Sincerely yours,
Linda Rosenstock, M.D., M.P.H. |

U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES
Public Health Service
Centers for Disease Control and Prevention
National Institute for Occupational Safety and Health
September 1999
Mention of any company name or product does not constitute endorsement by the National Institute for Occupational Safety and Health.
Copies of this and other NIOSH documents are available from
National Institute for Occupational Safety and
Health
Publications Dissemination
4676 Columbia Parkway
Cincinnati, OH 45226-1998
1-800-35-NIOSH (1-800-356-4674)
Fax number: (513) 533-8573
To receive other information about
occupational safety and health problems, call
1-800-35-NIOSH (1-800-356-4674), or
visit the NIOSH Web site at:
www.cdc.gov/niosh
DHHS (NIOSH) Publication No. 99-143
The use of respirators in the health care setting is a relatively new but important step forward in the efforts to prevent the transmission of tuberculosis (TB). Air purifying respirators provide a barrier to prevent health care workers from inhaling Mycobacterium tuberculosis. The level of protection a respirator provides is determined by the efficiency of the filter material and how well the facepiece fits or seals to the health care worker's face. A number of studies have shown that surgical masks will not provide adequate protection in filtering out the TB organism. Additionally, surgical masks are not respirators and therefore, are not NIOSH certified and do not satisfy OSHA requirements for respiratory protection. The proper use of respirators represents a significant improvement in employee protection against TB. NIOSH realizes that the use of respirators involves a number of new and perhaps confusing practices for the health care community. This manual is designed to serve as a practical guide for those individuals responsible for initiating and running a TB respiratory protection program in health care facilities.
Other areas of the hospital may also require the use of respirators but the program and respirators used may be different. If such a program exists in your facility and has an experienced program administrator, it would be effective to administer the TB respirator program under the existing program and use existing facilities for fit-testing, cleaning, maintenance, storage, etc.
This document is not designed to provide information on ventilation systems, negative pressure isolation rooms, and risk assessment methodologies, which should be included in a total TB prevention program. The TB respirator program described in this document does not supplant the respirator protection program necessary for other regulated hazards (e.g., formaldehyde or ethylene oxide) that may be found in health care facilities.
Sincerely yours,
Linda Rosenstock, M.D., M.P.H. |
| Disclaimer | |||
| Foreword | |||
| Abbreviations | |||
| Acknowledgments | |||
| Introduction | |||
| Respiratory Protection Program Elements For Health Care Workers Exposed To Tuberculosis | |||
| Respirator Program Administration | |||
| General | |||
| NIOSH Recommended Steps for Improving the Knowledge and Skills of the Program Administrator | |||
| Duties | |||
| Step 1 Conduct a TB Risk Assessment | |||
| Step 2 Select Respirators | |||
| Respirator Selection for Protection Against TB | |||
| Introduction | |||
| Types of Respirators for Protection Against TB | |||
| A. Disposable Particulate Respirators | |||
| B. Replaceable Particulate Respirators | |||
| C. PAPRs | |||
| D. Positive-Pressure Supplied-Air Respirators | |||
| Step 3 Write Standard Operating Procedures | |||
| Sample SOP | |||
| Step 4 Medically Screen All Users | |||
| Step 5 Provide Training | |||
| Respirator Training Program | |||
| Introduction | |||
| Who Should Receive Respirator Training | |||
| Who Should Conduct this Training | |||
| What Should the Training Include | |||
| OSHA Training Requirements Under 29 CFR 1910.139 | |||
| Tips For Training | |||
| Establish Specific Training Objectives | |||
| Make the Objectives Measurable and Observable | |||
| Make the Objectives Known to the Trainee | |||
| Actively Involve the Trainee in the Training | |||
| Allow Time for Adjustment | |||
| Provide Feedback | |||
| Provide Refresher Training | |||
| Tips For Reducing Resistance and Promoting Safety Behaviors | |||
| Safety Management | |||
| Supervisory Practices | |||
| Additional Responsibilities of the Supervisor | |||
| Environmental and Organizational Supports | |||
| Step 6 User Seal Check, Fit-Test, and Issue Respirators | |||
| Respirator Face Fitting Procedures | |||
| Fit-Testing Procedures | |||
| User seal checking Procedures | |||
| Step 7 Inspect, Clean, Maintain, and Store Respirators | |||
| Routine Respirator Inspection | |||
| Introduction | |||
| Inspection Before and After Each Use | |||
| Inspection During Cleaning | |||
| Cleaning, Repairing, and Storing Respirators Used For Protection Against TB | |||
| Introduction | |||
| Cleaning | |||
| A. Disassembly | |||
| B. Cleaning and Sanitizing | |||
| C. Cleaning and Sanitizing Solutions | |||
| D. Loose-Fitting PAPRS | |||
| Repair | |||
| Storage | |||
| Sample SOP | |||
| Step 8 Evaluate the Program | |||
| Respirator Program Evaluation | |||
| Annual Evaluation | |||
| Additional Evaluation | |||
| References | |||
| Appendix A | 1910.139 Respiratory Protection for M. tuberculosis | ||
| Appendix B | OSHA Instruction CPL 2.106 (TB Enforcement) | ||
| Appendix C | 1910.1020 Access to Employee Exposure and Medical Records | ||
| Appendix D | Names and Addresses of Respirator Manufacturers and Distributors | ||
| Appendix E | Respiratory Protection Checklist | ||
| Appendix F | CDC Guidelines (Pages 4-6) | ||
| Appendix G | Memorandum for OSHA Regional Administrators | ||
| Appendix H | Appendix A to 1910.134: Fit Testing Procedure | ||
| ACGIH | American Conference of Governmental Industrial Hygienists |
| AFB | Acid-fast bacilli |
| AIHA | American Industrial Hygiene Association |
| ANSI | American National Standards Institute |
| APF | Assigned Protection Factor |
| ATS | American Thoracic Society |
| cc | Cubic centimeter(s) |
| CDC | Centers for Disease Control and Prevention |
| CFR | Code of Federal Regulations |
| CNC | Condensation nuclei counter |
| CNP | Controlled negative pressure |
| DHHS | Department of Health and Human Services |
| FF | Fit-factor |
| g | Gram(s) |
| HCWs | Health Care Workers |
| HEPA filter | High-efficiency particulate air filter |
| hr | Hour(s) |
| in. | Inch(es) |
| L | Liter(s) |
| L/min | Liter(s) per minute |
| M. tuberculosis | Mycobacterium tuberculosis |
| mg | Milligram(s) |
| min | Minute(s) |
| ml | Milliliter(s) |
| NIOSH | National Institute for Occupational Safety and Health |
| OSHA | Occupational Safety and Health Administration |
| QLFT | Qualitative fit-testing |
| QNFT | Quantitative fit-testing |
| PAPR | Powered air-purifying respirator |
| PEL | Permissible exposure limit |
| PPD | Purified protein derivative |
| RPA | Respirator Program Administrator |
| sec | Second(s) |
| SOPs | Standard operating procedures |
| TB | Tuberculosis |
| USP | United States Pharmacopeia |
| µm | Micrometer |
This document was developed by Nancy Bollinger, Jeff Bryant, Walter Ruch, Jerry Flesch, Edward Petsonk, Thomas Hodous, Brian Day, Teri Palermo, Michael Colligan, Linda Martin, and Robert Mullan. Technical review and assistance were provided by Larry Reed, Roland Berry Ann, and Larry Murphy. Kim Clough, Brian Day, and Dorothy Tan-Wilhelm produced the poster. We thank Anne Hamilton and Chris Ellison for editing and Kim Clough for the cover design, photography, and formatting of the document.
Cover photographs courtesy of MSA, Alpha Pro Tech, and NIOSH.
NIOSH thanks the many reviewers who helped in completing this document, particularly the following agencies and their representatives:
CDC Hospital Infections Program, Elizabeth Bolyard
CDC Division of TB Elimination, Patricia Simone
CDC National Center for Infections Disease, Walter Bond
OSHA Demetra Collia and John Steelnack
Ruby Memorial Hospital Staff
Go back to the Table of ContentsRespiratory Protection Program Elements For Health Care Workers Exposed To Tuberculosis |
From 1985 to 1992, the incidence of tuberculosis (TB) in the general U.S. population increased approximately 14 percent, reversing a 30-year downward trend. In 1993, 25,313 new cases of TB were reported in the U.S. [CDC 1994]. Associated with this resurgence were hospital outbreaks of TB, and the emergence of multiple-drug-resistant TB. In response to these public health threats, extensive efforts were taken across the nation to improve TB-prevention and TB-control programs. As a result of these measures, since 1992, there has been a consistent decline in the number and incidence of TB (i.e., 7.4 cases per 100,000 population and 19,855 total cases in 1997) and a decline in multiple-drug-resistant TB [CDC 1998b]. The public health and the occupational risks of TB thus appear to be once again decreasing, but they remain very significant.
Health care workers exposed to patients with infectious TB require protection from that disease. Because the use of engineering controls (such as isolation rooms and ventilation) may not completely control the TB hazard, respiratory protection is needed.
When respirators are used, the Occupational Safety and Health Administration (OSHA) standard for respiratory protection for M. tuberculosis* [29 CFR 1910.139]** must be followed. OSHA has stated that it will promulgate a separate standard for TB; but until then, the use of respirators for TB exposures will be enforced under the original respiratory protection program prescribed by OSHA in 29 CFR 1910.139 (see Appendix A).
* Code of Federal Regulations. See CFR in references.
** [29 CFR 1910.139] was formerly codified at [29 CFR 1910.134]
This program requires the following:
NOTE: Each of these requirements will be addressed in more detail throughout this document.
Respirator Program Administration |
For a respirator program to be properly established and effective on a continuing basis, written SOPs must be established. One person (the program administrator) must be in charge of the program and be given the authority and responsibility to manage all aspects of the program. The administrator must have sufficient knowledge (obtained by training or experience) to develop and implement a respiratory protection program. Preferably, he or she should have a background in industrial hygiene, safety, health care, or engineering. The program administrator should report to the highest official possible (manager of the safety department, supervisor of nurses, worker health manager, infection control manager, etc.) and should be given sufficient time to administer the respirator program in addition to any other duties assigned.
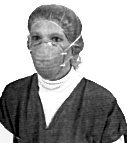 |
| N95 Disposable Respirator Photo Courtesy of Geiss. |
The administrator must be responsible for the entire program and ensure that the program is written, reviewed, and implemented. The administrator should:
Remember: Everything concerning the respirator program must be written.
Go back to the Table of ContentsConduct a risk assessment for the entire facility and for specific areas within the facility. The elements of the risk assessment are included below for complete information on how to conduct the assessment. Perform a follow-up risk assessment at the intervals indicated by the most recent risk assessment [CDC 1994, Figure 1, Table 2]. Determine who must wear a respirator and be included in the program.
