

Agenda for Quarterly Meeting on MDUFMA / MDUFA Performance
9:00 – 11:00, Thursday, October 30, 2008
Room 020B, 9200 Corporate Blvd.
________________________________________
Welcome
FDA MDUFMA / MDUFA Performance — Actions through Sept. 30, 2008
Qualitative Update on Finances
Overview of Medical Device Guidance Issued During FY 2008 Q4
Guidance Initiatives for FY 2009
The Role of the Matrix at CDRH
Training
OIVD Initiatives
GAO and Congressional Oversight Relating to FDAAA
Update on recent GAO studies and FDA reports to Congress. Phil Dejardins, CDRH-OCD.
Update on the Third-party Inspection Program
IT
Discussion
| Source | FY 2008 Authorized | FY 2008 Fee Revenues | Surplus (Deficit) | |||
|---|---|---|---|---|---|---|
| Receipts | Refunds | Net | % of Expected | |||
| Establishment Registration Fees 1 | $ 21,751,500 | $ 23,708,410 | $ 82,771 | $ 23,625,639 | 108.6% | $ 1,874,139 |
| Application / Reporting Fees 2 | $ 26,679,500 | $ 26,037,989 | $ 1,254,536 | $ 24,783,453 | 92.9% | $ (1,896,047) |
| Uncategorized 3 | $ 322,154 | $ - | $ 322,154 | $ 322,154 | ||
| Total 4 | $ 48,431,000 | $ 49,746,399 | $ 1,337,307 | $ 48,731,246 | 100.6% | $ 300,246 |
Notes:
Comparison: Medical Device User Fee Collection in Prior Years Excludes Unearned Fees, Includes Refunds |
||||
| FY 2003 | FY 2004 | FY 2005 | FY 2006 | FY 2007 |
|---|---|---|---|---|
| $21,620,549 | $ 25,309,853 | $31,801,091 | $34,567,188 | $26,893,394 |
During FY 2008, FDA issued 38 medical device guidance documents.
Fourth Quarter (July 2008 – September 2008) — 11 Publications
Draft Guidance for Industry and FDA Staff - Clinical Investigations of Devices Indicated for the Treatment of Urinary Incontinence — 09/19/2008
Available at http://www.fda.gov/cdrh/ode/guidance/1636.pdf
Guidance for Industry and FDA Staff - Information for Manufacturers Seeking Marketing Clearance of Diagnostic Ultrasound Systems and Transducers — 09/09/2008
Available at http://www.fda.gov/cdrh/ode/guidance/560.pdf
Guidance for Industry and FDA Staff - Establishing Safety and Compatibility of Passive Implants in the Magnetic Resonance (MR) Environment — 08/21/2008
Available at http://www.fda.gov/cdrh/osel/guidance/1685.pdf
Guidance for Industry - Medical Device Tracking; Guidance for Industry and FDA Staff — 08/15/2008
Available at http://www.fda.gov/cdrh/comp/guidance/169.pdf
Guidance for Industry and FDA Staff: Clinical Study Designs for Catheter Ablation Devices for Treatment of Atrial Flutter — 08/05/2008
Available at http://www.fda.gov/cdrh/ode/guidance/1678.pdf
Draft Guidance for HDE Holders, Institutional Review Boards (IRBs), Clinical Investigators, and FDA Staff - Humanitarian Device Exemption (HDE) Regulation: Questions and Answers — 08/05/2008
Available at http://www.fda.gov/cdrh/ode/guidance/1668.pdf
Guidance for Industry, FDA, and Foreign Governments - FY 2009 Medical Device User Fee Small Business Qualification and Certification — 08/01/2008
Available at http://www.fda.gov/cdrh/mdufma/guidance/2009.pdf
Guidance for Industry and FDA Staff - Class II Special Controls Guidance Document: Bone Sonometers — 07/16/2008
Available at http://www.fda.gov/cdrh/ode/guidance/1547.pdf
Guidance for Industry and FDA Staff - Surveillance and Detention Without Physical Examination of Surgeons’ and/or Patient Examination Gloves — 07/11/2008
Available at http://www.fda.gov/cdrh/comp/guidance/1141.pdf
