ISSN: 1080-6059
Volume 13, Number 1–January 2007
Research
Subtypes of Cryptosporidium parvum in Humans and Disease Risk
Paul R. Hunter,* Stephen J. Hadfield,† Dawn Wilkinson,*
Iain R. Lake,* Florence C.D. Harrison,* and Rachel M. Chalmers† ![]()
*University of East Anglia, Norwich, United Kingdom; and †National Public
Health Service for Wales, Swansea, United Kingdom
Suggested citation for this article
Abstract
The 2 main species of Cryptosporidium that infect humans are Cryptosporidium hominis and C. parvum.
Here, multilocus fragment analysis of 3 microsatellite loci (ML1, ML2, and gp60) was used to
subtype strains from sporadic cases of cryptosporidiosis in Wales and northwest
England. Of 72 strains of C. parvum, 63 were typeable at all 3 loci, forming 31 subtypes. These strains
formed 3 broad clusters, representing 74.6%, 20.6%, and 4.8% of typeable strains. Of 118 C. hominis strains, 106 were typeable at all 3 loci, forming 9
subtypes; however, 90% belonged to the same subtype. Analysis with
epidemiologic data found an association between strains from case-patients who
reported contact with farm animals and individual C. parvum microsatellite alleles. The strongest association was
with ML1; all strains from case-patients that reported farm animal contact had
the same allele (ML1–242). Microsatellite typing of C. parvum provides valuable additional information
on the epidemiology of this pathogen.
Cryptosporidium species are intestinal parasites that infect a variety of animals; Cryptosporidium hominis (synonym: Cryptosporidium parvum genotype 1) and C. parvum (synonym: C. parvum genotype 2) are the 2 most commonly identified species that cause disease (cryptosporidiosis) in humans (1,2). The main symptom of cryptosporidiosis is diarrhea, which may be accompanied by dehydration, weight loss, abdominal pain, fever, nausea, and vomiting (3). In England and Wales, ≈5,000 cases are reported annually (4). Disease, although lasting for up to 2 weeks, is usually self-limiting in immunocompetent persons but may be chronic and more severe in immunocompromised patients (5). Furthermore, C. hominis is associated with increased risk of postinfection symptoms (6).
C. hominis primarily infects humans but has recently been reported to infect a dugong and a lamb, and other animals have been infected experimentally (7). Rare occurrences of low-level natural infection of cattle by C. hominis have also been reported (8). By contrast, C. parvum naturally infects several animal species that serve as reservoirs for zoonotic infection, including cattle, sheep, goats, and deer (7).
Several methods have been described by different research groups to investigate intraspecies variation within the genus Cryptosporidium, including microsatellite sequence analysis (9–12), minisatellite and microsatellite PCR fragment length analysis (13,14), single-strand conformation polymorphism analysis (15), gp60 sequence analysis (16,17), and telomere sequence analysis (18,19). A recent study that used minisatellite and microsatellite fragment analysis identified some C. parvum clones that may not be zoonotic (13,14); this study compared isolates from humans and bovines in a single Scottish county. However, no epidemiologic data were presented on case-patients. In the study described here, we investigated the subtypes of C. parvum and C. hominis and tested the association of subtypes with known epidemiologic factors.
Materials and Methods
Strains
The strains included in this analysis were collected during the case-control study of human cryptosporidiosis in Wales and northwest England (20). This study is to date the only case control-study of risk factors for cryptosporidiosis with species identification of infecting strains. Some 427 case-patients and controls were surveyed by mail questionnaire. The key findings were that travel abroad and changing diapers of children <5 years of age were associated with risk for C. hominis infections. For C. parvum, touching farm animals was associated with illness but eating raw vegetables and tomatoes was strongly negatively associated with illness.
