ISSN: 1080-6059
Volume 13, Number 9–September 2007
Research
Effect of Interventions on Influenza A (H9N2) Isolation in Hong Kong's Live Poultry Markets, 1999–2005
Eric H.Y. Lau,*1 Y.H. Connie Leung,*1 Li Juan Zhang,* Benjamin J. Cowling,* Sin Ping Mak,† Yi Guan,* Gabriel M.
Leung,* ![]() and J.S. Malik Peiris*
and J.S. Malik Peiris*
*The University of Hong Kong, Hong Kong Special Administrative
Region, People's Republic of China; and †Government of the Hong Kong Special
Administrative Region, People's Republic of China
Suggested citation for this article
Abstract
Live poultry markets (LPMs) are a recognized source of influenza
viruses. Since 2001 and 2003, respectively, a first and second monthly
"rest-day" has been implemented in Hong Kong's LPMs, when stalls are cleared of
unsold poultry and disinfected. We assessed the incremental effectiveness of
each rest-day and the banning of live quail sales in 2002 in reducing (H9N2)
subtype isolation rates for chickens and minor poultry, by using a
multivariable Poisson generalized linear model. There was a 58% reduction (p =
0.001) in virus isolation after 1 monthly rest-day in minor poultry compared
with 27% (p = 0.22) in chickens. Combining 1 rest-day with the removal of
quails further reduced virus isolation in chickens but not in minor poultry.
However, an additional rest-day each month did not appear to affect isolation
rates for either species.
The abundance and diversity of avian influenza viruses in live poultry markets (LPMs) have been recognized since the 1970s (1), and avian influenza viruses are recognized as key to the emergence of pandemic influenza (2). More recently, there has been increasing recognition of their pivotal role in the amplification and maintenance of avian influenza viruses, in introduction of infection to poultry farms (3–5), and in zoonotic transmission of avian influenza viruses to humans (2,6). Nevertheless, LPMs proliferate throughout south Asia and Southeast Asia as well as in other parts of the world, including parts of the United States.
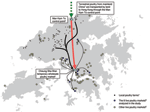 |
Figure 1. Supply chain of live terrestrial poultry in Hong Kong. *During the study period, 110–140 local poultry farms were registered in Hong Kong with ≈2 million chickens... |
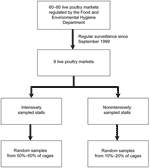 |
Figure 2. Sampling procedures from live poultry markets (LPMs) in Hong Kong. |
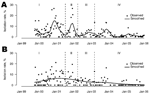 |
Figure 3. Weekly influenza A (H9N2) isolation rates for chickens (A) and minor poultry (B) in Hong Kong, September 1999–December 2005... |
 |
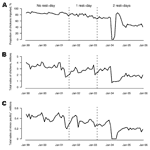
Figure 4. Average influenza A (H9N2) isolation rates by calendar day during the period with no rest-day and by days after a rest-day during the period with rest-days, for chickens (A) and minor poultry (B)... |
 |
Appendix Figure 1. A) Monthly imported chicken ratios (number of chickens imported to the total sales of chickens), January 1999–December 2005... |
 |
Appendix Figure 2. A) Weekly temperature and B) relative humidity, 1999–2005. |
In Hong Kong, LPMs were identified as a major risk for human influenza (H5N1) disease in 1997, when 6 of 18 infected persons died of this highly pathogenic novel strain (6). The territory-wide depopulation of all poultry stopped the outbreak. Again in 2001, early detection of multiple new genotypes of highly pathogenic influenza (H5N1) subtype led to another mass culling of poultry in markets before any zoonotic infection occurred. Since July 2001, a compulsory "rest-day" in these poultry markets has been imposed on day 25 of each month. The previous day, all remaining birds are sold or slaughtered, and the next day the stalls, free of poultry, are cleaned and disinfected. This rest-day has been synchronized with 1 of 3 standing monthly rest-days in the wholesale market (Cheung Sha Wan; Figure 1) (7). Since influenza A (H9N2) viruses found in quail were identified as the donor of the internal genes of the (H5N1) virus that caused human disease in Hong Kong in 1997 and because isolation rates of these viruses from quail were particularly high (8), the sale of live quails, together with any other live poultry at the same premises, was banned effective February 2002. Episodic reappearance of (H5N1) viruses in the LPMs in late 2002 and early 2003 led authorities to introduce a second rest-day on day 10 of each month beginning March 2003 (7).
