
Recent Advances in Wheat Head Scab Research in China
Li-Feng Chen, Gui-Hua Bai, and Anne E. Desjardins
Pathogen Biology
USDA, Agricultural
Research Service
|
Pathogen Biology |
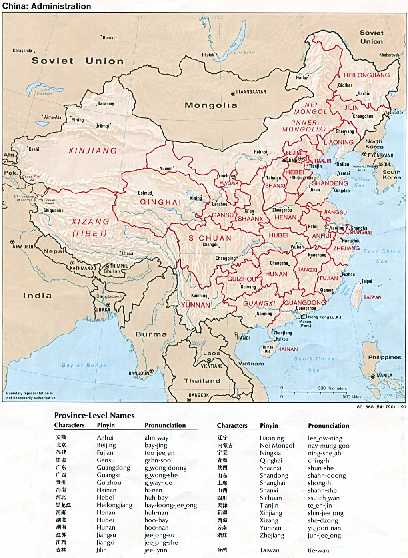
Species of Pathogens and Their Virulence
Similar WHS symptoms can be caused by several species of Fusarium. A total of 2,450 samples of scabbed spikes were collected by CWSCG from 21 provinces throughout the country, and 18 Fusarium species were identified as pathogens of WHS (CWSCG, 1984b). Among them, F. graminearum Schwabe (teleomorph=Gibberella zeae (Schw.) Petch) was the major species of Fusarium. In 18 out of 21 provinces where samples were collected, F. graminearum accounted for more than 90% of the isolates from scabbed wheat spikes (Xu and Yang, 1980; Chen and Wang et al., 1982; CWSCG, 1984b; Hu and Zhou et al., 1992). However, F. graminearum was much less frequently isolated in northwest China, including the provinces of Shaanxi and Qinghai, and Ningxia Autonomous Region. In these areas, only approximately 55 to 60% of the isolates were F. graminearum, 35% were F. culmorum, and F. avenaceum and F. tricinctum were also present (Chen, 1985; Chen and Zhao et al., 1996). F. culmorum were also found in other provinces, such as Guizhou, Yunnan, Sichuan, Jiangxi, and Jiangsu (CWSCG, 1984b). F. avenaceum was also found in the provinces of Henan, Jilin, Heilongjiang, Hubei, Fujian, Yunnan, Sichuan, and Jiangsu, and the municipality of Shanghai, where the frequency of occurrence was less than 3%, and in Ningxia
Autonomous Region where the frequency of
occurrence was 8% (Xu and Chen, 1993). F. tricinctum was
also isolated in the provinces of Jiangxi and Guizhou (CWSCG,
1984b). F. torulosum has not been reported from scabbed
wheat spikes in China, although it has been isolated from wheat
in Nepal (Desjardins AE, unpublished data).
There was a significant difference in ability to cause WHS among Fusarium species when tested either in the greenhouse or in the field by injecting fungal spores into single spikelets. The most virulent species included F. graminearum, F. culmorum, F. sambucinum (=F. sulphureum), F. camptocerus, and F. equiseti (CWSCG, 1984b). Because of its high frequency of occurrence and high virulence, F. graminearum is well accepted as the principle pathogen causing WHS in China (Xu and Chen, 1993).
Variation in Fusarium graminearum
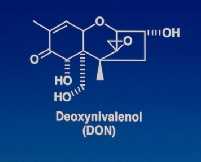
Variants of F. graminearum that differ significantly in their cultural characteristics, in DON production, and in virulence levels have been observed both in nature and in the laboratory (Chen, 1985; Xu and Chen et al., 1986; Lu and Wang et al., 1989; Wei and Lu et al., 1990; Xu and Yao et al., 1990; Wang and Zhang et al., 1997).
