Wildlife Health Bulletin #04-01
To: Natural Resource/Conservation Managers
From: Leslie Dierauf, Director, USGS National Wildlife Health Center
Title: Avian Influenza in Wild Birds
A strain of avian influenza virus (H5N1) in Asia has recently killed or resulted in the depopulation of
millions of domestic chickens. Human deaths due to H5N1 virus have also been reported. The potential spread of this virus is
of international concern.
While it is common for wild birds, particularly waterbirds, to carry strains of avian influenza virus,
there is little evidence that the new virulent H5N1 virus strain is affecting wild bird populations, or that
wild birds are able to spread this Highly Pathogenic Avian Influenza (HPAI) virus. Thus far in 2004, there is a report that out
of 6,000 wild birds tested in Hong Kong, one peregrine falcon was positive for the H5N1 strain. It is not known how the bird
became infected and reports are not clear if the bird actually died from the disease.
Currently, there is no evidence that humans have been infected with the H5N1 influenza virus through contact
with wild birds. All reported human infections have been associated with contact with domestic poultry.
Direct Infecion of Humans
Historically, it was considered very unusual for avian influenza to directly infect humans. However,
recent reports [Hong Kong (H5N1), 1997; Hong Kong/China (H9N2), 1999; Netherlands (H7N7), 2003; Asia (H5N1), 2003-2004]
indicate that at least some people who have had contact with domestic poultry have become directly infected with virulent
avian influenza virus. To date, these viruses have not acquired the capacity to effectively spread directly from human to
human.
Possible Impacts to Wild Birds
Historically, avian influenza viruses recovered from wild waterbirds have rarely caused disease. The
only reported die-off was in common terns in South Africa in 1961 (Friend, 1999). Little is known about the potential
impact the recent H5N1 avian influenza virus found in Asia may have on wild birds. There is a concern that a genertic
shift in the virus may have occurred during replication in domestic poultry, making this avian influenza virus virulent
to waterbirds.
In January 2004, ProMed (Archive No. 20040121.0243) reported that of the 6,000 wild birds tested during
extensive surveillance and testing of wild birds (unspecified species) in Hong Kong only one dead peregrine falcon was positive
for the H5N1 avian influenza virus. The falcon was found near two chicken farms and it is not known how it became infected. It
is also not clear if the bird actually died from the disease. A 2002 report
http://www.oie.int/eng/info/hebdo/AIS_07.HTM#Sec0 and
www.oie.int/eng/info/hebdo/AIS_35.HTM#Sec0 suggests that a strain of
H5N1 killed non-domestic birds in parks and a zoological collection in Hong Kong, including waterfowl, greater flamingos,
gray herons and egrets. There is no definite evidence that the 2003/2004 virulent H5N1 virus is affecting wild bird
populations, or that wild birds are able to disseminate this new Highly Pathogenic Avian Influenza (HPAI)
virus.
Virus Drift - High-density populations with high rates of virus replication
When a influenza virus is introduced to domestic fowl, multiple passages (generations) of virus occur
through sequential infection of individual birds in that population, which provides an excellent opportunity for the virus
to genetically mutate. This can happen quite rapidly when poultry are housed at high densities in confined quarters, allowing
the virus to spread quickly. As the virus progresses through a poultry flock, small changes in the virus genetic material,
known as "genetic drift", may result in the appearance of a virus strain that is highly pathogenic to poultry.
Some strains of HPAI have the ability to cause 90% mortality in domestic farmed fowl. Infected fowl can become virus pumps
producing and shedding large quantities of infectious virus that contaminated the local environment, facilitating transmission
of the virus within the population, which also increases the probability of virus spreading to sites/farms beyond the location
of the original outbreak. This geographic spread is rapid in situations of poor management practices or movement (smuggling)
of infected birds.