1. Review the community TB profile (from public health department data). 2. Review the number of TB patients who were treated in each area of the facility (both inpatient and outpatient). (This information can be obtained by analyzing laboratory surveillance data and by reviewing discharge diagnoses or medical and infection-control records.) 3. Review the drug-susceptibility patterns of TB isolates of patients who were treated at the facility. 4. Analyze purified protein derivative (PPD)-tuberculin skin-test results of health care workers (HCWs), by area or by occupational group for HCWs not assigned to a specific area (e.g., respiratory therapists). 5. To evaluate infection-control parameters, review medical records of a sample of TB patients seen at the facility. Calculate intervals from:
Obtain the following additional information:
6. Perform an observational review of TB infection control practices. 7. Review the most recent enviromental evaluation and maintenance procedures. |
Copies of the Morbidity and Mortality Weekly Report (October 28, 1994/Vol. 43/No. RR-13) entitled "Guidelines for Preventing the Transmission of Mycobacterium tuberculosis in Health Care Facilities, 1994" may be obtained by calling 1-800-843-6356 or is available through the CDC homepage at http://www.cdc.gov.
Go back to the Table of ContentsRespirator Selection For Protection Against TB |
Surgical masks are not respirators and are not certified as such; they do not protect the user adequately from exposure to TB. Disposable respirators (e.g., N-95s) are commonly used in TB isolation rooms, in transport of TB cases, or in other areas of the health care facility. However, when high-risk procedures such as bronchoscopy or autopsy are conducted, respiratory protection exceeding the CDC standard performance criteria may be needed. This protection includes full facepiece negative-pressure respirators, powered air-purifying respirators (PAPRs), or positive-pressure airline respirators equipped with a half-mask or full facepiece.
In addition, individual medical conditions such as latex allergy, can influence respirator selections. Latex-free respirators are available.
Additional information is provided below.
I. Consideration for Selection of Respirators Personal respiratory protection should be used by a) persons entering rooms where patients with known or suspected infectious TB are being isolated, b) persons present during cough-inducing or aerosol-generating procedures performed on such patients, and c) persons in other settings where administrative and engineering controls are not likely to protect them from inhaling infectious airborne droplet nuclei. These other settings should be identified on the basis of the facility's risk assessment. Although data regarding the effectiveness of respiratory protection from many hazardous airborne materials have been collected, the precise level of effectiveness in protecting HCWs [health care workers] from M. tuberculosis transmission in health care settings has not been determined. Information concerning the transmission of M. tuberculosis is incomplete. Neither the smallest infectious dose of M. tuberculosis nor the highest level of exposure to M. tuberculosis at which transmission will not occur has been defined conclusively (59, 151, 152). Furthermore, the size distribution of droplet nuclei and the number of particles containing viable M. tuberculosis that are expelled by infectious TB patients have not been defined adequately, and accurate methods of measuring the concentration of infectious droplet nuclei in a room have not been developed. Nevertheless, in certain settings the administrative and engineering controls may not adequately protect HCWs from airborne droplet nuclei (e.g., in TB isolation rooms, treatment rooms in which cough-inducing or aerosol-generating procedures are performed, and ambulances during the transport of infectious TB patients). Respiratory protective devices used in these settings should have characteristics that are suitable for the organism they are protecting against and the settings in which they are used. A. Performance Criteria for Personal Respirators for Protection Against Transmission of M. tuberculosis Respiratory protective devices used in health care settings for protection against M. tuberculosis should meet the following standard criteria. These criteria are based on currently available information, including a) data on the effectiveness of respiratory protection against noninfectious hazardous materials in workplaces other than health care settings and on an inter pretation of how these data can be applied to respiratory protection against M. tuberculosis; b) data on the efficiency of respirator filters in filtering biological aerosols; c) data on face-seal leakage; and d) data on the characteristics of respirators that were used in conjunction with administrative and engineering controls in outbreak settings where transmission to HCWs and patients was terminated. 1. The ability to filter particles 1 µ in size in the unloaded state with a filter efficiency of > 95% (i.e., filter leakage < of 5%), given flow rates of up to 50 L per minute. Available data suggest that infectious droplet nuclei range in size from 1 mm to 5 mm; therefore, respirators used in health care settings should be able to efficiently filter the smallest particles in this range. Fifty liters per minute is a reasonable estimate of the highest airflow rate an HCW is likely to achieve during breathing, even while performing strenuous work activities. 2. The ability to be qualitatively or quantitatively fit tested in a reliable way to obtain a face-seal leakage of < 10% (54, 55). 3. The ability to fit the different facial sizes and characteristics of HCWs, which can usually be met by making the respirators available in at least three sizes. 4. The ability to be checked for facepiece fit, in accordance with OSHA standards and good industrial hygiene practice, by HCWs each time they put on their respirators (54, 55). In some settings, HCWs may be at risk for two types of exposure: a) inhalation of M. tuberculosis and b) mucous membrane exposure to fluids that may contain bloodborne pathogens. In these settings, protection against both types of exposure should be used. When operative procedures (or other procedures requiring a sterile field) are performed on patients who may have infectious TB, respiratory protection worn by the HCW should serve two functions: a) it should protect the surgical field from the respiratory secretions of the HCW and b) it should protect the HCW from infectious droplet nuclei that may be expelled by the patient or generated by the procedure. Respirators with expiration valves and positive-pressure respirators do not protect the sterile field; therefore, a respirator that does not have a valve and that meets the criteria in Supplement 4, Section I.A, should be used. References reprinted from supplement 4 [CDC 1994, page 108 & page 112]
|
NOTE: Allow users to choose from a variety of respirators (several manufacturers and sizes) to obtain the best and most comfortable fit possible.§ The minimum level of respiratory protection for TB recommended by NIOSH is the N-95 half-mask respirator.
§ See Appendix D for a list of manufacturers.
 |
| Disposable Particulate
Respirator. Photo courtesy of Alpha Pro Tech. |
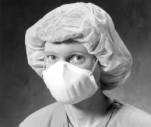 |
| Disposable Particulate
Respirator with fixed straps and no exhalation valve. Photo courtesy of MSA. |
A. Disposable Particulate Respirators
The NIOSH-certified disposable respirators labeled N, R, or P meet CDC criteria and may be obtained with or without exhalation valves. Most manufacturers also produce them in different sizes. A face shield may also be used in conjunction with a half-mask disposable respirator for protection against body fluids.
Advantages
1. The respirator is disposable and most models require
no cleaning or maintenance (See page 28).
2. The respirator is light weight and fairly comfortable to wear.
Disadvantages
1. The respirator is a negative-pressure device using the suction
produced by inhalation to draw air through the filter. The
inhalation process, even under the best of circumstances, will
allow some contaminated air to leak into the facepiece.
2. A respirator with exhalation valves cannot be used when working in a sterile field such as an operating room. The exhalation valve allows droplets and particles exhaled by the user to escape and potentially contaminate the surgical field. These respirators are also available without exhalation valves.
 |
| Disposable Particulate
Respirator with adjustable straps and exhalation valve. Photo courtesy of MSA. |
B. Replaceable Particulate Filter Respirators
The half-mask respirator also meets CDC requirements. This respirator has single or dual filters made of the same material as the N, R, and P disposable respirators (HEPA filters can also be used). Most manufacturers produce more than one size. A face shield may also be used in conjunction with a half-mask particulate filter respirator for protection against body fluids.
 |
 |
Half-Mask
Replaceable |
|
Photo (left)
courtesy of Neoterik |
|
NOTE: Manufacturer A's small size is not necessarily the same as Manufacturer B's small size.
Advantages
A. The respirator is lightweight and does not restrict mobility.
B. The respirator is made of rubber or elastomer and is durable. Only the filters need to be replaced when necessary.
Disadvantages
1. The respirator must be routinely inspected, cleaned,
disinfected, and repaired (See Step 7).
2. The respirator is a negative-pressure device using the suction produced by inhalation to draw air through the filter. The inhalation process, even under the best of circumstances, will allow some contaminated air to leak into the facepiece.
3. Communication may be difficult.
4. The respirator cannot be used in areas where a sterile field is required (surgical suite).
The full facepiece respirator also meets CDC requirements for respiratory protection against exposure to TB. The respirator can be equipped with the N, R, or P filters (HEPA filters can also be used). It is also manufactured in more than one size.
Advantages
1. The respirator provides a better seal than the half-mask and
with HEPA or 100 series filter is more protective.
2. The respirator is durable.
3. The respirator provides eye protection.
Disadvantages
1. The respirator cannot be used in areas where a sterile field
is required.
 |
| Full Facepiece Replaceable Particulate Filter Respirator. Photo courtesy of NIOSH. |
2. The respirator must be inspected, cleaned, and repaired.
3. The respirator is a negative-pressure device using the suction produced by inhalation to draw air through the filter. The inhalation process, even under the best of circumstances, will allow some contaminated air to leak into the facepiece.
4. Communication may be difficult.
5. Special lens kits are required for those respirator users who wear glasses.
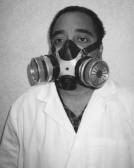
C. PAPRs
These respirators also meet CDC guidelines for protection against TB exposure. The equipment is battery operated, consists of a half or full facepiece, breathing tube, battery-operated blower, and particulate filters (HEPA only). A PAPR uses a blower to pass contaminated air through a HEPA filter, which removes the contaminant and supplies purified air to a facepiece. A PAPR is not a true positive-pressure device because it can be over-breathed when inhaling. A face shield may also be used in conjunction with a half-mask PAPR respirator for protection against body fluids.
 |
| Tight-Fitting PAPR. Photo courtesy of NIOSH. |
Advantages
1. The respirator is more protective than a half-mask respirator.
2. The respirator is usually more comfortable because air is forced into the mask by the blower, producing a cooling effect.
3. The respirator is durable.
4. Breathing resistance is lower.
Disadvantages
1. The respirator cannot be used where a sterile field is
required because it has an exhalation valve and in some cases air
can exit around the face seal.
2. Batteries must be recharged and maintained to assure proper flow rates into the mask.
3. The respirator must be inspected, cleaned, and repaired.
4. Communication may be a problem.
5. A PAPR may be bulky and noisy.
This respirator consists of a hood or helmet, breathing tube, battery-operated blower, and HEPA filters. It meets CDC guidelines.
 |
| Loose-Fitting PAPR. Photo
courtesy of Neoterik |
Advantages
1. More protective than a half-mask respirator.
2. The respirator is more comfortable because it is loose-fitting.
3. Provides a cooling effect in the hood or helmet.
4. The respirator is durable.
5. Breathing resistance is lower.
6. Vision may be better.
7. Can be worn with facial hair as long as facial hair does not interfere with valve or function of the respirator.
Disadvantages
1. The equipment cannot be used where a sterile field must be
maintained because air exits around the hood or helmet.
2. Batteries must be charged and maintained.
3. The respirator must be inspected, cleaned, and repaired.
4. Communication may be difficult.
5. A PAPR may be bulky and noisy.
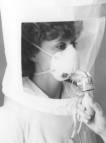
D. Positive-Pressure Supplied-Air Respirators
Supplied-air respirators use compressed air from a stationary source delivered through a hose under pressure to a half-mask or a full facepiece. A face shield may also be used in conjunction with a half-mask airline respirator for protection against body fluids.
Advantages
1. The respirator is much more protective because it provides
positive pressure in the facepiece and almost all leakage is
outward. A positive-pressure supplied-air respirator should be
used when disposable respirators, replaceable respirators, or
PAPRs do not provide adequate protection.
