ISSN: 1080-6059
Volume 14, Number 8–August 2008
Letter
Mycobacterium setense Infection in Humans
Alexandre Toro,* Toidi Adekambi,† François Cheynet,‡ Pierre-Edouard
Fournier,* and Michel Drancourt* ![]()
*Université de la Méditerranée, Marseille, France; †Centers for Disease
Control and Prevention, Atlanta, Georgia, USA; and ‡Assistance Publique
Hôpitaux de Marseille, Marseille, France
Suggested citation for this article
To the Editor: A 66-year-old man had a bone graft for treatment of an oroantral fistula in March 2007 in Marseille, France. The surgery consisted of a bilateral maxillary sinus filling with a parietal osseous graft to close the fistula (position 24–25). Painful edema of the hemiface and mild fever developed in the patient in July 2007. Computed tomography showed areas of hypodensity in the osseous graft in the left maxillary sinus consistent with osteolysis. Microscopic examination of a bone biopsy specimen after gram staining did not reveal any organisms but this specimen did grow Enterobacter cloacae and colonies of a gram-positive bacillus after a 2-day inoculation on 5% blood agar incubated at 37°C in an atmosphere of 5% CO2. Tentative identification of this catalase-positive, oxidase-negative gram-positive rod (isolate 74023791) by an API Coryne strip (API bioMérieux, La Balme-les Grottes, France) remained inconclusive. Isolate 74023791 exhibited acid fastness and was further identified by comparing its 16S rDNA (1) and heat shock protein 65 (hsp65) (2) sequences with those in the GenBank database and its RNA polymerase subunit B (rpoB) sequences (3) with those of Mycobacterium spp. in our local rpoB database.
Antimicrobial susceptibility testing using E-test (AB Biodisk, Bruz, France) indicated that isolate 74023791 was susceptible to ciprofloxacin with an MIC of 0.047 μg/mL and resistant to amoxicillin (MIC >256 μg/mL), ceftriaxone (MIC >256 μg/mL), erythromycin (MIC >256 μg/mL), clarithromycin (MIC >256 μg/mL), and rifampin (MIC >32 μg/mL). Disk testing and reference broth dilution method (4) showed that isolate 74023791 was susceptible to imipenem after a 3-day incubation. However, E-test showed heterogeneous resistance with colonies exhibiting an MIC >256 μg/mL. The same observations were made for Mycobacterium setense type strain CIP109395T (Collection de l'Institut Pasteur, Paris, France) (5). Daily treatment with 2 g imipenem and 1.5 g ciprofloxacin was prescribed for 1 month before the imipenem E-test and broth dilution results were available, and was followed by 1.5 g/day ciprofloxacin for 3 months, resulting in a favorable clinical and radiologic evaluation at 5-month follow-up.
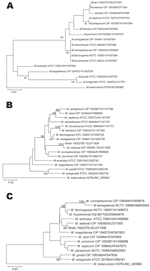 |
Figure. Phylogenetic position of isolate 74023791 and 16 rapidly growing Mycobacterium species based on A) 16S rDNA, B) partial RNA polymerase subunit B... |
Phylogenetic analyses indicated that isolate 74023791 belonged to the M. fortuitum group, along with M. porcinum and M. conceptionense, and was most closely related to M. setense, a recently described species of this group (5) (Figure). Isolate 74023791 shared 100% 16S rDNA (GenBank accession no. EU371507), 99.5% hsp65 (accession no. EU371508), and 99.0% rpoB (accession no. EU371509) sequence similarity with M. setense (accession nos. EF138818 and EU371504, EF138819 and EU371505, and EF414447 and EU371506, respectively). Because the 99% rpoB sequence similarity of the patient's isolate was above the 97% rpoB sequence similarity cut-off value used to identify rapidly growing mycobacteria (3), isolate 74023791 was therefore identified as M. setense.
M. setense, in association with an E. cloacae strain susceptible to antimicrobial drug therapy, was an agent of infection in our patient. It is noteworthy that M. setense and the closely-related species M. conceptionense were isolated from patients with posttraumatic osteitis (5,6); M. porcinum was isolated from 7/46 cases of osteomyelitis and additional cases of postsurgical infection, respectively (7); M. fortuitum osteomyelitis has also been reported (8). These data emphasize the role of M. fortuitum group organisms in posttraumatic and postsurgical osteitis.
In a later interview, the patient disclosed that he rinsed his mouth with well water during the weeks after receiving the bone graft. We initially suspected that the water was the source of M. setense, as previously suspected for M. conceptionense (6) and reported for M. porcinum (7). However, neither M. setense nor M. setense DNA were detected in the well water in October 2007.