Guidance for Industry and FDA Staff - Surveillance and Detention Without Physical Examination of Condoms — 07/11/2008
Available at http://www.fda.gov/cdrh/comp/guidance/1139.pdf
Guidance for Industry and FDA Review Staff - Intravascular Administration Sets Premarket Notification Submissions [510(k)] — 07/11/2008
Available at http://www.fda.gov/cdrh/ode/guidance/1189.pdf
Third Quarter (April 2008 – June 2008) — 9 Publications
FDA and Industry Actions on Premarket Approval Applications (PMAs): Effect on FDA Review Clock and Performance Assessment - Guidance for Industry and FDA Staff — 06/30/2008
Available at http://www.fda.gov/cdrh/mdufma/guidance/1218.pdf
Draft Guidance for Industry and FDA Staff: Class II Special Controls Guidance Document: Full Field Digital Mammography System — 05/30/2008
Available at http://www.fda.gov/cdrh/ode/guidance/1616.pdf
Guidance for Industry and FDA Staff: Display Accessories for Full-Field Digital Mammography Systems-Premarket Notification (510(k)) — 05/30/2008
Available at http://www.fda.gov/cdrh/ode/guidance/1617.pdf
Draft Guidance for Industry and FDA Staff - Class II Special Controls Guidance Document for Certain Percutaneous Transluminal Coronary Angioplasty (PTCA) Catheters — 05/30/2008
Available at http://www.fda.gov/cdrh/ode/guidance/1608.pdf
Guidance for Industry and FDA Staff: Class II Special Controls Guidance Document: Plasmodium Species Antigen Detection Assays — 05/20/2008
Available at http://www.fda.gov/cdrh/oivd/guidance/1646.pdf
Guidance for Industry and FDA Staff: Administrative Procedures for CLIA Categorization — 05/07/2008
Available at http://www.fda.gov/cdrh/oivd/guidance/1143.pdf
Guidance for Industry and FDA Staff: Hemodialysis Blood Tubing Sets – Premarket Notification [510(k)] Submissions — 04/23/2008
Available at http://www.fda.gov/cdrh/ode/guidance/1649.pdf
Guidance for Industry and FDA Staff: Investigational Device Exemptions (IDEs) for Devices Indicated for Nocturnal Home Hemodialysis — 04/15/2008
Available at http://www.fda.gov/cdrh/ode/guidance/1650.pdf
Guidance for Industry and FDA Staff: Preparation and Review of Investigational Device Exemption Applications (IDEs for Total Artificial Discs — 04/11/2008
Available at http://www.fda.gov/cdrh/ode/guidance/1637.pdf
Second Quarter (January 2008 – March 2008) — 11 Publications
Guidance for Industry: Coronary Drug-Eluting Stents— Nonclinical and Clinical Studies — 03/27/2008 Available at http://www.fda.gov/cdrh/ode/guidance/6255.pdf
Guidance for Industry: Coronary Drug-Eluting Stents - Nonclinical and Clinical Studies
(Companion to above, providing additional and more detailed guidance) — 03/27/2008
Available at http://www.fda.gov/cdrh/ode/guidance/6255comp.pdf
Expedited Review of Premarket Submissions for Devices - Guidance for Industry and FDA Staff — 02/29/2008
Available at http://www.fda.gov/cdrh/mdufma/guidance/108.pdf
Guidance for Industry and FDA Staff: Interactive Review for Medical Device Submissions: 510(k)s, Original PMAs, PMA Supplements, Original BLAs, and BLA Supplements — 02/28/2008
Available at http://www.fda.gov/cdrh/ode/guidance/1655.pdf
Guidance for Industry: Container and Closure System Integrity Testing in Lieu of Sterility Testing as a Component of the Stability Protocol for Sterile Products — 02/25/2008
Available at http://www.fda.gov/cber/gdlns/contain.pdf
