1. DIVIDED SQUARE FORMS
a. Over-the-Counter versus Prescription Designation
(1) Over-the-counter, designated by "OTC".
(2) Prescription, designated by "Rx".
(3) The Over-The-Counter or Prescription letter designa-
tion should appear on all symbols regardless of size of
the square in the center of the symbol. The location of
the letter designation is not interchangeable with other
positions in the symbol.
(4) The "Rx" symbol should appear on the labeling of those
animal drugs that are required to bear the veterinary
prescription legend "Caution: Federal law restricts this
drug to use by or on the order of a licensed
veterinarian." The "OTC" symbol should appear on the
labels of animal drugs indicated for cattle that are
not required to bear the veterinary prescription legend.
b. Indications for Use by Class of Cattle
(1) Lactating Dairy Cattle: An adult female dairy cow
producing milk at the time of drug administration,
designated by the letter "L".
(2) Dry Cows: An adult female dairy cow in a non-
lactating, i.e. "dry", state, at the time of drug
administration, designated by the letter "D".
(3) Heifers: Female dairy cattle under 20 months of
age, designated by the letter "H".
(4) Beef Cattle: Beef Cattle including dairy breed
steers and bulls, designated by the letter "B".
(5) Veal Calves: Bob Veal and Fancy Veal Calves,
designated by the letter "V".
(6) Calves: Beef and Dairy Calves excluding Veal
Calves, designated by the letter "C'.
(7) Multiple classes of cattle should be represented for
a particular drug product by shading and/or lettering the
appropriate triangular sections. All sections within the
symbol should be outlined as shown below. The triangular
sections are not interchangeable and each individual
position represents a particular class of cattle. For
those labels which are too small to bear the cattle
class lettering, shading only the appropriate triangular
area(s) is acceptable. CVM encourages sponsors to
incorporate the narrative description information into
the labeling that is likely to accompany the unit being
distributed to the user of the product.
c. Recommended Symbol Locations
(1) Front Main Panel: center bottom of carton.
(2) Package Insert: top center, page one of the insert.
(3) Vials: withdrawal symbols have priority.

2. CIRCULAR FORM a. For drug residue warnings (1) Milk discard symbol. (2) Slaughter withdrawal symbol. b. Symbol Location (1) Back carton panel near Residue Warning Statement or (2) Package Insert below written residue warning statement and centered. (3) Front Center - Upper 1/4 of 50 lb. bag Medicated Feeds (only where written Residue Warning statements are required). (4) Vial label where possible. c. Other Considerations (1) The circular symbol and accompanying printed test should be used in addition to rather than in place of other residue warning symbols and information present on the label. Other residue warning symbols and information must remain on the label. (2) The circular residue warning symbols and accompanying printed text should be employed on all drugs even if the milk withholding and/or slaughter withdrawal times are zero. 
NOTE: The Residue Warning symbols for Lactating and Dry cows products should have light and dark shaded backgrounds, respectively. The purpose of these different shaded backgrounds is to control the accidental misuse of a lactating product in place of a dry cow product and vice versa. The color of the dark background is optional. SPATIAL AND SIZE RELATIONSHIPS OF SYMBOLS AND STATEMENTS 1. The divided square symbol should be isolated from the circular forms. Their size should be as large as possible but not so small that they are ineffective. Divided Square Forms 1/2 inch X 1/2 inch minimum (basic square box) 2. The circular residue warning symbols should be placed in proximity to the written residue warning statements where possible. Their size should be as large as possible but not so small that they are ineffective. a. 1 inch in diameter b. number in the wording should be at least 1/4 inch wide and 1/4 inch high. 3. Type Size - All information to appear on a label needs to be clearly displayed and easily discernible to the user under regular conditions of use. A user with 20/20 vision should be able to read all information without difficulty. For use of the symbols on small vials and containers, the divided square forms and circular residue warning symbols may be reduced as necessary to be accommodated on the vial label. CVM recommends that if a vial or container is too small to accommodate the symbols, the sponsor propose (in a supplemental application) to use the symbols on single vial container and multiple vial labeling. CVM encourages sponsors to arrange vial labels to accommodate these symbols. 4. Color - The color and background contrasting, positioning and spacing of information should be considered. The color scheme presented in this guideline as well as the additional symbol coloring is not a mandatory feature. Sponsors should use contrasting colors to accentuate the symbol features using the colors already employed on the product label.