As part of that study, clinical laboratories were encouraged to send fecal samples positive for Cryptosporidium by microscopy to the UK Cryptosporidium Reference Unit in Swansea. Confirmation that samples were positive by microscopy was performed when required by using a modified Ziehl-Neelsen method as described by Casemore et al. (21). To extract Cryptosporidium DNA from microscopy-positive feces, oocysts were first separated from fecal matter by saturated-salt-solution centrifugation as described by Elwin et al. (22). The oocyst suspension was then incubated at 100°C for 60 min, digested with proteinase K and lysis buffer, and purified by using QIAamp DNA Mini Kit spin columns (QIAGEN Ltd, Crawly, UK) as described previously (2). DNA was stored at –20°C before species determination and subtyping, when appropriate.
Identification of Species or Genotype by PCR–Restriction Fragment Length Polymorphism Analysis (PCR-RFLP)
Cryptosporidium sp. was determined by PCR-RFLP analysis of the Cryptosporidium oocyst wall protein (COWP) and small subunit (SSU) rRNA genes using methods based on those described by Spano et al. (23) and Xiao et al. (24), respectively. For PCR-RFLP analysis of the COWP gene, PCR was carried out by using the forward primer 5´-GTAGATAATGGAAGAGATTGTG-3´ and reverse primer 5´-GGACTGAAATACAGGCATTATCTTG-3´ to produce an amplicon of ≈550 bp. The PCR products were digested by using the restriction enzyme RsaI to differentiate between most Cryptosporidium spp.
For nested PCR-RFLP analysis of the SSU rRNA gene, the primary PCR produced fragments of ≈1,325 bp by using the forward primer 5´-TTCTAGAGCTAATACATGCG-3´ and the reverse primer 5´-CCCATTTCCTTCGAAACAGGA-3´. The secondary PCR, which produced fragments of ≈830 bp, used the forward primer 5´-GGAAGGGTTGTATTTATTAGATAAAG-3´ and the reverse primer 5´-AAGGAGTAAGGAACAACCTCCA-3´. The products of the secondary PCR were digested with SspI and VspI. Digested fragments from SSU rRNA and COWP genes were separated by electrophoresis on 3% agarose gels, visualized by SYBR Green I (Sigma, Gillingham, UK) staining, and images were recorded with a digital imaging system (Alpha Imager, Kodak, Hemel Hempstead, UK).
Confirmation of Species or Genotype by SSU rRNA Gene Sequence Analysis
After PCR-RFLP analysis, unusual species and equivocal samples were confirmed by amplifying a fragment of the SSU rRNA gene and DNA sequencing in both directions. Briefly, amplicons of ≈830 bp were produced from each sample by using the nested primer set described above (23), and an ≈298-bp fragment was sequenced (Genetic Research Instrumentation, Braintree, UK) by using the forward primer 5´-AGTGACAAGAAATAACA ATACAGG-3´ and the reverse primer 5´-CCTGCTTTAAGCACTCTAATTTTC-3´ (25). The forward and reverse sequences of these fragments were then aligned and analyzed with a CEQ 8000 Genetic Analysis System (Beckman Coulter, High Wycombe, UK) to obtain a consensus sequence. This sequence was then compared with all GenBank, EMBL, DDBJ, and PDB sequences by using the National Center for Biotechnology Information BLASTN tool (available from www.ncbi.nlm.nih.gov/BLAST/).