The effects of the first rest-day on avian influenza virus carriage in LPMs were previously demonstrated by showing that the virus isolation rates of (H9N2) virus (a subtype endemic in poultry in southern China) within poultry markets were significantly reduced soon after the market rest-day (3). Here we assessed the impact of the first and second monthly market rest-days. We also addressed the question of the marginal effect of the second rest-day on the isolation rates of H9N2 virus, after adjusting for other important covariables such as temperature, relative humidity, market ventilation system, importation, and sales of poultry stratified by type. Additionally, we tested for the effects of the removal of quails from poultry markets in February 2002.
Low-pathogenic influenza A (H9N2) is endemic in poultry across Asia (8,9) and can serve as an indicator of the dynamics of influenza transmission in poultry. Because (H5N1) virus infection is uncommon in Hong Kong, especially after universal inoculation with inactivated (H5N2) subtype vaccines of all local and imported chickens was introduced in June 2003 and January 2004, respectively, we analyzed the dynamics of (H9N2) virus activity to provide an indicator of influenza virus transmission in LPMs. In addition to being an indicator of influenza virus (including [H5N1] subtype) transmission within poultry in general, (H9N2) viruses have been transmitted to humans and are themselves regarded as viruses with pandemic potential (10).
Materials and Methods
Sources of Data
Since September 1999, we have collected fecal samples from 8 LPMs, from a total of 60–80 regulated by the Food and Environmental Hygiene Department in Hong Kong. (The exact number varied, and mostly decreased, during the period of observation.) We tested these samples for influenza viruses as part of an ongoing longitudinal epizootic surveillance program. The 8 selected sites were a convenience sample with geographic representativeness (Figure 1); each site services a large catchment area covering major regions of Hong Kong. Data from 76 consecutive months, September 1999–December 2005, comprising periods with 0, 1, and 2 monthly rest-days, were analyzed.
Live terrestrial poultry from local farms or those imported from mainland China are collected initially at a single wholesale market and redistributed to retail LPMs (Figure 1). In the 8 selected LPMs, the number of stalls in each market was 3–24 in 2000 and was down to 1–16 in 2006. The number of poultry cages in each stall was 20–50. We selected 1 stall from each market for intensive sampling, in which 1 random fresh fecal sample was swabbed from each cage. Approximately 50%–60% of the cages in the stall were sampled. For the other stalls in each market, ≈10%–20% of cages were randomly sampled, 1 swab per cage.
We sampled chickens, which comprise most (80%) poultry consumption in Hong Kong, and other avian species collectively termed "minor poultry." These included pigeons, pheasants, silkie chickens, guinea fowls, and chukar partridges. Quails were not included in the analysis because live sales of these birds have been banned since 2002. The number of samples specific to quails was also very small (≈3% of all samples), precluding separate analyses due to lack of statistical power. Quails sold in markets were raised locally or imported from farms in mainland China. Isolation rates of influenza (H9N2) subtype in quail at the wholesale market before their entry into retail markets were compared for 6 months. The (H9N2) subtype isolation rate from cloacal swabs in the wholesale market was ≈3% compared to an isolation rate of 17% from fecal droppings in retail markets at the corresponding periods (unpub. data). This finding suggests that virus transmission was amplified within the retail markets in quail. In view of the common practice of stacking cages with different poultry species one above the other or placing cages side by side, transmission of virus across and within species in the market through the fecal-oral route was highly possible. Hence, we also examined the effect of removing quails from LPMs on isolation rates in other species. Waterfowl, ducks, and geese are recognized as the natural reservoirs of influenza viruses (1,2); ducks yield especially high virus isolation rates. Because of this, since 1998, after the 1997 (H5N1) zoonotic incident in Hong Kong, ducks and geese had been removed from LPMs in Hong Kong, imported separately, and sold already slaughtered and chilled. Figure 1 shows the live poultry supply chain, and Figure 2 summarizes our sampling procedure.