2. Breathing resistance is minimal.
3. The respirator is relatively comfortable to wear.
|
||
| Positive-Pressure Supplied-Air
Respirators. Photo courtesy of NIOSH. |
Disadvantages
1. The airline hose restricts the user's mobility.
2. This respirator exhausts air contaminated by the user and should not be worn during sterile procedures.
3. The respirator must be inspected, cleaned, and repaired.
4. Communication may be difficult.
5. Requires installation and maintenance of a regulated compressed air supply for Grade D breathing air.
6. Maintenance requires highly skilled, technically trained personnel.
7. Length of hose and connection point must be adequate to prevent exposure to TB when removing the respirator.
Go back to the Table of ContentsThe importance of written Standard Operating Procedures (SOPs) is emphasized by OSHA in 29 CFR Part 1910.139 which specifies the first requirement for a "minimal acceptable (respirator) program" as establishment of written SOPs governing the selection and use of respirators. Part 1910.139 does not provide any guidance for preparing these procedures and does not differentiate between large and small users.
 |
| Photo courtesy of Racal Health & Safety, Inc. |
An SOP is a detailed written procedure that describes an operation so thoroughly that it can be accomplished repeatedly and can consistently arrive at the same end point. No room for interpretation exists. The SOPs should contain all information needed to maintain an effective respirator program to meet the user's individual requirements. SOPs should be written to be useful to those directly involved in the respirator program, the program administrator, those fitting the respirators and training the workers, respirator maintenance workers, and the supervisors responsible for overseeing respirator use on the job. Generally, the procedures should cover the following topics:
| 1. | Administrative procedures: |
| A. Employer responsibilities | |
| B. Employee responsibilities | |
| C. Purchase of certified respirators | |
| D. Inventory control | |
| E. Issuance of respirators | |
| F. Special problems (beards, etc.) | |
| 2. | Respirator selection |
| 3. | Medical surveillance |
| 4. | Respirator training program |
| 5. | Respirator face-fitting procedures |
| 6. | Maintenance procedures: |
| A. Cleaning and sanitizing | |
| B. Inspection | |
| C. Repair | |
| D. Storage | |
| 7. | Program evaluation |
Guidance for an SOP follows on the next page. Additional information on SOPs may also be available from the manufacturer of the respirator.
Purpose:
The purpose of this standard operating procedure is to ensure the protection of all employees from respiratory hazards caused by exposure to TB, through the proper use of respirators.
Responsibility:
The Respirator Program Administrator (RPA) is __________________________. She/he is solely responsible for all aspects of this program and has full authority to make the necessary decisions to ensure its success. This authority includes (but is not limited to) hiring personnel, purchasing the necessary equipment to implement the program, and operate the respiratory protection program. The RPA (or designee) will develop written detailed instructions covering each of the basic elements in this program, and is the only person authorized to amend these instructions.
The ABC health care facility has expressly authorized the RPA to audit and change respirator usage procedures whenever there is a chance of exposure to TB. This includes designating mandatory respirator usage areas.
Program Elements:
| 1. | The RPA (or designee) will develop detailed written standard operating procedures governing the selection and use of respirators, using the OSHA regulations and the NIOSH Respirator Decision Logic as guidelines. Outside consultation, manufacturers assistance, and other recognized authorities will be consulted if there is any doubt regarding proper selection and use of respirators. These detailed procedures will be included as appendices to this respirator program. Only the RPA may amend these procedures. |
| 2. | Respirators will be selected on the basis of CDC guidelines. All selections will be made by the RPA (or designee). Only NIOSH certified respirators will be selected and used. |
| 3. | The user will be instructed and trained in the proper
use of respirators and their limitations. Both
supervisors and workers will be trained by the RPA (or
designee). The training should provide the employee an
opportunity to handle the respirator, have it fitted
properly, test its facepiece-to-face seal, wear it in
normal air for a long familiarity period, and finally to
wear it in a test atmosphere. Every respirator wearer
will receive fitting instructions, including
demonstrations and practice in how the respirator should
be worn, how to adjust it, and how to determine if it
fits properly. Respirators should not be worn when conditions prevent a good face seal. Such conditions may be a growth of beard, sideburns, a skull cap that projects under the facepiece, or temple pieces on glasses. No employees of this facility, who are required to wear tight fitting respirators may wear beards. Also the absence of one or both dentures can seriously affect the fit of a facepiece. The workers diligence in observing these factors will be evaluated by periodic checks. To assure proper protection, the user seal check will be done by the wearer each time she/he puts on the respirator. The manufactures instructions will be followed. |
| 4. | Where practicable, the respirators will be assigned to individual workers for their exclusive use. |
| 5. | Nondisposable respirators will be regularly cleaned
and disinfected. Those issued for the exclusive use of
one worker will be cleaned after each days use, or more
often if necessary. Those used by more than one worker
will be thoroughly cleaned and disinfected after each
use. The RPA will establish a respirator cleaning and
maintenance facility and develop detailed written
cleaning instructions. Disposable respirators will be discarded if they are soiled or are no longer functional. See the manufacturers instructions. |
| 6. | The central respirator cleaning and maintenance facility will store respirators in a clean and sanitary location. |
| 7. | Respirators used routinely will be inspected during cleaning. Worn or deteriorated parts will be replaced. |
| 8. | Appropriate (e.g., quarterly) surveillance of work area conditions and degree of employee exposure will be maintained. |
| 9. | There will be regular (e.g., annually) inspections and evaluations to determine the continued effectiveness of the program. The RPA will make frequent inspections of all areas where respirators are used to ensure compliance with the respiratory protection programs. |
| 10. | Persons will not be assigned to tasks requiring use of respirators unless it has been determined that they are physically able to perform the work and use the equipment. The ABC health care facility physician will determine what health and physical conditions are pertinent. The respirator users medical status will be reviewed annually. |
| 11. | NIOSH certified respirators will be used. |
Respirator Program Evaluation Checklist
In general, the respiratory protection program should be evaluated for each job or at least annually, with program adjustments, as appropriate, made to reflect the evaluation results. Program function can be separated into administration and operation.
A. Program Administration
| ____________ | (1) Is there a written policy which acknowledges employer responsibility for providing a safe and healthful workplace, and assigns program responsibility, accountability, and authority? | |
| ____________ | (2) Is program responsibility vested in one individual who is knowledgeable and who can coordinate all aspects of the program at the health care facility? | |
| ____________ | (3) Can administrative and engineering controls eliminate the need for respirators? | |
| ____________ | (4) Are there written procedures/statements covering the various aspects of the respirator program, including: | |
| ____________ | (a) designation of an administrator; | |
| ____________ | (b) respirator selection; | |
| ____________ | (c) purchase of NIOSH certified respirators; | |
| ____________ | (d) medical aspects of respirator usage; | |
| ____________ | (e) issuance of equipment; | |
| ____________ | (f) fitting; | |
| ____________ | (g) training; | |
| ____________ | (h) maintenance, storage, and repair; | |
| ____________ | (i) inspection; | |
| ____________ | (j) use under special conditions; and | |
| ____________ | (k) work area surveillance? | |
B. Program Operation
| (1) Respiratory protective equipment selection | |||
| ____________ |
|
||
| ____________ |
|
||
| ____________ |
|
||
| ____________ | (2) Are only NIOSH certified respirators purchased and used; do they provide adequate protection for the specific hazard? | ||
| ____________ | (3) Has a medical evaluation of the prospective user been made to determine physical and psychological ability to wear the selected respiratory protective equipment? | ||
| ____________ | (4) Where practical, have respirators been issued to the users for their exclusive use, and are there records covering issuance? | ||
| (5) Respiratory protective equipment fitting | |||
| ____________ |
|
||
| ____________ |
|
||
| ____________ |
|
||
| ____________ |
|
||
| ____________ |
|
||
| (6) Respirator use in the work area | |||
| ____________ |
|
||
| ____________ |
|
||
| (7) Maintenance of respiratory protective equipment | |||
| Cleaning and Disinfecting | |||
| ____________ |
|
||
| ____________ |
|
||
| Storage | |||
| ____________ |
|
||
| ____________ |
|
||
| ____________ |
|
||
| Inspection | |||
| ____________ |
|
||
| ____________ |
|
||
| ____________ |
|
||
| Repair | |||
| ____________ |
|
||
| ____________ |
|
||
| (8) Training and Feedback | |||
| ____________ |
|
||
| ____________ |
|
||
| ____________ |
|
||
| ____________ |
|
1. TYPE________________________________2.
NO._______________________
C.
DATE:____________________________________________________________
|
|
||||||||||||||||||||||||||||||||||||||||||
Medical Evaluation of Health Care Workers to Determine Fitness to Wear Respirators |
Respirators place several physiological stresses on wearers-stresses that particularly involve the pulmonary and cardiac systems. However, respirators typically used by health care workers are generally lightweight, and the physiological stresses they create are usually small. Therefore, most workers can safely wear respirators.
OSHA has exempted the respirator requirements of the current 1910.134 for prevention of occupational transmission of TB in health care settings. Current OSHA regulations (29 CFR 1910.139) state that workers should not be assigned tasks requiring respirators unless they have been determined to be physically able to perform the work while using the equipment. The regulations also note that a physician should determine the criteria on which to base this determination.
No general consensus exists about what elements to include in medical evaluations for respirator use in general industry. Some institutions use only a questionnaire as a screening tool; others routinely include a physical examination and spirometry; and some include a chest X-ray. No generally accepted criteria exist for excluding workers from wearing respirators. Specifically, no spirometric criteria exist for exclusion. However, several studies have shown that most workers with mild pulmonary function impairment can safely wear respirators. Thus, some journal articles and organizations recommend the following:
NOTE: Some respirators have a latex component and should not be worn by those who are allergic to latex.
Because most health care workers wear the very light, disposable half-mask respirator, CDC Guidelines [CDC 1994] recommend that a health questionnaire be the initial step in the evaluation. If results from this evaluation are essentially normal, the employee can be cleared for respirator wear. Further evaluation, possibly including a directed physical examination and/or spirometry, should be considered in cases in which potential problems are suggested on the basis of the questionnaire results.
Medical reviews of this issue including practical recommendations from several groups, including the American National Standards Institute (ANSI), AIHA, and the American Thoracic Society (ATS) are included in the references [ANSI 1992; AIHA 1993; American Thoracic Society 1996]. Sample questionnaires that have been used in health care settings are in Appendix E.
OSHA has issued a compliance memorandum (see Appendix B) and is developing a separate standard which will address the medical evaluation issue. Health care administrators should be alert to developments in this area.
If a health care facility uses respirators for worker protection against other regulated hazards (e.g., formaldehyde, ethylene oxide, etc.), then a respirator program must be implemented for these hazards under 1910.134 including medical evaluations by a physician or other licensed health care professional.
Go back to the Table of ContentsRespirator Training Program |
Equally important to selecting the appropriate respirator is using the selected device properly. Proper use can be achieved by carefully training both supervisors and workers in selection, use, and maintenance of respirators.
Provide the supervisor, the person issuing the respirators, and the respirator user with adequate training by qualified persons to ensure that the respirator is used correctly (see Respirator Program Administration in the Introduction).
The supervisor is defined as the person who oversees one or more workers who need to wear respirators. The supervisors must be in close contact with the workers to ensure that the workers are wearing respirators when necessary and that they are being worn properly. The training provided should emphasize that health and safety is an important part of the management function. It is recommended that the proper use of respirators should also be included in the supervisor's and worker's annual evaluation.