Analysis of C. hominis and C. parvum Subtypes
Subtypes were identified by using a multilocus fragment-size–analysis approach to target 3 microsatellite markers (ML1, ML2, and gp60 [synonymous with gp15]) as previously described (26). The ML1 fragment was amplified by using the forward primer 5´-CTAAAAATGGTGGAGAATATTC-3´ and the reverse primer 5´-CAACA AAATCTATATCCTC-3´ (10,11). The ML2 fragment was amplified by using the forward primer 5´-CAATGTAAGTTTACTTATGATTAT-3´ and the reverse primer 5´-CGACTATAAAGATGAGAGAAG-3´ (11). The gp60 fragment was amplified by using the forward primer 5´-GCCGTTCCACTCAGAGGAAC-3´ and the reverse primer 5´-CCACATTACAAATGAAGTGCCGC-3´ (13). Reverse primers were supplied that were labeled with Beckman Coulter WellRED D3 dye (Proligo, Paris, France). The 50-μL PCR mixture for each primer set contained PCR buffer (QIAGEN Ltd), 2.5 mmol/L of MgCl2, 200 μmol/L of each dNTP, 500 nmol/L of each primer, 2.5 U of HotStar Taq DNA polymerase (QIAGEN Ltd), and 5 μL of template DNA. The cycling conditions for each PCR were an initial denaturing step of 15 min at 95°C, then 40 cycles of 95°C for 50 s, 50°C (60°C for gp60) for 50 s, and 72°C for 60 s before a final extension of 10 min at 72°C. The fragment sizes of amplified products were then analyzed with a CEQ 8000 Genetic Analysis System (Beckman Coulter). Allele nomenclature was based on the median fragment size of each natural group rounded to the nearest probable base pair number. The combined results of fragment-size analysis at all 3 markers were used to create a multilocus fragment type for subtypes within C. parvum and C. hominis as described elsewhere (26,27).
Statistical Analysis
Data analysis was carried out by using SPSS 12.0 (SPSS Inc., Chicago, IL, USA). Subclusters were identified by using the SPSS clustering algorithm, a hierarchical algorithm that clusters strains and other clusters together on the basis of their similarity.
χ2 tests (or Fisher exact test when data were sparse) were used to identify significant trends between C. parvum cluster 1 and C. parvum clusters 2 and 3 combined, with epidemiologic parameters. A final multivariable model was derived by using logistic regression as previously described (20) and including all the different strains of C. parvum; the model was recalculated including only the strains that possessed the ML1–242 allele.
The Hunter-Gaston index of discriminatory power was calculated by using StatsDirect (28). This index was proposed as a measure of the discriminatory power of microbial typing schemes. By using the typing scheme under investigation, it calculates the probability of randomly picking 2 unrelated strains and finding them to be different.
Results
A total of 190 sporadic strains of Cryptosporidium were included in this analysis: 118 were C. hominis, of which 106 were typeable at all 3 microsatellite loci; 72 were C. parvum, of which 63 were typeable at all 3 loci. The distribution of these types is shown in Table 1.
Of the 106 strains of C. hominis typeable at all 3 loci, 95 (90%) were indistinguishable at all 3 loci, having the ML1 allele 233 (ML1–233), ML2–180, and gp60–371. This lack of diversity of C. hominis as demonstrated by these 3 markers did not allow further analysis.
Much greater diversity in allele size at all 3 microsatellite loci was displayed by C. parvum than by C. hominis. The discriminatory power of the 3-loci typing method for C. parvum using the Hunter-Gaston index of discriminatory power was 0.957 (95% confidence interval [CI] 0.937–0.977). For C. hominis, the discriminatory power was 0.197 (95% CI 0.096–0.298).
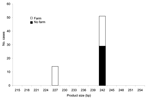 |
Figure 1. Product size at microsatellite locus ML1 with number of Cryptosporidium parvum case-patients who touched or handled farm animals before onset of illness. |
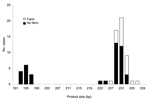 |
Figure 2. Product size at microsatellite locus ML2 with number of Cryptosporidium parvum case-patients who touched or handled farm animals before onset of illness. |
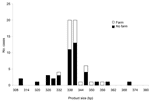 |
Figure 3. Product size at microsatellite locus gp60 with number of Cryptosporidium parvum case-patients who touched or handled farm animals before onset of illness. |
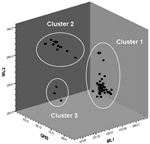 |
Appendix Figure. Three-dimensional scatter plot of Cryptosporidium parvum strains typeable at all 3 loci. |
The Appendix Figure shows a 3-dimensional scatterplot of the strains of C. parvum. Considerable variation can be seen in microsatellite length, and 3 broad subclusters are identifiable. Strains belonging to the 2 smaller clusters had the same ML1–227 allele, whereas all strains belonging to the larger cluster had the ML1–242 allele.