In addition, we collected potential confounding covariable data, including the total sales of chickens and minor poultry, proportion of chickens imported as a ratio to the total (all minor poultry analyzed were imported from mainland China except for some locally raised pigeons), temperature and relative humidity, and type of ventilation system used in LPMs. Weekly average temperature and relative humidity were obtained from the Hong Kong Observatory (11). Older LPMs are naturally ventilated, whereas the newer markets have installed either a market economic air treatment (MEAT) system, which lowers the temperature by 3°C when it rises to >25°C, or an air-conditioning system, which operates on a thermostat maintaining ambient temperature at 23°C.
Laboratory Procedures
Fecal swabs were collected and transported in vials containing 2.0 mL transport medium containing M199 (9.5 g/L), penicillin G (2× 106 U/L), polymyxin B (10× 106 U/L), gentamicin (2,500 mg/L), nystatin (0.5× 106 U/L), ofloxacin HCl (100 mg/L), and sulfamethoxazole (1 g/L). An aliquot of 200 mL from each swab sample was injected into the allantoic cavity of a 9- to 11-day-old chicken embryo egg and incubated for 3 days at 35°C. Positive isolates were subtyped by hemagglutination-inhibition tests and neuraminidase inhibition test with standard antisera (1,8).
Statistical Analysis
We fitted a Poisson generalized linear model (12) for the outcome variable influenza (H9N2) subtype weekly isolation counts adjusted for the proportion of chickens imported; total sales of chickens and minor poultry; period with 0, 1 (with and without live quails being sold in the markets) and 2 monthly rest-days; ventilation system; weekly average temperature; relative humidity; seasonal variations; and sample size. The antilog of the estimated parameters corresponds to the relative risk (RR) for that factor.
The full Poisson regression model for the number of isolations in a particular week can be represented as log(no. of positive isolates) = log(no. of samples) + β0 + β1 (indicator of period II) + β2 (indicator of period III) + β3 (indicator of period IV) + β4 (chicken imported ratio) + β5 (total sales of chicken) + β6 (total sales of minor poultry) + β7 (indicator of MEAT system) + β8 (indicator of air-conditioned market) + β9 (temperature) + β9 (relative humidity) + S(t) + interaction + residual error.
Weekly isolation counts were analyzed from September 22, 1999–December 20, 2005. The indicator variables for periods II, III, and IV take the value 1 within the period with 1 rest-day with quail sales, 1 rest-day without quail sales, and the period with 2 rest-days, respectively, and otherwise take the value 0. S(t) represents a seasonal term and comprises harmonic terms, which are linear combinations of sine and cosine terms similar to Serfling regression (13). We investigated second-order interaction terms between periods, chicken imported ratios, total sales of chicken and minor poultry, temperature, and humidity. We only retained statistically significant interaction terms in the final model.
We fitted separate models for chickens and minor poultry to explore the potential heterogeneity of effects across poultry species strata. To verify model goodness-of-fit, 2 coauthors independently viewed residual plots and verified that the model fit was adequate. All analyses were implemented in R version 2.3.1 (14).
Results
Figure 3 shows the overall isolation rates by week for chickens and minor poultry from 1999 through 2005. Large fluctuations are most likely attributable to seasonality and stochasticity.
Figure 4 shows average isolation rates
by calendar day of the month and by number of days after the rest-day(s) for
chickens and minor poultry. Overall, mean crude isolation rates for chickens
and minor poultry for the period before the introduction of the rest-day were
5.9% and 6.0%, respectively. Similarly, the crude isolation rates for the
period in which 1 rest-day per month was implemented were 5.8% and 4.8% before
quails were removed and 3.2% and 3.1% afterwards, and when 2 rest-days per
month were enforced, they were 2.0% and 2.0%, respectively. The timing of the
sample collection in relation to the rest-days is summarized in the Technical Appendix (![]() 18 KB, 1 page).