Initially, M. setense was reported to be susceptible to imipenem by the disk diffusion method, which is not the reference method (5). In this report, the disk and reference broth dilution methods showed that both clinical and reference M. setense strains were initially susceptible to imipenem but the E-test disclosed that both strains exhibited heterogeneous resistance to imipenem; colonies exhibited an MIC >256 μg/mL. This result was unexpected because the E-test showed that related species M. fortuitum CIP104534T, M. conceptionense, CIP 108544T and M. porcinum CIP105392T were susceptible to imipenem (6). Likewise, the modified broth dilution method showed that the MIC for imipenem was <4 μg/ml for M. fortuitum (9). However, when a broth dilution method was used, the MIC for imipenem ranged from 0.5 μg/mL to 8 μg/mL in 42 M. porcinum strains (7). Together, these data challenge the susceptibility to imipenem in organisms of the M. fortuitum group.
M. setense is an emerging organism of the M. fortuitum group that must be added to the growing list of rapidly growing mycobacteria isolated from humans. The initial gram-positive rod appearance of M. setense may delay its accurate identification. Determination of antimicrobial drug susceptibility needs to be conducted by the reference broth dilution method. Further reports are warranted to characterize the role of M. setense in infection.
This report was supported by Unité des Rickettsies CNRS IRD UMR 6236.
References
- Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173:697–703.
- Ringuet H, Akoua-Koffi C, Honore S, Varnerot A, Vincent V, Berche P, et al. hsp65 sequencing for identification of rapidly growing mycobacteria. J Clin Microbiol. 1999;37:852–7.
- Adekambi T, Colson P, Drancourt M. rpoB-based identification of nonpigmented and late-pigmenting rapidly growing mycobacteria. J Clin Microbiol. 2003;41:5699–708. PubMed DOI
- National Committee for Clinical Laboratory Standards. Susceptibility testing of mycobacteria, Nocardiae and other aerobic actinomycetes: approved standard M24–A. Wayne (PA): The Committee; 2003.
- Lamy B, Marchandin H, Hamitouche K, Laurent F. Mycobacterium setense sp. nov., a Mycobacterium fortuitum–group organism isolated from a patient with soft tissue infection and osteitis. Int J Syst Evol Microbiol. 2008;58:486–90. PubMed DOI
- Adekambi T, Stein A, Carvajal J, Raoult D, Drancourt M. Description of Mycobacterium conceptionense sp. nov., a Mycobacterium fortuitum group organism isolated from a posttraumatic osteitis inflammation. J Clin Microbiol. 2006;44:1268–73. PubMed DOI
- Wallace RJ Jr, Brown-Elliott BA, Wilson RW, Mann L, Hall L, Zhang Y, et al. Clinical and laboratory features of Mycobacterium porcinum. J Clin Microbiol. 2004;42:5689–97. PubMed DOI
- Tejan-Sie SA, Robin K, Avery SH, Mossad SB. Mycobacterium fortuitum osteomyelitis in a peripheral blood stem cell transplant recipient. Scand J Infect Dis. 2000;32:94–6. PubMed DOI
- Lee SM, Kim J, Jeong J, Park YK, Bai GH, Lee EY, et al. Evaluation of the broth microdilution method using 2,3-diphenyl-5-thienyl-(2)-tetrazolium chloride for rapidly growing mycobacteria susceptibility testing. J Korean Med Sci. 2007;22:784–90.
Figure
Suggested Citation for this Article
Toro A, Adekambi T, Cheynet F, Fournier P-E, Drancourt M. Mycobacterium setense infection in humans [letter]. Emerg Infect Dis [serial on the Internet]. 2008 Aug [date cited]. Available from http://www.cdc.gov/EID/content/14/8/1330.htm
DOI: 10.3201/eid1408.080179
Please use the form below to submit correspondence to the authors or contact them at the following address:
Michel Drancourt, Unité des Rickettsies, Faculté de Médecine, Université de la Méditerranée, 27 Blvd Jean Moulin, 13385 Marseille CEDEX 5, France; email: michel.drancourt@medecine.univ-mrs.fr
Please contact the EID Editors at eideditor@cdc.gov
The opinions expressed by authors contributing to this journal do not necessarily reflect the opinions of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.
This page posted July 25, 2008
Centers for Disease Control and Prevention, 1600 Clifton Rd, Atlanta, GA 30333, U.S.A
Tel: (404) 639-3311 / Public Inquiries: (404) 639-3534 / (800) 311-3435