We further looked at the association between polymorphisms at the 3 loci and reported case-patient contact with animals. For this analysis, all strains were included, whether or not they were typeable at all 3 loci. Significantly more persons with strains with ML1–242 (22/52, 43%) had touched or handled farm animals than those with ML1–227 strains (0/14, 0%) (Mann-Whitney U test, p = 0.000 (Figure 1). Similarly, at ML2, significantly more strains with alleles between 223 and 237 (42%, 22/52) were from case-patients who had touched or handled farm animals than were strains with alleles 193 and 197 (0%, 0/13) (Mann-Whitney U test, p = 0.000) (Figure 2). Alleles of gp60 (Figure 3) varied from 311 to 371 bp and peaked at 340 to 341 bp. Case-patients who had contact with farm animals yielded significantly greater product sizes at this locus than those who reported no animal contact before onset of illness (Mann-Whitney U test, p = 0.003).
To test further the association between the ML1–242 polymorphism and contact with animals, the final logistic regression model for C. parvum presented in our earlier article (20) was re-run but included only those strains with the 242-bp allele. The positive association with farm animals and the negative associations with eating raw vegetables all are stronger in the model with just ML1–242 allele strains than in the model containing all C. parvum strains (Table 2).
Each typeable strain was also categorized by local environment, based on postal code of patient's residence. These categories were urban, town or town fringe, village, and hamlet or isolated dwelling. The attack rates per 100,000 population for each of the 2 ML1 types of C. parvum are shown in Table 3. The incidence of ML1–242 strains increased as the home environment became increasingly rural, whereas ML1–227 strains were largely restricted to urban and town environments (Mann-Whitney U test, p = 0.005).
Discussion
At these 3 microsatellite loci, much greater genetic diversity was detected among C. parvum strains than among C. hominis strains. For C. parvum the 3 loci were highly discriminatory (Hunter-Gaston index 0.957), but for C. hominis, they were poorly discriminatory (0.197). These 3 loci by themselves are unlikely to be sufficient for subtyping C. hominis but are adequate for subtyping C. parvum.
Using all 3 loci, the typeability for C. hominis was 90% and for C. parvum 87.5%. The presence of nontypeable strains in any one of the 3 single loci reduced the overall typeability and therefore discriminatory power of the typing method. However, strains that did not type at every locus could still be compared. For example, 70 (96%) strains of C. parvum were typed at the ML1 locus, which improved the power of analyses using just this locus. We are unable to say whether nontyping at a particular locus was because of an unusual allele or because of the sensitivity of the method.
The low diversity of C. hominis is to be expected because it is a species-specific parasite. Hunter and Fraser (29) noted that species adapted to single host species were likely to be less genetically diverse than those with a wider host range, as predicted by the theory of adaptive polymorphism. Greater genetic variation was also found among C. parvum (type 2) than C. hominis (type 1) isolates in a previous study that used minisatellite and microsatellite loci (13). This apparently low genetic diversity among strains of C. hominis might make it difficult to develop discriminatory and reproducible typing methods for C. hominis. However, recent investigation of isolates from global sources at multiple minisatellite and microsatellite loci showed increased polymorphism, particularly over many minisatellite loci (30). On the other hand, the use of only 3 loci gives good discriminatory power for C. parvum.
Using just 3 microsatellite loci, we have shown that 3 major groupings of C. parvum can be found, which supports the similar findings of Mallon et al. (13), who used 7 loci. These researchers reported that the largest cluster contained strains isolated from both humans and animals, while the 2 smaller clusters contained strains isolated only from humans. In our study, all strains isolated from persons reporting contact with animals came from cluster 1, which supports the suggestion of 2 clones of human-adapted strains of C. parvum.