Draft Guidance for Industry and FDA Staff: Establishing the Performance Characteristics of In Vitro Diagnostic Devices for the Detection or Detection and Differentiation of Influenza Viruses — 02/15/2008
Available at http://www.fda.gov/cdrh/oivd/guidance/1638.pdf
Guidance for Industry and FDA Staff: Coronary and Carotid Embolic Protection Devices - Premarket Notification [510(k)] Submissions — 02/15/2008
Available at http://www.fda.gov/cdrh/ode/guidance/1658.pdf
Guidance for Industry and FDA Staff: Recommendations for Clinical Laboratory Improvement Amendments of 1988 (CLIA) Waiver Applications for Manufacturers of In Vitro Diagnostic Devices — 01/30/2008
Available at http://www.fda.gov/cdrh/oivd/guidance/1171.pdf
Guidance for Industry and FDA Staff - Medical Glove Guidance Manual — 01/22/2008
Available at http://www.fda.gov/cdrh/ocer/guidance/1661.html
Guidance for Industry and FDA Staff - The Review and Inspection of Premarket Approval Application Manufacturing Information and Operations — 01/08/2008
Available at http://www.fda.gov/cdrh/comp/guidance/1566.pdf
Guidance for Industry and FDA Staff - The Review and Inspection of Premarket Approval Applications under the Bioresearch Monitoring Program — 01/08/2008
Available at http://www.fda.gov/cdrh/comp/guidance/1602.pdf
First Quarter (October 2007 – December 2007) — 7 Publications
Guidance for Industry and FDA Staff: Interactive Review for Medical Device Submissions: 510(k)s, Original PMAs, PMA Supplements, Original BLAs, and BLA Supplements — 12/28/2007
This edition superseded by revised guidance on 02/28/2008.
Class II Special Controls Guidance Document: Automated Blood Cell Separator Device Operating by Centrifugal or Filtration Separation Principle — 11/29/2007
Available at http://www.fda.gov/cber/gdlns/autobldcell.pdf
Draft Guidance for Industry and FDA Staff: Impact-Resistant Lenses: Questions and Answers — 10/26/2007
Available at http://www.fda.gov/cdrh/dsmica/guidance/23.pdf
Draft Guidance for Industry and FDA Staff - In Vitro Diagnostic (IVD) Device Studies – Frequently Asked Questions — 10/25/2007
Available at http://www.fda.gov/cdrh/oivd/guidance/1587.pdf
Guidance for Industry and FDA Staff: Class II Special Controls Guidance Document: Remote Medication Management System — 10/19/2007
Available at http://www.fda.gov/cdrh/ode/guidance/1621.pdf
Guidance for Industry and FDA Staff - Biological Indicator (BI) Premarket Notification [510(k)] Submissions — 10/04/2007
Available at http://www.fda.gov/cdrh/ode/guidance/1320.pdf
Draft Guidance for Industry and FDA Staff: Class II Special Controls Guidance Document: Electrocardiograph Electrodes — 10/04/2007
Available at http://www.fda.gov/cdrh/ode/guidance/1597.pdf
Jonathan Sackner-Bernstein, M.D.
Associate Director, Post Market Operations
Center for Devices and Radiological Health
October 2008
First in a series of three slides illustrating how the CDRH Matrix works. This slide "sets the stage" by simply listing each Matrix Network down the Y-axis and listing CDRH Offices across the X-axis. No relationships are shown.
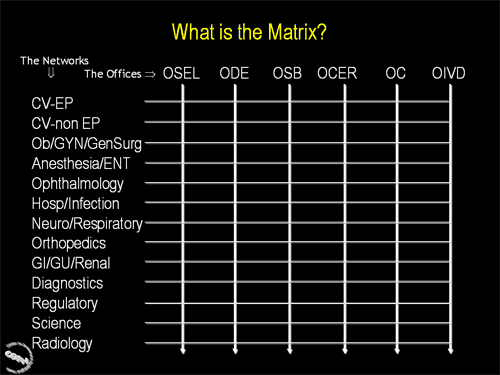
Second in a series of three slides illustrating how the CDRH Matrix works. This slide illustrates the point that every Matrix Network will work with each CDRH Office.

Third in a series of three slides illustrating how the CDRH Matrix works. This slide illustrates the point that different processes may involve different mixes of Matrix Networks and CDRH Offices.
