

 |
|
Medical Device Safety
|
||||||||||||||||||||||||
|
|||
|
|||
| Problems with Medtronic Intrathecal Catheters | |||
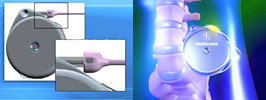 Medtronic has alerted healthcare professionals about the potential for misconnections between certain sutureless intrathecal catheters and the implanted infusion pumps to which they're attached. The affected catheters are used in conjunction with implanted Medtronic infusion pumps to deliver medications directly to the intrathecal space in the spine. They are used primarily to treat chronic, intractable pain and severe spasticity... Medtronic has alerted healthcare professionals about the potential for misconnections between certain sutureless intrathecal catheters and the implanted infusion pumps to which they're attached. The affected catheters are used in conjunction with implanted Medtronic infusion pumps to deliver medications directly to the intrathecal space in the spine. They are used primarily to treat chronic, intractable pain and severe spasticity... |
|||
| Potential Problems with Insulin Pens in Hospitals | |||
 In a recent article, the Institute for Safe Medication Practices (ISMP) highlighted several potential safety problems when hospitals switch from multiple dose vials of insulin to insulin pens. ISMP points out that there are certain safety advantages in using the pens. For example, the pens may reduce the chance of drug mix-ups, since each pen is pre-labeled with product name and strength, and the patient's name can be on the label as well. But ISMP also notes a number of potential safety problems to watch out for when hospitals switch to pens... In a recent article, the Institute for Safe Medication Practices (ISMP) highlighted several potential safety problems when hospitals switch from multiple dose vials of insulin to insulin pens. ISMP points out that there are certain safety advantages in using the pens. For example, the pens may reduce the chance of drug mix-ups, since each pen is pre-labeled with product name and strength, and the patient's name can be on the label as well. But ISMP also notes a number of potential safety problems to watch out for when hospitals switch to pens... |
|||
Updated December 1, 2008
![]()
CDRH Home Page | CDRH A-Z Index | Contact CDRH | Accessibility | Disclaimer
FDA Home Page | Search FDA Site | FDA A-Z Index | Contact FDA | HHS Home Page
Center for Devices and Radiological Health / CDRH