The most intriguing finding was that of an association between strains of C. parvum that may be human-adapted or zoonotic and particular alleles of the microsatellites. While this association included all 3 loci, the strongest association was with alleles at the ML1 locus. This observation was even more dramatic, given that only 2 alleles were found at this locus. None of the case-patients whose strains yielded ML1–227 reported contact with farm animals, while 43% of those whose strains yielded ML1–242 reported such contact. This finding is strengthened by the observation that most of the case-patients yielding cluster 2 or 3 strains were more likely to live in urban areas where the possibilities for animal contact are lower than for those yielding cluster 1 strains. In a related study, all 28 strains isolated from animals were ML1–242, which further supports this hypothesis (27,31).
Although the ML2 locus is more variable than the ML1 locus, the 2 loci correlate very closely. This linkage disequilibrium between the 2 loci has already been noted by other researchers (11), although we must emphasize that our results differ from those of Cacciò et al. (11), who detected 3 alleles at the ML1 locus (ML1–238, ML1–226, and ML1–220). By sequencing PCR products, these authors also found all 3 alleles in isolates from animals. These discrepancies are not likely to be due to the different methods used for sizing of PCR fragments.
We cannot yet conclude that our findings indicate human-adapted strains of C. parvum exist or if all strains are potentially zoonotic. ML1–227 strains do not appear to be zoonotic in the United Kingdom but have been identified as such by other workers in Italy (11), for example. If such strains are zoonotic in other countries, they likely would have spread into the UK human population through imported foods or during foreign travel and subsequently spread among humans. However, they may not have yet made the transition to UK animals.
Microsatellite fragment analysis of C. parvum would appear to provide a discriminatory and rapid means of distinguishing strains. This technique would be useful in outbreak settings to determine whether outbreaks were due to single or multiple strains and, if the former, may indicate the source of contamination. The microsatellites used in this work would not be discriminatory enough for routine use for C. hominis, although others may prove to be of more value.
Acknowledgments
We thank Kristin Elwin, Anne Thomas, Cathy Bentley, and David Gomez for maintenance of the National Collection of Oocysts and Cryptosporidium species determination, and Guy Robinson for provision of additional subtyping data.
This project was funded by the Department for Food, Environment and Rural Affairs and managed by the Drinking Water Inspectorate.
Dr Hunter is professor of health protection at the University of East Anglia. His main interests are in the epidemiology of waterborne disease, especially that caused by Cryptosporidium.
References
- McLauchlin J, Amar C, Pedraza-Díaz S, Nichols GL. Molecular epidemiological analysis of Cryptosporidium spp. in the United Kingdom: results of genotyping Cryptosporidium spp. in 1705 fecal samples from humans and 105 fecal samples from livestock animals. J Clin Microbiol. 2000;38:3984–90.
- The development of a national collection for oocysts of Cryptosporidium. Foundation for Water Research, Marlow, Bucks, UK, 2002 [cited 2006 Oct 11]. Available from http://www.fwr.org/
- Chen X-M, Keithly JS, Paya CV, LaRusso NF. Cryptosporidiosis. N Engl J Med. 2002;346:1723–31.
- Health Protection Agency, 2006 [cited 2006 Oct 11]. Available from http://www.hpa.org.uk/
- Hunter PR, Nichols G. The epidemiology and clinical features of Cryptosporidium infection in immune-compromised patients. Clin Microbiol Rev. 2002;15:145–54.
- Hunter PR, Hughes S, Woodhouse S, Raj N, Syed Q, Chalmers RM, et al. Health sequelae of human cryptosporidiosis in immunocompetent patients. Clin Infect Dis. 2004;39:504–10.
- Xiao L, Rayer R, Ryan U, Upton SJ. Cryptosporidium taxonomy: recent advances and implications for public health. Clin Microbiol Rev. 2004;17:72–97.