The training must be given by a qualified person-usually the Respirator Program Administrator. As an alternative, the Administrator can have someone trained to do the job or hire a consultant. See Respirator Program Administration in the Introduction for more information about respiratory protection training.
1. Describe the nature, extent, and specific hazards of TB in your health care facility (See Appendix F).
2. Explain the risk assessment and its relationship to the respirator program. The risk assessment should define facility areas requiring the use of respirators and the level of protection required. For example: normal operations might require only disposable N95 respirators. Higher-risk areas, such as autopsy rooms, could require a higher level of protection such as full facepiece negative-pressure respirators, PAPRs, or half-mask positive-pressure airline respirators.
Also, advise the trainees that risk assessment will be done periodically. CDC recommends that risk assessment be conducted at least yearly in the minimal-risk, very-low-risk, and low-risk areas; every 6-12 months for intermediate-risk areas; and every 3 months in high risk areas. The workers should also be trained to recognize signs and symbols used to show that respirators are required in an area.
3. Explain the reason for using respirators. For example, the respirator needs to be used to minimize exposure to the hazards in the workplace (in this case, TB).
Some individuals (e.g., those that are immunocompromised are at higher risk for TB (see appendix F). These individuals need to understand the risk and the need to wear their respirator.
4. Describe existing engineering controls. Engineering controls are methods used to prevent the spread and reduce the concentration of infectious droplet nuclei. Examples are ventilation controls (e.g., negative-pressure isolation rooms) and laboratory hoods. Because engineering controls may not entirely eliminate the TB hazard, the respirator wearer must be trained to know when to wear a respirator.
5. Explain the reason for selecting a particular respirator for a given hazard (see Step 2). In most cases, the N95 disposable respirator will be selected. This NIOSH-certified respirator meets minimum CDC criteria for respiratory protection in TB areas. For high-risk areas, more protective respirators may be needed (e.g., full facepiece respirators with PAPRs and positive-pressure airline respirators). The respirator chosen depends on the severity of exposure.
6. Explain how the respirator works, its capabilities, and limitations. Negative-pressure air-purifying respirators (e.g., disposable, half-mask, and full facepiece respirators) work by drawing ambient air through the filter element during inhalation. Inhalation causes a negative pressure to develop in the tight-fitting facepiece and allows air to enter while the particles are captured on the filter. Air leaves the facepiece during exhalation because a positive pressure develops in the facepiece and forces air out of the mask through the filter (disposable) or through an exhalation valve (replaceable and some disposable). PAPRs are equipped with a blower that draws air through the filters into the facepiece. PAPRs can be equipped with a tight-fitting facepiece or loose-fitting helmet or hood. Airline (supplied-air) respirators are provided with air from a stationary source (compressor) or a bottle. (See Step 2 for a discussion about the advantages and disadvantages of different respirator classes).
7. During the training session, give the user the chance to handle and wear the respirator until the user is proficient. Also teach the user how to perform the user seal check and wear the respirator in an uncontaminated environment for a period of time. Instruct the user to follow the manufacturer's instructions provided with the respirator. Give workers a copy of the manufacturers instructions.
8. Teach the user the importance of and how to properly store disposable respirators. Teach the user the importance of and how to clean, maintain, and store replaceable filter respirators (unless there is a central maintenance facility that provides this service for cleaning, see Step 7).
9. Explain that facial hair between the wearer's skin and the sealing surfaces of the tight-fitting respirator will prevent a good seal. A respirator that permits negative-air pressure inside the facepiece during inhalation may allow leakage and, in the case of positive pressure devices, will either reduce service time or waste breathing air.
10. Provide the trainees with the lecture materials (or a summary) developed by the program administrator to use as quick reference materials.
11. Instruct trainees to refer all respirator problems immediately to the respirator pro-gram administrator.
12. Discuss the OSHA standard [29 CFR 1910. 139] (see Appendix A) in detail with the trainee. Everyone must know the mandatory regulations.
Training may need to be repeated yearly to maintain the respirator skills of the users.
The training recommendations listed in the preceding section should provide the basis for an adequate training program and summarize methods for satisfying the OSHA requirements listed in 29 CFR 1910.139. These requirements are reprinted as a summary below and reproduced fully in Appendix A.
| 1910.139 (a)(3) The employee shall
use the provided respiratory protection in accordance
with instructions and training received. 1910.139 (b)(3) The user shall be instructed and trained in the proper use of respirators and their limitations. 1910.139 (e)(2) The correct respirator shall be specified for each job. The respirator type is usually specified in the work procedures by a qualified individual supervising the respiratory protective program. The individual issuing them shall be adequately instructed to insure that the correct respirator is issued. 1910.139 (e)(5) For safe use of any respirator, it is essential that the user be properly instructed in its selection, use, and maintenance. Both supervisors and workers shall be so instructed by competent persons. Training shall provide the workers an opportunity to handle the respirator, have it fitted properly, test its face-piece-to-face seal, wear it in normal air for a long familiarity period, and, finally, to wear it in a test atmosphere. 1910.139 (e)(5)(i) Every respirator wearer shall receive fitting instructions including demonstrations and practice in how the respirator should be worn, how to adjust it, and how to determine if it fits properly. Respirators shall not be worn when conditions prevent a good face seal. Such conditions may be a growth of beard, sideburns, a skull cap that projects under the facepiece, or temple pieces on glasses. Also, the absence of one or both dentures can seriously affect the fit of a facepiece. The worker's diligence in observing these factors shall be evaluated by periodic check. To assure proper protection, the facepiece fit shall be checked by the wearer each time he puts on the respirator. This may be done by following the manufacturer's facepiece fitting instructions. |
Tips For Training |
The OSHA training requirements under 29 CFR 1910.139 (see preceding section and Appendix A) consist of the "bare bones" training responsibilities of the respiratory protection program manager. A trainer can use various tips and strategies to meet these obligations and to enhance the effectiveness of the program. These tips and strategies are discussed in the following subsections.
Make sure that the trainer has a definite understanding of exactly what the trainee should know or be able to do as a result of the training. If this objective is not clear to the trainer, it will never be clear to the trainee. Also, state the objectives in such a way that the trainer can measure whether they have been achieved.
Objectives such as "the trainee will be made aware of the need for respiratory protection" or "the trainee will know how to inspect a respirator" cannot be measured directly. Instead, state the objectives using action terms or tasks the trainee should be able to do. For example: (1) "the trainee will be able to name the areas of the facility where respirators are required," or (2) "the trainee will be able to inspect a respirator and identify a defective valve." Measurable training objectives allows the trainer to determine whether people are learning what they need to know.
Inform trainees about the objectives of the training. This knowledge provides them with a framework for understanding the information that will follow and motivates them to keep their attention focused on the important points.
People generally learn better by doing than by watching or listening. Ask the trainees to put on and remove respirators, inspect respirators, replace filters, discuss respiratory protection issues, etc. Such activities are much more effective than having trainees read about these procedures or merely listen to a description. Films and demonstrations are useful in modeling the desired behavior, but it is important to have the trainee actively replicate what was just witnessed.
Wearing a respirator for the first time can be a strange and even traumatic experience for the new user, particularly if the user is given a respirator and immediately put to work. The barriers of respirator usage noted earlier (such as labored breathing and impaired vision) coupled with the demands of the job may be too great for many first-time users, increasing their intolerance to the respirator and reducing the likelihood of future compliance.
Allow the user to gradually adjust to the respirator by wearing it for short periods in a relaxed, non-work setting. Tell new users that it is normal to feel a little strange and frightened the first time they wear a respirator. This information lets them know that their initial adverse reaction does not indicate a chronic personal intolerance to respirators and that their tolerance will improve. Once users become accustomed to the physical and psychological effects of wearing a respirator, it will be easier for them to perform their normal work routine.
Give feedback to workers during the initial training and in the workplace to tell them what they are doing right or wrong. The feedback should always be positive, constructive, and specific. Thus comments such as "keep up the good work" or "good job" (although complimentary) provide little information. Instead say "good job in replacing your filter" or "John, you need to remember to check your seal every time you put on your respirator." The point is not to criticize or punish the individual but to provide corrective instruction.
Establish a schedule for periodically evaluating on-the-job performance and providing refresher training when indicated. Such training is needed because unfortunately, the effects of training do not last forever. Forgetting or relapse occurs over time-especially if the behaviors are not frequently practiced or rehearsed (that is, respirators are worn only occasionally), if the behavior is costly and complex (for example, inspection and maintenance), and if continuous monitoring and corrective feedback are not provided.
Tips For Reducing Resistance And Promoting Safety Behaviors |
No matter how much time and effort are put into developing a respiratory protection program, it is doomed to fail if workers do not wear the equipment properly under the prescribed conditions. Workers fail to wear respirators for a number of reasons, and it is important to understand the nature of this resistance to overcome it. The following are the most frequently cited reasons for not wearing respirators:
| 1) | They are hot and uncomfortable. |
| 2) | They produce "pain spots" if poorly fitted. |
| 3) | They interfere with communication and performance. |
| 4) | They are not easily accessible when you need them. |
| 5) | They put the burden of safety on the wearer rather than the company |
| 6) | They make the wearer look "funny," alarmist, not macho, or unattractive. |
| 7) | They produce labored breathing, increased heart rate, and perspiration. |
| 8) | They impair vision and can actually be a safety hazard. |
| 9) | They produce feelings of claustrophobia and anxiety. |
In addition to these numerous barriers to working while wearing a respirator, the benefits (that is, the avoidance of disease) may seem remote. Furthermore, since air contaminated with infectious droplet nuclei have no over-whelming noxious properties or physical effects, there is no immediate consequence for not wearing a respirator except that the user feels better without it. Therefore, the program manager must work hard to overcome worker resistance to wearing respirators and promote full compliance with the respiratory protection program.
For a worker to behave safely, three conditions must be met: (1) the worker must have the necessary knowledge, skills, and ability; (2) the worker must be properly motivated; and (3) the worker must receive the necessary environmental and organizational supports.
The first condition is addressed by the training program, the second by supervisory practice, and the third by organizational climate and policy. The first factor is addressed in the preceding section (Tips for Training) and the latter two factors are considered briefly as follows.