From 10/01/2007 to 09/30/2008 (as of 10/21/08)
FY 2008 1 st through 4 th Quarter MDUFA-Related Training
FDA continues to invest in internal and external training opportunities supporting the medical device review process. CDRH’s Staff College is a premier workforce development organization that designs and delivers training opportunities to meet the science, law, leadership and professional development needs of CDRH staff.
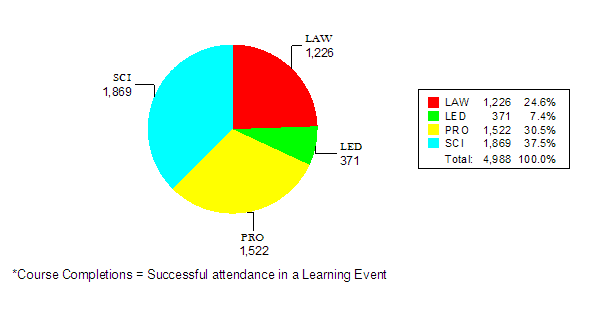
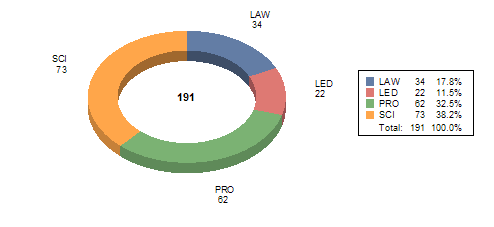
Table X attached provides a summary of internal training conducted between October 1, 2007 and September 30, 2008. The data shows 191 Staff College training courses and seminars focused on reviewer training, new scientific technologies, law, regulation and guidance updates or leadership and professional development designed to improve the device review process and support MDUFA goals and activities.
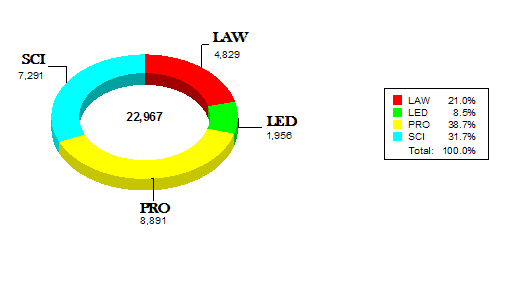
The remaining charts illustrate that 970 of the 1100 CDRH staff attended an average of 5 internal Staff College learning events representing approximately 23,000 contact hours. CDRH also had opportunities to attend other learning events with a focus on science and application review. Examples of these opportunities include:
In addition to the formal internal and external training opportunities, that CDRH provides staff, reviewers are provided informal training to assist with the application review process. These opportunities include:
Table X: MDUFA FY 08 CDRH Staff College Internal Training
Topical Area |
# of Courses |
Total # of Participants |
Examples of Internal Training Conducted/Attended Between October 1, 2007 – September 30, 2008 |
|---|---|---|---|
Science (SCI) |
73 |
1,869 |
--The CDRH Software Education Program – Modules 3-6 (14 Modules Total) --CDRH Ophthalmic and ENT Network Education Series – Sessions 1-5 of 8 --Electromagnetic Compatibility – Lecture & Lab Tour --Statistics for Clinical Trials --Meet the Experts: Cardiovascular, Asymptomatic and Symptomatic Carotid Artery Stenosis --Clinical Update in Cardiology --History of Medical Devices --Epidemiology Grand Rounds --Finite Element Analysis --Neurology Grand Rounds --Atrial Fibrillation and Drug Therapies |