18 KB, 1 page).
In the period before market rest-days were introduced, there
was no obvious secular trend over calendar days. During the period with 1
rest-day before live quails sales were banned, a substantial reduction in virus
isolation for both chickens and minor poultry occurred immediately after the
rest-day, followed by a drift back up to the period-specific baseline 1–3 weeks
later. Precisely describing the time course of virus isolation is difficult,
given the lack of samples during the intervening period. After quails were
removed with 1 rest-day, the average isolation rates for both species groups
declined further. In particular, when comparing the isolation rates in weeks 3
and 4 after the rest-day, levels were substantially lower in the period without
quails than that with quails. Again, the lack of samples during the first week
or so after the rest-day precluded any direct inference about the time trend of
virus isolation. However, extrapolating the near-zero isolation rates observed
in the period with 1 rest-day in the presence of live quails sales, we might
speculate that the average isolation rates were overestimated during the period
of 1-rest-day without quails. When there were 2 rest-days, isolation rates were
relatively constant throughout each day of the month, with slightly higher
isolation prevalence during the week immediately preceding the rest-days. Of
note, the 95% confidence intervals were fairly wide during the first period
with no rest-days because of the smaller number of specimens available (Technical Appendix [![]() 8 KB, 1 page]) for the number of samples tested.
8 KB, 1 page]) for the number of samples tested.
The proportion of chickens that was imported declined from 90% to 40% during the period of observation, with a short-lived complete ban in February 2004 due to highly publicized avian influenza outbreaks in Guangdong, Anhui, and Shanghai. The total sales of chickens and particularly minor poultry also showed a downward trend: they decreased ≈50% overall from 1999 to the end of 2005 (Appendix Figure 1). In terms of the other covariables, there are clear seasonal patterns in temperature and relative humidity (Appendix Figure 2).
The Table gives the parameter estimates of the final fitted models, which were adjusted for the effect of a number of potential co-variables that may affect virus isolation rates. No second-order interaction terms, except that between total sales of chickens and minor poultry, were found to be statistically significant. For chickens, compared to the reference category of no rest-day, the period with 1 rest-day with quails was associated with an insignificantly lower average isolation rate of (H9N2) virus. With quails removed, the isolation rate showed a 39% decline from baseline (i.e., no rest-day) that was of borderline significance (p = 0.06). However, the later period with 2 rest-days demonstrated little additional effect (p = 0.74, comparing the additional effect of 2 rest-days vs. the effect of 1 rest-day without quails). Naturally ventilated LPMs and those with MEAT system had similar isolation rates; the air-conditioned LPM had a lower rate, albeit with borderline significance.
Findings from the minor poultry model were generally similar. Compared to baseline, the effect of the first rest-day (with or without quails) was significantly more marked. However, there appeared to be little change in the average isolation rate (adjusted RR 0.42 vs. 0.40, p = 0.88) associated with banning live quail sales. The additional effect of the second rest-day was also marginal (p = 0.80, comparing the additional effect of 2 rest-days vs. the effect of 1 rest-day without quails). The isolation rates were not significantly associated with the type of ventilation system used.
For both models, although the proportion of chickens imported was not associated with isolation rates, the number of chickens and minor poultry sold (a reflection of the composite of imported and domestically raised poultry entering the LPMs) appeared to be important determinants of H9 isolation rates. There was significant statistical interaction between chicken sales and minor poultry sales as the 2 trends closely tracked each other (Appendix Figure 1).
Of note, the abrupt cessation of chicken and minor poultry imports in early-2004 (Appendix Figure 1) could have introduced a considerable amount of additional variability in the import and total sales parameters, which might have affected the model results. We tested model sensitivity to this effect by omitting those 4 months (February–May 2004) with exceptionally low (or zero) total imports. All the estimates remained robust and did not change appreciably (data not shown).
Discussion
We found that isolations in chickens and minor poultry showed different time-dependent contours and variance, indicating that transmission dynamics may differ between the types of poultry examined in this study (15), independent of the confounding effects of quails and waterfowl. Such findings reinforce the importance of examining different bird species separately. The scatter plots of isolation rates show large stochastic fluctuations, in addition to strong seasonal variability, which suggest that intervention effects must be studied with statistical methods that can take into account such variability and the confounding influence of relevant environmental covariables. To the best of our knowledge, the present analysis is the first to have implemented both of these principles.