Virus Shift - Different species acting as mixing vessels for virus genes
Increased virulence may occur when genes from two different influenza strains reassort during co-infection
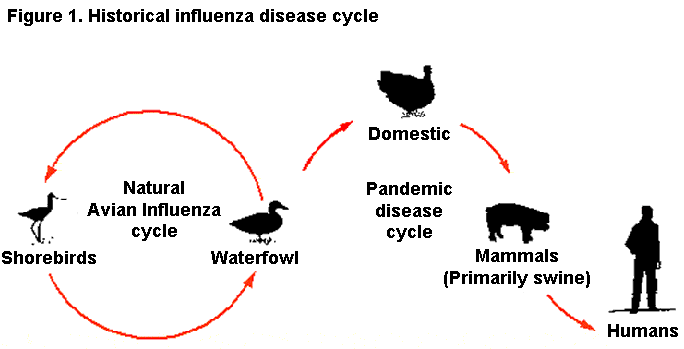
in a single host, expanding the range of animals the virus can infect. A classic example of a viral mixing vessel for this
gene reassortment is swine (Figure 1). If a swine influenza virus and an avian influenza virus simultaneously infect a pig,
the two different influenza viruses can swap genetic material as they both replicate in the pig host. This is an example of
genetic shift. If the avian influenza virus acquires genes required for mammalian infection and transmission, the new avian
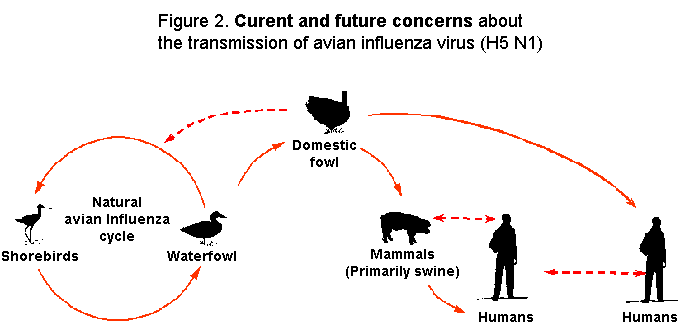
influenza virus may gain the unique ability to spread easily between mammals, possibly including humans (Figure 2).
Evidence suggests that domestic quail can also become mixing vessels for avian influenza viruses because
they have the potential to become infected by multiple avian influenza subtypes. One report proposes taht the simultaneous
infection of domestic quail with influenza subtypes from geese, ducks, and quail provided the opportunity for genetic shift in
the quail virus resulting in increased virulence in new hosts, including poultry and humans (Webby and Webster, 2001). This is
believed to have been the source for avian influenza viruses that infected humans in Hong Kong in 1997 and 1999.
There is concern that humans could assume a role similar to swine and dometic quail by becoming
the "vessel" for mixing avian and human influenza genes. This would provide the opportunity for a genetic shift
in the avian influenza virus to a virus that is highly virulent and transmissible to and among humans, possibly sparking the
next influenza pandemic.
There are situations that provide an ideal opportunity for individuals to become simultaneously infected
with multiple influenza virus strains. Human contact with live bird markets that house numerous bird species in close conact,
co-located poultry (backyard flocks) and swine, and high density poultry operations are ideal for potential genetic shift,
allowing the virus to jump species.
What is needed
New information is needed that addresses the potential for the current H5N1 virus to kill wild waterbirds, and determine whether wild waterbirds play a significant role in disseminating of this virus. Surveillance of wild birds, particularly during mortality events, needs to be conducted to monitor for the appearance of H5N1 subtypes, followed by laboratory tests to assess the virulence of this virus in the wild birds, and to determine the potential role wild birds may play in disseminating this new virus.
The outbreak of avian influenza in Asia, along with the recent emergence of other high-profile zoonotic diseases such as West Nile virus and severe acute respiratory syndrome (SARS) has increased the importance of understanding the interactions among humans, domestic animals,and wildlife in the maintenance and transmission of disease and illustrates the need for collaborating among wildlife, agricultural and human health agencies.
To obtain further information, contact Christopher Brand, 608-270-2440, or Paul Slota, 608-270-2420, at the National Wildlife Health Centar.
USGS WILDLIFE HEALTH BULLETINS are distributed to natural resource/conservation agencies to provide and promote information exchange about significant wildlife health threats in their geographic region.
References
Friend, M. 1999, Field Manual of Wildlife Diseases. General Field Procedures and Diseases of Birds, U.S. Department of the Interior, U.S. Geological Survey Information and Technology Report 1999-001.
Webby, R.J., Webster, R.G. 2001 Emergence of influenza A viruses. Philosphical Transactions of the Royal Society of London B Biological Science 356, 1817-1828.
Swayne, D.E., Haverson, D.A., 1999. Inflenza in Diseases of Poultry 11th Edition, pp. 135-160. Edited by Y.M. Saif, Iowa State Press, Blackwell Publishing Company, USA.
Links to more information:
Chapter 22 of The Field Guide to Wildlife Diseases (pdf file)
Center for Disease Control - Avian Influenza (Bird Flu) Outbreak
Frequently Asked Questions about Avian Influenza and Wild Birds
USDA Avian Influenza Factsheet
OIE World Organization for Animal Health
|