Variation in Cultural Characteristics
In the laboratory, variation in cultural characteristics and in virulence quite often occurs in F. graminearum following culture under artificial conditions or during long-term storage at 0 to 4 C. Sectors of the culture with a changed form of growth are a common visible phenomenon in cultures grown on potato dextrose agar plates. In one study, the frequency of culture plates with visible sectors was 4 to 11%, with an average of 8% (Xu and Xu et al., 1992). Compared with their corresponding parents, sectorial variants were characterized by a decrease or absence of aerial mycelia; deeper carmine red color on substratum; slower growth of colonies; decreased production of conidia and perithecia; and decreased virulence on wheat spikes (Xu and Xu et al., 1992). After one to ten successive transfers to new plates of medium, all 15 sectors tested generally showed the same cultural characteristics as their parents, but occasionally produced new sectors (Xu and Xu et al., 1992). Thus, the variation seems not to be a stably inheritable trait. The cause of variation in culture is not well understood. To avoid variation after successive transfers in the laboratory, isolates can be maintained for long-term storage by using either F. graminearum infected wheat spikes or F. graminearum infested wheat grain as preservation substrates at low temperature (4 C) under dry conditions. This method can keep isolates stable up to 13 years with 100% fungal survival rate, and stability of cultural characteristics, sporulation, ability to produce toxins, and virulence on wheat spikes (Xu and Lu et al., 1992).
Variation in Virulence
The virulence of F. graminearum on wheat spikes is a quantitative trait. Isolates of F. graminearum from different geographical locations or from different sources can differ greatly in virulence (Xu and Chen et al., 1986; Wang and Zhang et al., 1997). Isolates can be categorized into three types when tested by the single spikelet inoculation technique: highly virulent isolates causing scab of more than 50% of the spikelets of a spike; moderately virulent isolates causing scab of two or more spikelets but less than 50% of the spikelets of a spike; and weakly virulent isolates causing only limited disease in the inoculated spikelet and rachis (Wang and Miller, 1987; Wang and Chen et al., 1990; Xu and Chen, 1993). In a single experiment, isolates may be distinguished from each other by their virulence ratings on cultivars with different levels of resistance, but the virulence ratings of these isolates may vary in replicate tests. Extensive data have provided no evidence that stable physiological races of F. graminearum exist naturally in China (Chen, 1985; Xu and Chen, 1986; Wang and Miller, 1987; Xiao and Wu et al., 1989).
Cytological Studies on Variation
The nature of variation in F. graminearum has been investigated by a variety of methods. F. graminearum isolates, including single ascospore isolates and single germ tube isolates, were examined microscopically and nuclei were identified following ferric ammonium sulfate-hematoxylin staining (Xu and Xu et al., 1990). In this study, anastomosis (hyphal fusion) was observed between different isolates; between different hyphae of the same isolate; between different parts of a single hypha; between germ tubes of different ascospores or conidia; and between different germ tubes from different cells of the same ascospore (Xu and Xu et al., 1990). Data from this study indicated that each cell of a multicelled ascospore or conidium has one nucleus. Only 2% of the cells of a conidiophore have two nuclei, whereas the other 98% are mononucleate. All cells from germ tubes have two to seven (mostly three to five) nuclei and hyphal cells have one to seven (usually less than three) nuclei. In the anastomosis bridge, one or two nuclei can be observed, but it is not known whether the single nucleus in the bridge results from the fusion of the two nuclei. These preliminary results indicate that anastomosis between hyphae of genetically different isolates might be a source of asexual variation of the fungus (Xu and Xu et al., 1990).
Production and analysis of protoplasts and auxotrophic mutants
should facilitate further study of genetic variation in F.
graminearum. Protoplasts have been generated from mycelium
and conidia of F. graminearum treated with either
snail digestive enzymes or a combination of cellulase, snail
digestive enzymes, and lysozyme at 28 C (Xu and Hu et al., 1997).
Four auxotrophic mutants were induced by ultraviolet light, gamma
rays, or N-methyl-N'-nitro-N-nitrosoguanidine treatments (Hu
and Xu et al., 1994). Among the four auxotrophs, one was arginine
deficient, one was methionine deficient, and the other two mutants
have not yet been characterized. All four auxotrophs showed characteristics
of slower growth, less aerial mycelia, paler pigmentation in
the media, lower production of conidia, inability to produce
perithecia, and low virulence on wheat. So far, however, no experiments
on the nature of variation in F. graminearum have been
conducted using these protoplast-derived strains and auxotrophs.