There was a significant 58% reduction (adjusted RR 0.42, p<0.01) in virus isolation in minor poultry after the first monthly rest-day (with live quail sales) was introduced, compared to only 27% (adjusted RR 0.73) in chickens (not significant). With the removal of quails, the effect size became larger in chickens (adjusted RR 0.61) and almost reached significance at the 0.05 level, but not in minor poultry (adjusted RR 0.40). However, an additional rest-day every 2 weeks did not appear to be effective in further reducing isolation rates significantly for either species group, after other contributing factors were adjusted for. A previous study in which (H9N2) virus isolation rates in individual markets immediately before and after the market rest-day were compared demonstrated that the rest-day was associated with a reduction in virus isolation rates (3).
Total sales of live birds in LPMs were also a major determinant of transmission, where the effects of chickens and minor poultry were different. In addition, minority poultry, although fewer in numbers (by a whole order of magnitude), appeared to have exerted a greater effect on positive isolation frequencies. These 2 observations suggest that influenza virus transmission in minor poultry within LPMs is more sensitive to changes in environmental conditions than virus transmission in chickens. This could be due to interspecies biologic differences or different market practices. For instance, minor poultry, because of their higher price and lower popularity compared with chickens, tend to have an increased market life and remain in cages longer than chickens, which typically have a more rapid turnover (1–2 days). Also, minor poultry, which tend to come to market at a younger age, may have higher levels of virus carriage.
In any case, from a policy perspective, perhaps an appropriate response could be to separate the sales of live chicken and minor poultry. This would have the additional benefit of preventing cross-species infection to chickens, which are the main poultry consumed (16). Indeed, we observed that the effect of 1 rest-day in chickens became larger and more significant statistically after quails were removed. The sales of live ducks and geese in LPMs had already been banned since 1998 in Hong Kong, and the sales of live quails were banned in 2002 (7).
The data also suggest that reducing the volume of sales in LPMs reduces virus isolation rates. This finding may be counter-intuitive to the extent that a high turnover is likely to be associated with shorter holding time of the poultry within the market, and one would expect this to be associated with reduced virus isolation rates. On the other hand, decreased volume of sales decreases the risk for introduction of virus into a market, and therefore the risk of establishing transmission within the market.
Analysis of the data by calendar day (Figure 4)
confirmed earlier results (3,17)
that rest-days led to an immediate decline in positive isolates
by interrupting the amplification cycle. Our findings further suggest that
the effect of very low isolation rates can likely be sustained for up to
2 weeks, although we caution that we had little data during the second week
to provide definitive support to this observation (Technical
Appendix [![]() 8
KB, 1 page] and Figure 4). Moreover,
the analysis in Figure 4 is unadjusted for other
covariables and therefore cannot be directly compared with the
multivariable model results, which suggest the second rest-day had marginal
effects on further reducing virus isolation.
8
KB, 1 page] and Figure 4). Moreover,
the analysis in Figure 4 is unadjusted for other
covariables and therefore cannot be directly compared with the
multivariable model results, which suggest the second rest-day had marginal
effects on further reducing virus isolation.
This finding does not necessarily imply that the number of market rest-days in Hong Kong should be reduced in frequency from twice to once per month. In addition to the impact on overall viral load, the frequency in rest-days is predicated on minimizing the duration of the circulation of a potentially pathogenic avian virus (e.g., [H5N1]) within markets after its occasional introduction. Meanwhile, the diminishing marginal effectiveness for each additional rest-day may prompt the implementation of centrally slaughtering of all live poultry for further reduction in transmission risk. This is probably a more important intervention to aim for than removal of another species of poultry from the poultry markets.
The study was conducted on low pathogenic avian influenza (H9N2) viruses because they are endemic in poultry in the region. While the (H9N2) subtype is itself important as a zoonotic pathogen and may be a candidate for the next pandemic virus, the transmission dynamics of this virus may also provide insights into the transmission and control of the highly pathogenic avian influenza (HPAI) (H5N1) viruses that are currently a major threat to animal and human health across Asia. Therefore, our results suggest that for countries confronting endemic (H5N1) subtype infection in poultry, introducing even 1 rest-day per month in these LPMs is likely to provide definite benefit. Interventions designed to interrupt virus transmission in poultry markets may have even greater effects in retail poultry markets in mainland China and elsewhere in Asia, where aquatic and terrestrial poultry are both present within the same markets, because aquatic poultry appear to be the more important carriers of HPAI (H5N1) viruses.