Motivating workers to behave safely is a major responsibility of the supervisor or program administrator. Workers must not only know how to maintain and wear respirators, they must actually wear them when working in a hazardous environment. To convince a worker to wear a respirator, the worker must see that the benefits of respirator use outweigh the barriers. The first step in this direction is the training program, which describes workplace hazards, their consequences, and the role of respirators in reducing these hazards. Although different models exist for presenting this information, one of the most popular is the Health Belief Model developed by Becker [1974]. To use this model to foster respirator use, a worker must:
| Feel susceptible to the disease or condition related to the hazard |
The worker must understand that the disease is related to exposure, and that symptom onset may be delayed. In explaining TB, tell the worker how the inhaled droplet nuclei from an infectious person's cough or sneeze lodge themselves in the alveoli of the lungs, where infection begins. No immediate symptoms will occur as the infection spreads to other areas in which TB is most likely to develop. The immune system usually intervenes within 2 to 10 weeks after infection and halts the multiplication of tubercle bacilli. Approximately 10% of those infected will develop the disease. The other 90% will remain infected but free of disease for the rest of their lives. Many workers do not feel susceptible because they have no firsthand experience with TB and do not understand how it develops. Cause and effect must be established in a straightforward, concrete fashion.
| Believe the illness poses serious consequences to health and well-being |
In addition to feeling susceptible to TB, workers must also understand its consequences to health and well-being. Training programs often describe the illness in abstract medical terms or use scare tactics to exaggerate the symptoms. Instead, describe the disease in a realistic and imaginable fashion. Describe the impact of the illness on life-style factors such as family interactions, hobbies, and recreational activities. Use case histories, testimonies from illness victims, and illness simulations to help workers identify with the impact of the disease on themselves and others. Relate that preventive drug therapy can result in serious health effects (e.g., liver damage).
| Believe that the respirator can control the risk |
The worker must understand that the use of a respirator can effectively reduce the risk of exposure. To convey this information, explain the way respirators work and the importance of proper use, fit-testing, and maintenance. Use the fit-testing exercise to simulate leakage, and give the worker a sense of how a toxic agent can be inhaled if the seal is not sufficient. Workers need to know the basic principles of respirator operation in order to accept them and believe they can provide protection if used properly.
| Believe that the benefits of respirator use outweigh the barriers |
Wearing a respirator is a major inconvenience to most workers. A training program that ignores this point lacks credibility. Instead, emphasize that despite the drawbacks, wearing a respirator and protecting one's health are worth the inconvenience.
In addition to providing workers with the kind of information described above, the immediate supervisor must take steps to assure that workers' intentions to wear respirators are carried over and sustained in the workplace. This is a continuing responsibility of the supervisor-much like work scheduling and production oversight. The supervisor must convey to the worker his or her commitment to the respirator program through actions as well as words. Examples of what this requires from the supervisor are as follows:
Make workers aware of their own role in motivating others to wear respirators. Peer influences are often effective in encouraging self-protective behavior. Inform physicians that they often serve as role models for other hospital workers, and that by the simple act of wearing a respirator, they may be encouraging many other workers to wear respirators. Unfortunately, the opposite may also be true.
A safety management program cannot succeed without the sincere support and commitment of the highest levels of the organization. A token or superficial endorsement of the safety program is quickly perceived by members of the organization as manipulative and hypocritical; it is likely to produce resentment and resistance. Steps an organization can take to demonstrate its level of commitment to the respiratory protection program might include the following:
Respirator Face Fitting Procedures |
The following step presents procedures for user seal checking and fit-testing respirators used by health care workers exposed to TB. All respirators (excluding loose-fitting models) must be fit-tested and user seal checked. A user seal check is a method for determining whether a respirator has been put on and adjusted to fit properly and is performed every time a respirator is worn. A fit-test is a method used to select the respirator that provides an adequate and comfortable fit. Fit tests should be completed at regular, periodic intervals (e.g., annually) to ensure continued adequate fit.
Note: Respirator users who are not clean-shaven, should not be fit-tested with tight-fitting respirators because facial hair between the skin and the sealing surfaces of the respirator will prevent a good seal. Tight-fitting respirators cannot be assigned to or used by workers with facial hair that interferes with the seal.
 |
| Saccharin or Bitrex
Qualitative Fit Testing. Photo courtesy of 3M. |
A fit-test must be conducted to determine which brand, model, and size of respirator fits the user adequately and to ensure that the user knows when the respirator fits properly. Such knowledge is important because TB aerosol can leak around the facepiece into the respirator and be inhaled if the respirator does not fit the user's face. In the December 11, 1998, MMWR article, NIOSH found that fit testing " N95 respirators is essential in programs employing these respirators and can eliminate poorly fitting respirators, ensuring at least the expected level of protection. Without surrogate fit testing, average exposure for the 25-person panel was reduced to 33% of the ambient level, which is much less protection than expected of this class of respirators (i.e., exposure reduced to <10% of ambient levels) However, when fit tested first, the panel received substantially greater protection than normally expected (the average exposure was reduced to 4% of the ambient level). Without fit testing, persons unknowingly may have poor face seals, resulting in excessive leakage and exposure" [CDC 1998a]. Fit-testing is also required by OSHA [29 CFR 1910.139(e)(5)]. Determining facepiece fit involves qualitative fit-testing (QLFT) or quantitative fit-testing (QNFT). A QLFT test relies on the wearer's subjective response to taste, odor, or irritation. A QNFT uses another means of detecting facepiece leakage and does not require the wearer's subjective response.
 |
| Portacount unit. Photo courtesy of 3M. |
Respirator models have inherently different fitting characteristics. Moreover, each of the several brands that are marketed has slightly different fitting characteristics. Although every manufacturer designs facepieces to fit the broadest possible section of the working population, no single respirator fits everyone. Therefore, more than one brand or model, and various sizes of a given type of respirator should be purchased to take advantage of the different fitting characteristics of each and to increase the chances of properly fitting all workers. Having more than one facepiece from which to choose also gives the worker a better chance of finding a respirator that provides reasonable comfort and good protection.
The respirator program administrator must decide whether to use QLFT or QNFT procedures.
After fit-testing, a wallet-sized card should be provided to the respirator user showing worker's name, date, type, brand, model, and size of respirator.
NOTE: For facilities conducting QNFTs on disposable and replaceable half-masks, OSHA requires a minimum fit-factor (FF) of 100. When an individual passes a QLFT, a minimum FF of 100 will be assumed to have been achieved. (See Appendix G, OSHA Memorandum For Regional Administrators Regarding Fit-Testing and User Seal Checking Procedures).
NIOSH does not recommend qualitative fit testing using irritant smoke because of the health risk associated with exposure to the irritant fume.
Fit-checking procedures that have been accepted by OSHA in 1910.134 can be found in Appendix H. When the TB standard is promulgated, specific guidance will be included.
To ensure adequate protection, the user of a respirator equipped with a tight-fitting facepiece must check the seal of the facepiece routinely before each entry into areas with potential TB exposures. This check may be accomplished by using the seal-check procedures recommended by the manufacturer or by using those described in Appendix H.
 |
| Negative-pressure user seal check. |
 |
| Positive-pressure user seal
check. Both photos courtesy of NIOSH. |
Routine Respirator Inspection |
Scrupulous respirator inspection and maintenance must be an integral part of the overall respirator program. Follow the manufacturer's instructions for inspection, cleaning, and maintenance to ensure that the respirator continues to function properly. Wearing poorly maintained or malfunctioning respirators may be more dangerous than not wearing a respirator at all. The worker who wears a defective device may falsely assume that protection is being provided.
The OSHA respirator regulations require the following [29 CFR 1910.139]:
| 1. [b] (7) Respirators used
routinely shall be inspected during cleaning. Worn or
deteriorated parts shall be replaced. Respirators for
emergency use such as self-contained devices shall be
thoroughly inspected at least once a month after each
use. 2. (f) (2) (i) All respirators shall be inspected routinely before and after each use. A respirator that is not routinely used but is kept ready for emergency use shall be inspected after each use and at least monthly to assure that it is in satisfactory working conditions. 3. (f) (2) (iii) Respirator inspection shall include a check of the tightness of connections and the condition of the facepiece, headbands, valves, connecting tube, and canisters. Rubber or elastomer parts shall be inspected for pliability and signs of deterioration. Stretching and manipulating rubber or elastomer parts with a massaging action will keep them pliable and flexible and prevent them from taking a "set" during storage. |
A. Examine the facepiece of the disposable respirator to determine whether it is functional and has structural integrity. If the filter material is physically damaged or soiled, discard the respirator. Also discard the respirator if there are nicks, abrasions, cuts, or creases in the facepiece-to-face sealing material.
B. Check the respirator straps to be sure they are not cut or otherwise damaged. The straps should be attached at all connection points.
C. Make sure that the metal nose clip (if applicable) is in place and functions correctly.
D. Make sure that the respirator is NIOSH approved (NIOSH approval will be marked on the filter, filter package, or respirator box).
A. Check the integrity of the facepiece to be sure it is not cut, torn, modified, deteriorated, or dirty. The elastomer should not be abraded, and the sealing surface should be smooth and undamaged.
 |
Check straps and buckles. Photo courtesy of NIOSH |
B. Check to see that the straps on the respirator are elastic, pliable, and have not been knotted to shorten them. The buckles and any attachment must be present and working correctly.
C. Inspect the inhalation and exhalation valves to see that they are in place and pliable, functioning properly, and lying flat on the surface of the valve seat. The sealing surfaces must be clean and not chipped, scratched, or broken.
D. Make sure that the exhalation valve covers are present and attached to the respirator.
E. An approved half-mask respirator includes the facepiece and filters. Check the respirator to be sure the correct filters for the hazard are in place. The filter and filter holder threads should not be scratched, chipped, or otherwise damaged. If gaskets are required between the filter and filter holder be sure they are in place and in good condition. Remove the gaskets to check for dirt under them.
F. Make sure that the gaskets fit properly in the filter holders.
G. If the filters seal directly against the facepiece, be sure that the sealing surface is not torn, chipped, cut, or otherwise damaged.
H. Inspect the filters to be sure that the threads are not scratched, chipped, dented, or otherwise damaged.
I. The strap assembly will usually have corrugations in the rubber that holds the strap tightly once it is placed on the head and tightened. Be sure that the corrugations are not worn off, all clips are present, and the straps are attached to the mask.
A. Check to see that the lens in a full facepiece respirator is not scratched, cracked, broken, or otherwise damaged. The lens should be completely sealed around the facepiece.
B. If the respirator has a speaking diaphragm, make sure that it is in place, not punctured, and that the gasket is in place.
C. Check the integrity of the facepiece to be sure it is not cut, torn, modified, deteriorated, or dirty. The elastomer should not be abraded and the sealing surface should be smooth and undamaged.
D. Make sure that all the required clamps are in place and are specific for the respirator being inspected.
E. Inspect the inhalation and exhalation valves to see that they are in place and pliable, functioning properly, and lying flat on the surface of the valve seat. The sealing surfaces must be clean and not chipped, scratched, or broken.
F. An approved full facepiece respirator includes the facepiece and the filters. Check the respirator to be sure the correct filters for the hazard are in place. The filter and filter holder threads should not be scratched, chipped, or otherwise damaged. If gaskets are required between the filter and filter holder be sure they are in place and in good condition. Remove the gaskets to check for dirt under them.
G. The strap assembly will usually have corrugations in the rubber that holds the strap tightly once it is placed on the head and tightened. Be sure that the corrugations are not worn off, all clips are present, and the straps are attached to the mask.
H. Check to see that the straps on the respirator are elastic, pliable, and have not been knotted to shorten them. The buckles and any attachment must be present and working correctly.
I. Make sure that the exhalation valve covers are present and attached to the respirator.
J. Make sure that the gaskets fit properly in the filter holders.
K. If the filters seal directly against the facepiece, be sure that the sealing surface is not torn, chipped, cut, or otherwise damaged.
L. Inspect the filters to be sure that the threads are not scratched, chipped, dented, or otherwise damaged.
A. Stretch out the corrugated breathing tube to inspect it for cuts, abrasions, and pinholes.
B. Inspect the blower assembly and batteries as described by the manufacturer.
C. The inspection procedures for half-masks and full facepieces used with PAPRs are the same as those described above.
D. If the PAPR is equipped with a hood or helmet, inspect according to the manufacturer's instructions.
A. The inspection procedures for half-masks and full facepieces used with supplied-air devices are the same as those described for air-purifying respirators (excluding filter cartridges). If the respirator is equipped with a corrugated hose, stretch it out and inspect for cuts, abrasions, and pinholes.