- Smith HV, Nichols RAB, Mallon M, Macleod A, Tait A, Reilly WJ, Browning LM, Gray D, Reid SWJ, Wastling JM. Natural Cryptosporidium hominis infections in Scottish cattle. Veterinary Record. 2005;156:710–711.
- Aeillo AE, Xiao L, Limor JR, Liu C, Abrahamson MS, Lal AA. Microsatellite analysis of the human and bovine genotypes of Cryptosporidium parvum. J Eukaryot Microbiol. 1999;46:46S–7.
- Cacciò S, Homan W, Camilli R, Traldi G, Kortbeek T, Pozio E. A microsatellite marker reveals population heterogeneity within human and animal genotypes of Cryptosporidium parvum. Parasitology. 2000;120:237–44.
- Cacciò S, Spano F, Pozio E. Large sequence variation at two microsatellite loci among zoonotic (genotype C) isolates of Cryptosporidium parvum. Int J Parasitol. 2001;31:1082–6.
- Enemark HL, Ahrens P, Juel CD, Petersen E, Petersen RF, Andersen JS, et al. Molecular characterization of Danish Cryptosporidium parvum isolates. Parasitology. 2002;125:331–41.
- Mallon M, MacLeod A, Wastling J, Smith H, Reilly B, Tait A. Population structures and the role of genetic exchange in the zoonotic pathogen Cryptosporidium parvum. J Mol Evol. 2003;56:407–17.
- Mallon ME, MacLeod A, Wastling JM, Smith H, Tait A. Multilocus genotyping of Cryptosporidium parvum Type 2: population genetics and sub-structuring. Infect Genet Evol. 2003;3:207–18.
- Gasser RB. Abs EL-Osta Y, Prepens S, Chalmers RM. An improved "cold SSCP" for the genotypic and subgenotypic characterisation of Cryptosporidium. Mol Cell Probes. 2004;18:329–32.
- Strong WB, Gut J, Nelson RG. Cloning and sequence analysis of a highly polymorphic Cryptosporidium parvum gene encoding a 60-kilodalton glycoprotein and characterization of its 15- and 45-kilodalton zoite surface antigen products. Infect Immun. 2000;68:4117–34.
- Alves M, Xiao L, Sulaiman I, Lal AA, Matos O, Antunes F. Subgenotype analysis of Cryptosporidium isolates from humans, cattle, and zoo ruminants in Portugal. J Clin Microbiol. 2003;41:2744–7.
- Blasdall SA, Ongerth JE, Ashbolt NJ. Differentiation of Cryptosporidium parvum subtypes in calves of four dairy herds by a novel microsatellite-telomere PCR with PAGE. Proceedings of Cryptosporidium from Molecules to Disease, 7–12 October 2001, Fremantle, Australia. Melbourne: Water Services Association of Australia; 2001.
- Blasdall SA, Ongerth JE, Ashbolt NJ. Sub-species differentiation among Type 2 bovine C. parvum isolates using a RAPD microsatellite + telomere primer scheme. Proceedings of IWA World Water Congress, Berlin, 2001. London: International Water Association; 2001.
- Hunter PR, Hughes LS, Woodhouse S, Syed Q, Verlander N, Chalmers RM. Sporadic cryptosporidiosis case-control study with genotyping. Emerg Infect Dis. 2004;10:1241–9.
- Casemore DP, Armstrong M, Sands RL. Laboratory diagnosis of cryptosporidiosis. J Clin Pathol. 1985;38:1337–41.
- Elwin K, Chalmers RM, Roberts R, Guy EC, Casemore DP. The modification of a rapid method for the identification of gene-specific polymorphisms in Cryptosporidium parvum, and application to clinical and epidemiological investigations. Appl Environ Microbiol. 2001;67:5581–4.