Reg Regulatory and (L Law (LAW) |
34 |
1,226 |
--MDUFMA II: Performance Goals and Interactive Review Guidance Training --510(k) Essentials Hands-on Exercise --Deficiency Writing: 4-Part Harmony --Good Guidance Practices Refresher Course --Medical Device Law --Product Codes --Intro to Medical Device Software Risk --From Enforcement to Recalls and Beyond: The Office of Compliance Explained --Advanced Topics in Regulatory Issues |
Leadership Education & Development (LED) |
22 |
371 |
--Leadership Forum -Judgment – How Winning Leaders Make Great Calls -Human Resource Practices for Supervisors -Behavioral Based Interviewing --Leadership Readiness Program -Critical Conversations -Effective Presentations |
Professional Development (PRO) |
62 |
1,522 |
--Achieving Oral Communication --Speaking Under Fire --Organizational Awareness --Creating an Environment and Culture of Collaboration --Effective Facilitation --Building a Winning Scientific and Technical Team --Critical Thinking |
TOTAL |
191 |
4,988 |
|
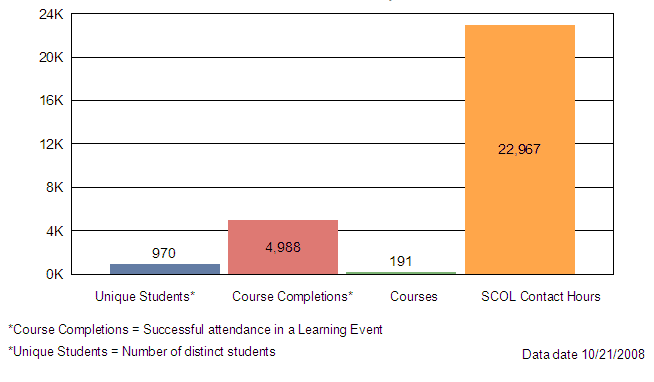
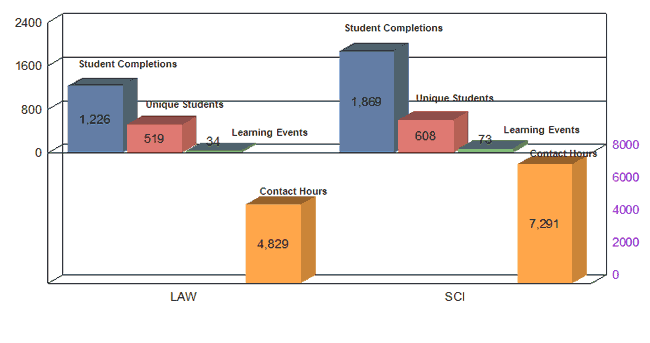
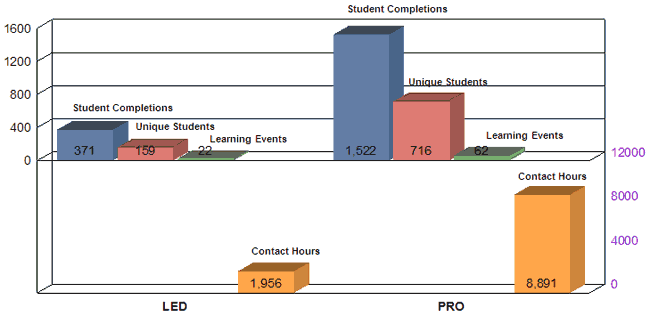
Contact Hours by Category
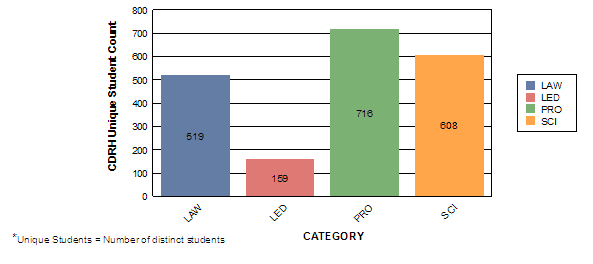
Bill Sutton
Deputy Director
Division of Small Manufacturers, International and Consumer Assistance (DSMICA)
UPDATE October 2008
Authorized under MDUFMA of 2002
Amended by FDAAA of 2007
( FDAAA changes in Italics)
Notification to FDA, Office of Compliance (CDRH or CBER)
Division of Small Manufacturers, International and Consumer Assistance (DSMICA)
http://www.fda.gov/cdrh/industry/support/
Email: DSMICA@FDA.HHS.GOV
Fax: 240-276-3151
Phone: 240-276-3150 or 800-638-2041
Updated December 5, 2008
![]()
CDRH Home Page | CDRH A-Z Index | Contact CDRH | Accessibility | Disclaimer
FDA Home Page | Search FDA Site | FDA A-Z Index | Contact FDA | HHS Home Page
Center for Devices and Radiological Health / CDRH