B. Check the condition of the air supply hose, including attachments and end fittings.
C. Check the regulator as described by the manufacturer.
The respirator must also be inspected during cleaning:
1. Use the same inspection procedures followed before and after each use, but remove all parts of the respirator from the mask and check for dirt, damage, and non-flexibility.
2. Replace defective parts.
3. Thoroughly wash, disinfect, and reassemble the parts and mask.
Cleaning, Repairing, And Storing Respirators Used For Protection Against TB |
The OSHA respirator regulation (see Appendix A) requires that respirators be properly cleaned, repaired, and stored. A proper maintenance program ensures that the worker's respirator remains as effective as when it was new.
Generally, disposable respirators do not need to be cleaned or maintained. If they are soiled or otherwise damaged they are discarded. However, some manufacturers make disposable respirators that look like replaceable respirators (filters cannot be removed or replacement parts are not available) and the facepiece may require some cleaning.
Replaceable filter respirators must be carefully maintained. The manufacturer's recommendations should be followed to ensure proper functioning of the respirator. The following discussion of maintenance procedures should help users understand the overall process:
A. Disassembly
Respirators cannot simply be immersed in cleaning solutions.
Before cleaning and sanitizing, remove the following parts from
the facepiece:
B. Cleaning and Sanitizing
Follow the manufacturer's instructions for cleaning and
sanitizing respirators, especially with regard to maximum
temperatures. These steps are generally as follows:
1. Wash the respirator in warm water containing a mild detergent at the temperature recommended by the manufacturer. A combination cleaner/sanitizer solution can also be used (see the following subsection, Cleaning and Sanitizing Solutions). NEVER use an organic solvent to clean a respirator.
2. The elastic straps are cleaned by using a bristle brush and mild detergent.
3. If a cleaner/sanitizer solution has not been used, sanitize and rinse the respirator in clean water. Use the manufacturers' recommended temperature.
4. Drain water from the respirator and allow it to air-dry in a clean and sanitary location.
5. Clean and sanitize all the parts previously removed from the respirator.
6. Wipe the respirator and all its components with a cloth to remove any remaining water.
When a large number of respirators must be cleaned, a commercial clothes washer and dryer can be used if they have been modified to hold the facepieces in a fixed position.
C. Cleaning and Sanitizing Solutions
Use cleaning and sanitizing solutions as follows:
1. Use any good detergent to clean a respirator or use specifically designed cleaners or sani-tizers (a class of liquid chemical germicides having surfactant action). A sanitizer is often a quaternary ammonium compound.
2. Follow the instructions on the sanitizer label for immersion times.
3. Rinse the cleaned and disinfected respirators thoroughly in clean water at the manufacturer's recommended temperature to remove all traces of detergent and sanitizer. This step is very important to prevent dermatitis in respirator users.
D. Loose-Fitting PAPRS
To clean loose-fitting PAPRs, remove the hood or helmet from the
respirator and clean with a detergent solution. Clean the
suspension inside the head gear in a similar fashion. Clean and
sanitize the protective face shield.
Repair respirators as follows:
1. Inspect the respirator and all its parts.
2. Replace defective parts with parts designed for that particular respirator. Use only replacement parts from the respirator manufacturer.
3. Reassemble the respirator and its parts.
4. Attach new filters to the respirator.
5. Inspect the entire respirator for completeness and tightness of parts.
Store respirators as follows:
1. Fulfill OSHA requirements by packing or storing the respirators so that the facepieces and exhalation valves rest in normal positions [29 CFR 1910.139(f)(5)(ii)]. Impaired function will result if the elastomer sets in an abnormal position.
2. Store disposable respirators at the entrance to designated TB areas so that users can pick them up when entering. One method for accomplishing this is to install a box with sufficient compartments for storing all the respirators required in that area. The storage bin would look like a mail box with slots for each user's respirator. Each slot would be labeled with the user's name.
3. Never store disposable respirators in pockets, plastic bags, or other confined areas.
4. Store replaceable filter half-mask and full facepiece respirators in plastic bags after drying and keep them in storage cabinets. Store them in a single layer with the facepieces and exhalation valves in normal positions to prevent the elastomer from taking a permanent "set."
NOTE: Always read and follow the manufacturer's instructions for cleaning, sanitizing, repairing, inspecting, and storing the respirator.
Two example SOPs for maintenance, cleaning, and storage follow:
Standard Operating Procedure # ____
Maintenance Of Brand ___________ Replaceable Filter Half-Mask
Respirator
Hospital name: ________________________________
Address: ______________________________________
______________________________________
Program administrator (or other designated author)
_________________________
Date SOP written _______________
Date signed by program administrator _______________
Date to be reviewed ________________
Disassembly:
| 1. | Have central supply personnel collect used respirators at the designated collection points located in the hallway of each TB isolation room. |
| 2. | Take used respirators to the respirator maintenance area in central supply. |
| 3. | Remove all parts from the respirator as recommended
by the manufacturer: A. Filters and gaskets |
| 4. | Inspect the respirator for damaged and defective
parts; discard them if found. Replacement parts must be
identical to the original parts, or NIOSH-accepted
alternatives obtained from the respirator manufacturer. A. Check the filters for damage (dents or cracks), filter soiling, and damaged threads (scratched, chipped, or dented). Inspect the gaskets for pliability and wear. B. Check the exhalation valve cover for defects. C. Examine the inhalation and exhalation valves for cracks, tears, holes, or distortion in the valve material. E. Examine the headbands for breaks, loss of elasticity, and malfunctioning buckles and attachments. F. Check the facepiece for cracks, tears, holes, lack of flexibility, or distortion from improper storage. |
Cleaning and Sanitizing:
| 1. | Clean and sanitize the respirator and parts using ____________ at the following temperature: _________. Immerse respirator components for __________ minutes. Always follow the manufacturer's instructions. |
| 2. | Rinse the cleaned and disinfected respirators thoroughly in clean water at __________ temperature to remove all traces of detergent and sanitizer. This step is important in the prevention of dermatitis. |
| 3. | Allow the respirator and parts to air-dry in a clean, sanitary location. |
| 4. | The respirator and parts are wiped with cloth to remove any remaining water. |
Reassembly and Repair:
| 1. | Reassemble and inspect the clean, dry facepiece and parts. Replace filter. |
Storage:
| 1. | Place the freshly cleaned and dried respirator in a reusable plastic bag until reissue. Store it in a clean, dry location away from direct sunlight and with the facepiece in a normal position to prevent the rubber or plastic from taking a permanent "set (deforming)." |
Standard Operating Procedure # _____
Maintenance Of Brand ___________ Disposable N95 Half-Mask
Respirator
Hospital Name: ___________________________________
Address: _________________________________________
_________________________________________
Program Administrator (Or Other Designated Official)
____________________________
Date SOP Written _____________________________
Date Signed By Program Administrator
_____________________________
Date To Be Reviewed __________________________________
| 1. | Determine whether the respirator straps hold the
respirator tightly against the face. If not, discard the
respirator. Do not attempt to tighten the respirator by
knotting the straps. Note: Some manufacturer's disposable respirators may have adjustable straps. |
| 2. | Inspect the respirator to determine if it is soiled or damaged. If so, discard the respirator. |
| 3. | Store the respirator in a clean and dry location. Respirators should be labeled for each worker. Storing the respirator in a plastic sealable bag after use is not considered a good practice. The respirator may be damp after use and sealing prevents drying and encourages microbial growth. If plastic bags are used, respirators should be allowed to dry before storage. |
| Note: Always read the manufacturer's recommendations on maintenance procedures for these N-95 respirators. |
Respirator Program Evaluation |
The respirator program needs to be evaluated periodically to ensure that it continues to be effective. Review the entire program at least annually and modify the written operating procedures to reflect the evaluation results if necessary.
Take the following steps at least once each year:
A. Review the program using the respiratory protection checklist in Appendix E and make necessary changes to reflect current operations and procedures.
B. Using CDC protocol, conduct a risk assessment in all potential TB exposure areas to determine whether the level of exposure has changed.
C. Review the medical surveillance of respirator users.
D. Follow up evidence of excessive exposure to hazards (e.g., TB skin test conversion rates) to determine why inadequate protection was provided and what action should be taken to remedy the problem.
 |
| Photo courtesy of NIOSH. |
Take the following steps more frequently:
A. Use frequent inspections to determine whether the correct respirators are being used and worn properly.
B. Examine respirators in use and in storage to determine how well they are main-tained.
C. Consult users about their acceptance of respirators, including the discomfort, resistance to breathing, fatigue, interference with vision and communication, restriction of movement, interference with job performance, and their confidence in the respirator's effectiveness.
Go back to the Table of ContentsAIHA [1993]. Respiratory protection: a manual and guideline. 2nd ed. Fairfax, VA: American Industrial Hygiene Association.
American Thoracic Society [1996]. Respiratory protection guidelines. Am J Respir Crit Care Med 154:1153-1165.
ANSI [1980]. American national standard: respiratory protection. New York, NY: American National Standards Institute, ANSI Z88.2-1980.
ANSI [1992]. American national standard: respiratory protection. New York, NY: American National Standards Institute, ANSI Z88.2-1992.
Becker MH [1974]. The health belief model and personal health behavior. Thorofare, NJ: Charles B. Slack, Inc.
Bollinger NJ, Schutz RH [1987]. NIOSH guide to industrial respiratory protection. Cincinnati, OH: U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Institute for Occupational Safety and Health, DHHS (NIOSH) Publication No. 87-116.
CDC (Centers for Disease Control and Prevention) [1994]. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health care facilities, 1994. MMWR 43:RR-13.
CDC (Centers for Disease Control and Prevention) [1998a]. Laboratory performance evaluation of N95 filtering facepiece respirators, 1996. MMWR, 47(48):1045-1049.
CDC (Centers for Disease Control and Prevention) [1998b]. Tuberculosis morbidity--United States, 1997. MMWR 47:253-257.
CFR. Code of Federal Regulations. Washington, DC: U.S. Government Printing Office, Office of the Federal Register.
Go back to the Table of Contents§1910.139* Respiratory protection for M. tuberculosis.
This section applies only to respiratory protection against M. tuberculosis and applies in lieu of §1910.134.