- Spano F, Putignani L, McLauchlin J, Casemore DP, Crisanti A. PCR-RFLP analysis of the Cryptosporidium oocyst wall protein (COWP) gene discriminates between C. wrairi and C. parvum, and between C. parvum isolates of human and animal origin. FEMS Microbiol Lett. 1997;150:209–17.
- Xiao L, Singh A, Limor J, Graczyk TK, Gradus S, Lal A. Molecular characterization of Cryptosporidium oocysts in samples of raw surface water and wastewater. Appl Environ Microbiol. 2001;67:1097–101.
- Morgan UM, Constantine CC, Forbes DA, Thompson RCA. Differentiation between human and animal isolates of Cryptosporidium parvum using rDNA sequencing and direct PCR analysis. J Parasitol. 1997;83:825–30.
- Investigation of Cryptosporidium clinical isolates and analysis with epidemiological data. Foundation for Water Research, Marlow, Bucks, UK, 2005 [cited 2006 Oct 11]. Available from http://www.fwr.org/
- Establishing the relationship between farm restocking and cryptosporidia: the Caldew catchment study. Foundation for Water Research, Marlow, Bucks, UK, 2005 [cited 2006 Oct 11]. Available from http://www.fwr.org/
- Hunter PR, Gaston MA. A numerical index of the discriminatory ability of typing systems: an application of Simpson's index of diversity. J Clin Microbiol. 1988;26:2465–6.
- Hunter PR, Fraser CAM. Application of the theory of adaptive polymorphism to the ecology and epidemiology of pathogenic yeasts. Appl Environ Microbiol. 1990;56:2219–22.
- Tanriverdi S, Widmer G. Differential evolution of repetitive sequences in Cryptosporidium parvum and Cryptosporidium hominis. Infect Genet Evol. 2006;6:113–22.
- Robinson G. Investigating the public health significance of Cryptosporidium in the enivironment [PhD dissertation]. Cardiff (UK): University of Wales College of Medicine; 2005.
Figures
Figure 1. Product size at microsatellite locus ML1 with number
of Cryptosporidium parvum case-patients who
touched or handled farm animals before onset of illness.
Figure 2. Product size at microsatellite locus ML2 with number
of Cryptosporidium parvum case-patients who
touched or handled farm animals before onset of illness.
Figure 3. Product size at microsatellite locus gp60 with number
of Cryptosporidium parvum case-patients who
touched or handled farm animals before onset of illness.
Appendix Figure. Three-dimensional scatter plot of Cryptosporidium
parvum strains typeable at all 3 loci.
Tables
Table 1. Distribution of multilocus fragment types (MLFTs)
for Cryptosporidim strains typeable at all 3 loci
Table 2. Logistic regression model from case-control study...
Table 3. Association between subtype number and attack rate
per 100,000 population and residential land use
Suggested Citation for this Article
Hunter PR, Hadfield SJ, Wilkinson D, Lake IR, Harrison FCD, Chalmers RM. Subtypes of Cryptosporidium parvum in humans and disease risk. Emerg Infect Dis [serial on the Internet]. 2007 Jan [date cited]. Available from http://www.cdc.gov/ncidod/EID/13/1/82.htm
Please use the form below to submit correspondence to the authors or contact them at the following address:
Rachel M. Chalmers, UK Cryptosporidium Reference Unit, NPHS Microbiology Swansea (Velindre NHS Trust), Singleton Hospital, Sketty, Swansea SA2 8QA, UK; email: rachel.chalmers@nphs.wales.nhs.uk
Please note: To prevent email errors, please use no web addresses, email addresses, HTML code, or the characters <, >, and @ in the body of your message.
Please contact the EID Editors at eideditor@cdc.gov
The opinions expressed by authors contributing to this journal do not necessarily reflect the opinions of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.
This page posted December 1, 2006
This page last reviewed December 21, 2006
Centers for Disease Control and Prevention, 1600 Clifton Rd, Atlanta, GA 30333, U.S.A
Tel: (404) 639-3311 / Public Inquiries: (404) 639-3534 / (800) 311-3435