The winter increase in (H9N2) virus isolation rate and strong seasonal forcing observed parallel those seen for (H5N1) viruses in poultry markets in southern China (18,19). The reasons for this increase in virus carriage rates in the winter are unclear. However, the lower temperature and humidity may increase virus survival in the environment, thereby increasing virus transmission.
The lack of association with the proportion of chickens that were imported likely reflects the progressive culmination of an effective package of biosecurity measures (e.g., including universal vaccination with sentinel flocks, stringent surveillance from farm to market, segregation of species during transport) further up the supply chain, as detailed in Figure 1, panel A, such that there is little difference in risk for virus introduction into the LPMs between locally farmed and imported chickens.
We did not have information on some parameters that could affect transmission efficacy, such as market and stall designs, poultry density, and proximity of different species. Nevertheless, unless these changed substantially during the time series, which we do not believe to be the case, they should not have had a large effect on the results.
Future research should explore optimizing the number and timing of market rest-days and other interventions by using mathematical and statistical models, with parameters determined by empirical data. Field experiments studying the contextual effects of market conditions could also further inform the transmission dynamics of influenza in LPMs.
These findings and other studies documenting that LPMs can serve as a source of infection for farms (5) confirm that these markets maintain, amplify, and disseminate influenza viruses. Thus, while retail poultry markets are a dead-end for the poultry that are slaughtered there, they are not a dead-end for the virus. Indeed, these markets possibly help maintain infection in poultry flocks and provide a potential site for intervening to control virus transmission (16). Studies to address their role in maintaining influenza virus circulation in countries where (H5N1) HPAI is endemic are urgently needed. The LPM practice may differ between countries, and these differences may greatly affect the role of these markets in amplifying and disseminating avian influenza viruses. For example, poultry markets where unsold poultry are not held over to the next day are less likely to contribute to amplification of virus load. However, establishing that LPMs play a role in maintaining and disseminating virus in these environments (as they do in Hong Kong) would prove a focal point for strategic intervention to interrupt transmission of this virus.
Acknowledgments
We thank Mary Chow, Shirley Chuk, Rhonda Lo, and Chan Wing Shun for information regarding poultry importation and poultry markets; Martin Tsang, Hagi Ng, Isaac Chow, and Johnny Wong for sample collection and laboratory analysis; and 3 anonymous referees for their helpful comments and suggestions.
Research funding was provided by the Research Fund for the Control of Infectious Diseases of the Health, Welfare and Food Bureau of the Hong Kong SAR Government; The Wellcome Trust; and The Vice Chancellor's Research Fund, The University of Hong Kong.
Dr Lau is a postdoctoral fellow in statistical epidemiology of infectious disease.
References
- Shortridge KF, Butterfield WK, Webster RG, Campbell CH. Isolation and characterization of influenza A viruses from avian species in Hong Kong. Bull World Health Organ. 1977;55:15–20.
- Shortridge KF. Pandemic influenza—a zoonosis? Semin Respir Infect. 1992;7:11–25.
- Kung NY, Guan Y, Perkins NR, Bissett L, Ellis T, Sims L, et al. The impact of a monthly rest day on avian influenza virus isolation rates in retail live poultry markets in Hong Kong. Avian Dis. 2003;47:1037–41.
- Senne DA, Peason JE, Pahigrahy B. Live poultry markets: a missing link in the epidemiology of avian influenza. Proceedings of the Third International Symposium on Avian Influenza; 1992 May 27–29; Madison, WI, USA. Richmond (VA): Animal Health Association; 1992.
- Kung NY, Morris RS, Perkins NR, Sims LD, Ellis TM, Bissett L, et al. Risk for infection with highly pathogenic influenza A virus (H5N1) in chickens, Hong Kong 2002. Emerg Infect Dis. 2007;13:412–8.
- Mounts AW, Kwong H, Izurieta HS, Ho Y, Au T, Lee M, et al. Case-control study of risk factors for avian influenza A (H5N1) disease, Hong Kong, 1997. J Infect Dis. 1999;180:505–8.
- Sims LD, Ellis TM, Liu KK, Dyrting K, Wong H, Peiris M, et al. Avian influenza in Hong Kong, 1997–2002. Avian Dis. 2003;47:832–8.