*29 CFR 1910.134 is now codified for protection against TB at 29 CFR 1910.139.
| (a) | Permissible practice. | ||||||
| (1) In the control of those occupational diseases
caused by breathing air contaminated with harmful dusts,
fogs, fumes, mists, gases, smokes, sprays, or vapors, the
primary objective shall be to prevent atmospheric
contamination. This shall be accomplished as far as
feasible by accepted engineering control measures (for
example, enclosure or confinement of the operation,
general and local ventilation, and substitution of less
toxic materials). When effective engineering controls are
not feasible, or while they are being instituted,
appropriate respirators shall be used pursuant to the
following requirements. (2) Respirators shall be provided by the employer when such equipment is necessary to protect the health of the employee. The employer shall provide the respirators which are applicable and suitable for the purpose intended. The employer shall be responsible for the establishment and maintenance of a respiratory protective program which shall include the requirements outlined in paragraph (b) of this section. (3) The employee shall use the provided respiratory protection in accordance with instructions and training received. |
|||||||
| (b) | Requirements for a minimal acceptable program. | ||||||
| (1) Written standard operating procedures governing
the selection and use of respirators shall be
established. (2) Respirators shall be selected on the basis of hazards to which the worker is exposed. (3) The user shall be instructed and trained in the proper use of respirators and their limitations. (4) [Reserved] (5) Respirators shall be regularly cleaned and disinfected. Those used by more than one worker shall be thoroughly cleaned and disinfected after each use. (6) Respirators shall be stored in a convenient, clean, and sanitary location. (7) Respirators used routinely shall be inspected during cleaning. Worn or deteriorated parts shall be replaced. Respirators for emergency use such as self-contained devices shall be thoroughly inspected at least once a month and after each use. (8) Appropriate surveillance of work area conditions and degree of employee exposure or stress shall be maintained. (9) There shall be regular inspection and evaluation to determine the continued effectiveness of the program. (10) Persons should not be assigned to tasks requiring use of respirators unless it has been determined that they are physically able to perform the work and use the equipment. The local physician shall determine what health and physical conditions are pertinent. The respirator user's medical status should be reviewed periodically (for instance, annually). (11) Respirators shall be selected from among those jointly approved by the Mine Safety and Health Administration and the National Institute for Occupational Safety and Health under the provisions of 30 CFR part 11. |
|||||||
| (c) | Selection of respirators. Proper selection of respirators shall be made according to the guidance of American National Standard Practices for Respiratory Protection Z88.2-1969. | ||||||
| (d) | Air quality. | ||||||
| (1) Compressed air, compressed oxygen, liquid air,
and liquid oxygen used for respiration shall be of high
purity. Oxygen shall meet the requirements of the United
States Pharmacopoeia for medical or breathing oxygen.
Breathing air shall meet at least the requirements of the
specification for Grade D breathing air as described in
Compressed Gas Association Commodity Specification
G-7.1-1966. Compressed oxygen shall not be used in
supplied-air respirators or in open circuit
self-contained breathing apparatus that have previously
used compressed air. Oxygen must never be used with air
line respirators. (2) Breathing air may be supplied to respirators from cylinders or air compressors.
(3) Air line couplings shall be incompatible with outlets for other gas systems to prevent inadvertent servicing of air line respirators with nonrespirable gases or oxygen. (4) Breathing gas containers shall be marked in accordance with American National Standard Method of Marking Portable Compressed Gas Containers to Identify the Material Contained, Z48.1-1954; Federal Specification BB-A-1034a, June 21, 1968. Air, Compressed for Breathing Purposes; orInterim Federal Specification GG-B-00675b, April 27, 1965, Breathing Apparatus, Self-Contained. |
|||||||
| (e) | Use of respirators. | ||||||
| (1) Standard procedures shall be developed for
respirator use. These should include all information and
guidance necessary for their proper selection, use, and
care. Possible emergency and routine uses of respirators
should be anticipated and planned for. (2) The correct respirator shall be specified for each job. The respirator type is usually specified in the work procedures by a qualified individual supervising the respiratory protective program. The individual issuing them shall be adequately instructed to insure that the correct respirator is issued. (3) Written procedures shall be prepared covering safe use of respirators in dangerous atmospheres that might be encountered in normal operations or in emergencies. Personnel shall be familiar with these procedures and the available respirators.
(4) Respiratory protection is no better than the respirator in use, even though it is worn conscientiously. Frequent random inspections shall be conducted by a qualified individual to assure that respirators are properly selected, used, cleaned, and maintained. (5) For safe use of any respirator, it is essential that the user be properly instructed in its selection, use and maintenance. Both supervisors and workers shall be so instructed by competent persons. Training shall provide the workers an opportunity to handle the respirator, have it fitted properly, test its face-piece-to-face seal, wear it in normal air for a long familiarity period, and, finally, to wear it in a test atmosphere.
|
|||||||
| (f) | Maintenance and care of respirators. | ||||||
(1) A program for maintenance and care of respirators
shall be adjusted to the type of plant, working
conditions, and hazards involved, and shall include the
following basic services:
Equipment shall be properly maintained to retain its original effectiveness. (2)
(3) Routinely used respirators shall be collected, cleaned, and disinfected as frequently as necessary to insure that proper protection is provided for the wearer. Respirators maintained for emergency use shall be cleaned and disinfected after each use. (4) Replacement or repairs shall be done only by experienced persons with parts designed for the respirator. No attempt shall be made to replace components or to make adjustment or repairs beyond the manufacturer's recommendations. Reducing or admission valves or regulators shall be returned to the manufacturer or to a trained technician for adjustment or repair. (5)
|
|||||||
| (g) | Identification of gas mask canisters. | ||||||
| (1) The primary means of identifying a gas mask
canister shall be by means of properly worded labels. The
secondary means of identifying a gas mask canister shall
be by a color code. (2) All who issue or use gas masks falling within the scope of this section shall see that all gas mask canisters purchased or used by them are properly labeled and colored in accordance with these requirements before they are placed in service and that the labels and colors are properly maintained at all times thereafter until the canisters have completely served their purpose. (3) On each canister shall appear in bold letters the following:
(4) Canisters having a special high-efficiency filter for protection against radio nuclides and other highly toxic particulates shall be labeled with a statement of the type and degree of protection afforded by the filter. The label shall be affixed to the neck end of, or to the gray stripe which is around and near the top of, the canister. The degree of protection shall be marked as the percent of penetration of the canister by a 0.3-micron-diameter dioctyl phthalate (DOP) smoke at a flow rate of 85 liters per minute. (5) Each canister shall have a label warning that gas masks should be used only in atmospheres containing sufficient oxygen to support life (at least 16 percent by volume), since gas mask canisters are only designed to neutralize or remove contaminants from the air. (6) Each gas mask canister shall be painted a distinctive color or combination of colors indicated in Table I-1. All colors used shall be such that they are clearly identifiable by the user and clearly distinguishable from one another. The color coating used shall offer a high degree of resistance to chipping, scaling, peeling, blistering, fading, and the effects of the ordinary atmospheres to which they may be exposed under normal conditions of storage and use. Appropriately colored pressure sensitive tape may be used or the stripes |
| Atmospheric contaminants to be protected against | Colors assigned* |
| Acid gases ... | White. |
| Hydrocyanic acid gas ... | White with 1/2-inch green strip completely around the canister near the bottom. |
| Chlorine gas ... | White with 1/2- inch yellow stripe completely around the canister near the bottom. |
| Organic vapors ... | Black. |
| Ammonia gas ... | Green. |
| Acid gases and ammonia gas ... | Green with 1/2-inch white stripe completely around the canister near the bottom. |
| Carbon monoxide ... | Blue. |
| Acid gases and organic vapors ... | Yellow. |
| Hydrocyanic acid gas and chloropicrin vapor ... | Yellow with 1/2-inch blue stripe completely around the canister near the bottom. |
| Acid gases, organic vapors, and ammonia gases ... | Brown. |
| Radioactive materials, excepting tritium and noble gases ... | Purple (Magenta). |
| Particulates (dusts, fumes, mists, fogs, or smokes) in combination with any of the above gases or vapors... | Canister color for contaminant, as designated above, with 1/2-inch gray stripe completely around the canister near the top. |
| All of the above atmospheric contaminants ... | Red with 1/2-inch gray stripe completely around the canister near the top. |
| *Gray shall not be assigned as the main color for a canister designed to remove acids or vapors. | |
| NOTE: Orange shall be used as a complete body, or stripe color to represent gases not included in this table. The user will need to refer to the canister label to determine the degree of protection the canister will afford. | |
CPL 2.106 - Enforcement Procedures and Scheduling for Occupational Exposure to Tuberculosis.
------------------------------------------------------------------------
OSHA Instruction CPL 2.106
February 9, 1996
Office of Health Compliance Assistance
SUBJECT: Enforcement Procedures and Scheduling for Occupational Exposure to Tuberculosis (TB)
| A. | Purpose. This instruction provides uniform inspection procedures and guidelines to be followed when conducting inspections and issuing citations under Section 5(a)(1) of the OSHA Act and pertinent standards for employees who are occupationally exposed to tuberculosis. | |||||||||||||||||||||||||||||||||||||||||
| B. | Scope. This instruction applies OSHA-wide. | |||||||||||||||||||||||||||||||||||||||||
| C. | References. | |||||||||||||||||||||||||||||||||||||||||
| 1. | OSHA Instruction CPL 2.103, September 26, 1994, Field Inspection Reference Manual (FIRM). | |||||||||||||||||||||||||||||||||||||||||
| 2. | OSHA Instruction CPL 2.45B, June 15, 1985, The Revised Field Operations Manual (FOM). | |||||||||||||||||||||||||||||||||||||||||
| 3. | American Public Health Association - 1990 or current edition, Control of Communicable Diseases in Man. | |||||||||||||||||||||||||||||||||||||||||
| 4. | OSHA Instruction CPL 2-2.20B, CH-3, August 22, 1994. Occupational Safety and Health Administration Technical Manual Chapter No. 7. | |||||||||||||||||||||||||||||||||||||||||
| 5. | OSHA Instruction, ADM 1-31, the IMIS Enforcement Data Processing Manual. | |||||||||||||||||||||||||||||||||||||||||
| 6. | OSHA Instruction ADM 1-32, Enforcement User Skills Manual (for those Area Offices still using the NCR system). | |||||||||||||||||||||||||||||||||||||||||
| 7. | Centers for Disease Control and Prevention (CDC), Biosafety in Microbiological and Biomedical Laboratories, 3rd Edition, or current edition. | |||||||||||||||||||||||||||||||||||||||||
| 8. | Department of Health and Human Services, Public Health Service, 42 CFR Part 84; Final Rule. | |||||||||||||||||||||||||||||||||||||||||
| 9. | Centers for Disease Control and Prevention (CDC); Guidelines for Preventing the transmission of mycobacterium tuberculosis in Health Care Facilities, 1994; MMWR October 26, 1994 Vol. 43, No. RR-13. | |||||||||||||||||||||||||||||||||||||||||
| D. | Action. OSHA Regional Administrators and Area Directors will use this instruction to ensure uniformity when performing inspections for occupational exposures to tuberculosis (TB). The Directorate of Compliance Programs will provide support as necessary to assist the Regional Administrators and Area Directors in enforcing this directive. Issuance of this directive cancels the Memorandum to Regional Administers dated October 8, 1993, and entitled Enforcement Policy and Procedures for Occupational Exposure to Tuberculosis. | |||||||||||||||||||||||||||||||||||||||||
| E. | Federal Program Change. This is a federal program change which impacts state programs. | |||||||||||||||||||||||||||||||||||||||||
| 1. | The Regional Administrator (RA) will ensure that this change is promptly forwarded to each state designee using a format consistent with the Plan Change Two-way Memorandum in Appendix A, State Plan Policies and Procedures Manual (SPM). | |||||||||||||||||||||||||||||||||||||||||
| 2. | The RA shall explain the content of this change to the state designee as required. | |||||||||||||||||||||||||||||||||||||||||
| 3. | The state shall respond to this change within 70 days in accordance with paragraph I.1.a.(2).(a). and (b)., Part I, Chapter III of the SPM. | |||||||||||||||||||||||||||||||||||||||||
| 4. | The state's acknowledgment shall include (a) the state's plan to adopt and implement an identical change, (b) the state's plan to develop an alternative, which is as effective, or the reasons why no change is necessary to maintain a program which is as effective. The state shall submit a plan supplement within six months in accordance with I.1.a.(3).(c)., Part I, Chapter III of the SPM. | |||||||||||||||||||||||||||||||||||||||||
| 5. | The RA shall advise state designees of the following: | |||||||||||||||||||||||||||||||||||||||||
| a. | In order to ensure a sound and consistent national enforcement and litigation strategy in relation to complex issues addressed by this instruction, state implementation of the procedures in this instruction, or comparable state procedures, must be carefully coordinated with OSHA. | |||||||||||||||||||||||||||||||||||||||||
| b. | The state is also responsible for extending coverage under its procedures for addressing occupational exposure to tuberculosis to the public sector employees in workplaces covered by this instruction. | |||||||||||||||||||||||||||||||||||||||||
| c. | The Directorate of Technical Support is available to assist the states in locating expert witnesses (see paragraph M., expert witnesses). Also, the Directorate of Compliance Programs will provide support to the states through the RA to assist in the enforcement of this directive. | |||||||||||||||||||||||||||||||||||||||||
| 6. | The RA shall review policies, instructions, and guidelines issued by the state to determine that this change has been communicated to state compliance personnel. | |||||||||||||||||||||||||||||||||||||||||
| F. | Definitions. For a complete list of definitions applicable to tuberculosis please refer to the list of definitions in the 1994 CDC guidelines found in Appendix A beginning on page 113. | |||||||||||||||||||||||||||||||||||||||||
| G. | Background. Since 1985,
the incidence of tuberculosis (TB) in the general U.S.