Spore induction
Large numbers of spores are necessary for
biological studies of F. graminearum, for virulence tests,
and for resistance evaluation of wheat cultivars. F. graminearum
is able to produce both asexual spores (conidia) and sexual spores
(ascospores), and both types of spores can cause WHS. However,
this fungus often conidiates poorly on standard media, such as
potato dextrose agar, in the laboratory. F. graminearum
does produce large numbers of macroconidia on potato dextrose
agar in glass Petri dishes at 25 to 28 C under direct sunlight
(Jiang and Liu, 1982). Abundant conidia were also observed in
cultures irradiated at a distance of 17 cm below a 15 W ultraviolet
light tube for four hours. The most suitable temperature range
and relative humidity for conidiation were 25 to 28 C, and 50%,
respectively. Oxygen may also influence  macroconidium
production. In the presence of air, hyphae from 93% of germinated
conidia were able to produce conidia, while under vacuum conditions,
hyphae from only 20% of the germinated conidia were able to produce
conidia (Jiang and Liu, 1982). A new medium suitable for conidiation
of F. graminearum contains KH2PO4 1g, KNO3 1 g, sucrose
0.5 g, vitamin B1 3 mg, vitamin B2 1.5 mg, vitamin B6 0.2 mg,
calcium pantothenate 1 mg, nicotinamide 10 mg, vitamin C 100
mg, agar 20 g, and distilled water 1000 ml (Wang and Chen, 1995).
This medium is transparent so that conidiophores and spores can
be easily observed under the microscope (Wang and Chen, 1995).
Another simple method to produce conidia is to inoculate isolates
in mung bean liquid medium (Xu and Chen, 1993). The medium can
be easily made by boiling water with mung beans (40 g per liter)
for 10 minutes, filtering to remove the mung beans, and autoclaving
the filtrate. Most isolates of F. graminearum and other
Fusarium species produce large numbers of macroconidia
when they are incubated with shaking for three to four days on
this medium.
macroconidium
production. In the presence of air, hyphae from 93% of germinated
conidia were able to produce conidia, while under vacuum conditions,
hyphae from only 20% of the germinated conidia were able to produce
conidia (Jiang and Liu, 1982). A new medium suitable for conidiation
of F. graminearum contains KH2PO4 1g, KNO3 1 g, sucrose
0.5 g, vitamin B1 3 mg, vitamin B2 1.5 mg, vitamin B6 0.2 mg,
calcium pantothenate 1 mg, nicotinamide 10 mg, vitamin C 100
mg, agar 20 g, and distilled water 1000 ml (Wang and Chen, 1995).
This medium is transparent so that conidiophores and spores can
be easily observed under the microscope (Wang and Chen, 1995).
Another simple method to produce conidia is to inoculate isolates
in mung bean liquid medium (Xu and Chen, 1993). The medium can
be easily made by boiling water with mung beans (40 g per liter)
for 10 minutes, filtering to remove the mung beans, and autoclaving
the filtrate. Most isolates of F. graminearum and other
Fusarium species produce large numbers of macroconidia
when they are incubated with shaking for three to four days on
this medium.
To produce perithecia, wheat grain infested with F. graminearum
hyphae is placed on the surface of autoclaved sand or vermiculite
in pots. The sand or vermiculite is kept moist at 15 to 25 C
under either natural indirect light or black light. Three to
six days later, perithecia are produced on the grain (Liang and
Wang, 1981). Another four to six days later, ascospores develop
within asci in the perithecia, and are released from the perithecia.
This method is widely employed to provide ascospore inoculum
for wheat resistance tests and for fungal virulence tests.
.gif) |
USDA, ARS, National Agricultural
Library |