- Guan Y, Shortridge KF, Krauss S, Chin PS, Dyrting KC, Ellis TM, et al. H9N2 influenza viruses possessing H5N1-like internal genomes continue to circulate in poultry in southeastern China. J Virol. 2000;74:9372–80.
- Cameron KR, Gregory V, Banks J, Brown IH, Alexander DJ, Hay AJ, et al. H9N2 subtype influenza A viruses in poultry in Pakistan are closely related to the H9N2 viruses responsible for human infection in Hong Kong. Virology. 2000;278:36–41.
- Peiris M, Yuen KY, Leung CW, Chan KH, Ip PLS, Lai RWM, et al. Human infection with influenza H9N2. Lancet. 1999;354:916–7.
- Hong Kong Observatory HKSAR. Extract of meteorological observations for Hong Kong. [cited 2006 July 21]. Available from http://www.hko.gov.hk/wxinfo/pastwx/extract.htm
- McCullagh P, Nelder JA. Generalized linear models. London: Chapman and Hall; 1989.
- Serfling RE. Methods for current statistical analysis of excess pneumonia-influenza deaths. Public Health Rep. 1963;78:494–506.
- R Development Core Team. R: A language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing; 2004.
- Humberd J, Guan Y, Webster RG. Comparison of the replication of influenza A viruses in Chinese ring-necked pheasants and chukar partridges. J Virol. 2006;80:2151–61.
- Webster RG. Wet markets—a continuing source of severe acute respiratory syndrome and influenza. Lancet. 2004;363:234–6.
- Bulaga LL, Garber L, Senne DA, Myers TJ, Good R, Wainwright S. Epidemiologic and surveillance studies on avian influenza in live-bird markets in New York and New Jersey, 2001. Avian Dis. 2003;47:996–1001.
- Li KS, Guan Y, Wang J, Smith GJD, Xu KM, Duan L, et al. Genesis of a highly pathogenic and potentially pandemic H5N1 influenza virus in eastern Asia. Nature. 2004;430:209–13.
- Chen H, Smith GJD, Li KS, Wang J, Fan XH, Rayner JM, et al. Establishment of multiple sublineages of H5N1 influenza virus in Asia: implications for pandemic control. Proc Natl Acad Sci U S A. 2006;103:2845–50.
Figures
Figure 1. Supply chain of live terrestrial poultry in Hong
Kong. *During the study period, 110–140 local poultry farms were registered in
Hong Kong with ≈2 million chickens...
Figure 2. Sampling procedures from live poultry markets (LPMs) in Hong Kong.
Figure 3. Weekly influenza A (H9N2) isolation rates for
chickens (A) and minor poultry (B) in Hong Kong, September 1999–December 2005...
Figure 4. Average influenza A (H9N2) isolation rates by
calendar day during the period with no rest-day and by days after a rest-day
during the period with rest-days, for chickens (A) and minor poultry (B)...
Appendix Figure 1. A) Monthly imported chicken ratios
(number of chickens imported to the total sales of chickens), January
1999–December 2005...
Appendix Figure 2. A) Weekly temperature and B) relative humidity, 1999–2005.
Table
Suggested Citation for this Article
Lau EHY, Leung YHC, Zhang LJ, Cowling BJ, Mak SP, Guan Y, et al. Effect of interventions on influenza A (H9N2) isolation in Hong Kong's live poultry markets, 1999–2005. Emerg Infect Dis [serial on the Internet]. 2007 Sep [date cited]. Available from http://www.cdc.gov/EID/content/13/9/1340.htm
1These authors contributed equally to this article.
Please use the form below to submit correspondence to the authors or contact them at the following address:
Gabriel M. Leung, Department of Community Medicine and School of Public Health, Li Ka Shing Faculty of Medicine, The University of Hong Kong, 21 Sassoon Rd, Pokfulam, Hong Kong Special Administrative Region, People's Republic of China; email: gmleung@hku.hk
Please contact the EID Editors at eideditor@cdc.gov
The opinions expressed by authors contributing to this journal do not necessarily reflect the opinions of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.
This page posted August 29, 2007
This page last reviewed August 29, 2008
Centers for Disease Control and Prevention, 1600 Clifton Rd, Atlanta, GA 30333, U.S.A
Tel: (404) 639-3311 / Public Inquiries: (404) 639-3534 / (800) 311-3435