population has increased approximately 14 percent,
reversing a 30-year downward trend. In 1993, 25,313 new
cases of TB were reported in the United States. Increases
in the incidence of TB have been observed in some
geographic areas; these increases are related partially
to the high risk for TB among immunosuppressed persons,
particularly those infected with human immunodeficiency
virus (HIV). Other factors (e.g., socioeconomic) have
also contributed to these increases. Outbreaks have
occurred in hospitals, correctional institutions,
homeless shelters, nursing homes, and residential care
facilities for AIDS patients. During 1994 and 1995 there
has been a decrease in the number of TB cases in the
United States that is likely been due to increased
awareness and efforts in the prevention and control of
TB, including the implementation of TB control measures
recommended by the CDC and required by OSHA. Recently, drug resistant strains of M. tuberculosis have become a serious concern and cases of multi-drug-resistant (MDR) TB have occurred in forty states. In a recent New York City study, 33% of cases had organisms resistant to the two most effective drugs available for treating the disease. When organisms are resistant to both drugs, the course of the treatment increases from six months to 18-24 months, and the cure rate decreases from 100% to 60% or less. In a 1992 American Hospital Association survey/CDC survey, 90 of 729 (13%) respondents reported nosocomial TB transmission to health care workers. More than 80% of those facilities experienced TB skin test conversions among workers. More than 100 cases of active TB disease in health care workers were known to CDC and reported to Congress by Dr. William Roper in the Spring of 1993. Twelve (12) health care workers have died. Nationwide, at least several hundred employees have become infected and required medical treatment after workplace exposure to TB. In general, persons who become infected with TB have approximately a 10% risk for developing active TB in their lifetimes. M. tuberculosis is carried through the air in tiny infectious droplet nuclei of 1 to 5 microns in diameter. These droplets may be generated when a person with pulmonary and laryngeal TB disease coughs, speaks, sings, sneezes, or spits. When inhaled by susceptible persons, the mycobacteria in these droplets may become established in the lungs and, in some cases, spread throughout the body. After an interval of months, years, or even decades, the initial infection may then progress to clinical illness (i.e., tuberculosis disease). Transmission of TB is most likely to occur from persons with pulmonary or laryngeal TB that are not on effective anti-TB therapy and who have not been placed in respiratory isolation. In occupational health care settings, where patients with TB are seen, workers exposed to tuberculosis droplet nuclei are at increased risk of infection with exposure to TB. Certain high-risk medical procedures that are cough-inducing or aerosol generating can further increase the risk of infection in health care workers. The employer's obligations are those set forth in the Occupational Safety and Health Act (OSH Act) of 1970. Recommendations for preventing the transmission of TB for health care settings were originally established with the 1990 CDC Guidelines. In October, of 1994, those guidelines were revised and published (Appendix A). The new guidelines emphasize the control of TB through an effective TB infection control program. Under these guidelines the control of TB is to be accomplished through the early identification, isolation, and treatment of persons with TB, use of engineering and administrative procedures to reduce the risk of exposure, and through the use of respiratory protection. OSHA believes these guidelines reflect an industry recognition of the hazard as well as appropriate, widely recognized, and accepted standards of practice to befollowed by employers in carrying out their responsibilities under the OSH Act. |
|||||||||||||||||||||||||||||||||||||||||
| H. | Inspection Scheduling and Scope | |||||||||||||||||||||||||||||||||||||||||
| 1. | The evaluation of occupational exposure
to TB shall be conducted in response to employee
complaints, related fatality/catastrophes, or as part of
all industrial hygiene inspections conducted in
workplaces where the CDC has identified workers as having
a greater incidence of TB infection than in the general
population. The degree of risk of occupational exposure
of a worker to TB will vary based on a number of factors
discussed in detail by the CDC (Appendix A, pg. 4-5).
These workplaces have been the subject of reports issued
by the CDC which provide recommendations for the control
of tuberculosis. Specifically, these workplaces are as
follows: a. health care facilities |
|||||||||||||||||||||||||||||||||||||||||
| Note: Health care facilities include hospitals where patients with confirmed or suspect TB are treated or to which they are transported. Coverage of non-hospital health care settings (i.e., doctors' offices, clinics, etc.) includes only personnel present during the performance of high hazard procedures on suspect or active TB patients. Dental health care personnel are covered by the directive only if they treat suspect or active patients in a hospital or correctional facility. | ||||||||||||||||||||||||||||||||||||||||||
| 1. | Homeless shelters - due to a variety of circumstances, the control of TB in homeless shelters presents unique problems for the protection of workers. Shelters must establish protocols that provide for rapid early identification followed by immediate transfer of suspect cases if the shelters have elected not to treat these patients. | |||||||||||||||||||||||||||||||||||||||||
| 2. | All inspections in these workplaces shall include a review of the employer's plans for employee TB protection, if any. Such plans may include the infection control program, respiratory protection and skin testing. Employee interviews and site observations are an integral part of the process evaluation. | |||||||||||||||||||||||||||||||||||||||||
| 3. | Complaints received from state and local government employees who are outside federal jurisdiction in federal enforcement states shall be referred to the appropriate agency by the Area Office. | |||||||||||||||||||||||||||||||||||||||||
| I. | Inspection Procedures. The procedure given in the FIRM, Chapter II, shall be followed except as modified in the following sections: | |||||||||||||||||||||||||||||||||||||||||
| 1. | Health care facilities generally have internal infection control and employee health programs. This function may be performed by a team or individual. Upon entry, the CSHO shall request the presence of the infection control director and employee occupational health professional responsible for occupational health hazard control. Other individuals who will be responsible for providing records pertinent to the inspection may include: training director, facilities engineer, director of nursing, etc. | |||||||||||||||||||||||||||||||||||||||||
| 2. | The CSHO shall establish whether or not the facility has had a suspect or confirmed TB case within the previous six (6) months from the opening conference to determine coverage under the OSH Act. This determination may be based upon interviews and, in a hospital, a review of the infection control data. | |||||||||||||||||||||||||||||||||||||||||
| 3. | If the facility has had a suspect or confirmed TB case within the previous six months, the CSHO shall proceed with the TB portion of the inspection. The CSHO shall verify implementation of the employer's plans for TB protection through employee interviews and direct observation where feasible. Professional judgment shall be used to identify which areas of a facility must be inspected during the walk through (e.g., emergency rooms, respiratory therapy areas, bronchoscopy suites, and morgue). After review of the facility plans for worker TB protection, employee interviews combined with an inspection of appropriate areas of the facility, shall be used to determine compliance. | |||||||||||||||||||||||||||||||||||||||||
| 4. | CSHOs who perform smoke-trail visualization tests should review the protocol in Appendix B of this directive. | |||||||||||||||||||||||||||||||||||||||||
| 5. | CSHOs should be prepared to present to the employer the material safety data sheet (MSDS) for the smoke that is released on a smoke-trail visualization. | |||||||||||||||||||||||||||||||||||||||||
| J. | Compliance Officer Protection | |||||||||||||||||||||||||||||||||||||||||
| 1. | Area Directors or Assistant Area Directors shall ensure that CSHOs performing TB related inspections are familiar with the CDC Guidelines, terminology, and are adequately trained through either course work or field/work experience in health care settings. Consultation with the regional TB coordinators is encouraged prior to beginning such inspections. | |||||||||||||||||||||||||||||||||||||||||
| 2. | CSHOs shall not enter occupied respiratory isolation [AFB (acid fast bacilli)] rooms to evaluate compliance unless, in their determination entry is required to document a violation. Prior to entry CSHOs will discuss the need for entry with the Area Director. Photographs or video taping where practical shall be used for case documentation. Under no circumstances shall photographing or videotaping of patients be done. CSHO's must take all necessary precautions to assure and protect patient confidentiality. | |||||||||||||||||||||||||||||||||||||||||
| 3. | CSHOs shall exercise professional judgement and extreme caution when engaging in activities that may involve potential exposure to TB. CSHOs normally shall establish the existence of hazards and adequacy of work practices through employee interviews and shall observe them in a manner which prevents exposure (e.g., through an observation window where available). | |||||||||||||||||||||||||||||||||||||||||
| 4. | On rare occasions when entry into potentially hazardous areas is judged necessary (e.g., where the CSHO determines that direct observation of a high hazard procedure is necessary), the CSHO shall be properly equipped as required by the facility, this directive, and following consultation with the CSHO's supervisor. Since CSHOs' respiratory protection is used in more than one type of industry they shall use their negative pressure elastomeric face piece respirators equipped with HEPA filters as the minimum level of respiratory protection. | |||||||||||||||||||||||||||||||||||||||||
| 5. | CSHOs who conduct TB inspections shall
have been offered the TB skin tests. CSHOs exposed to an
individual(s) with active infectious TB shall receive a
follow-up examination and follow Sections J. and K. of
Appendix A beginning on page 37. Note: A "TB Skin Test" means the intradermal injection (Mantoux Method) of tuberculin antigen (usually PPD) with subsequent measurement of the induration by designated, trained personnel. |
|||||||||||||||||||||||||||||||||||||||||
| 6. | If an isolation room is occupied by a patient with confirmed or suspect TB or has not been adequately purged when a smoke-trail test is performed, then the CSHO should assume that the isolation room is not under negative pressure. Under such circumstances CSHOs shall wear a negative pressure HEPA respirator when performing air tests as described in Appendix B or if entry into the room is determined to be necessary. | |||||||||||||||||||||||||||||||||||||||||
| K. | Citation Policy. Relevant chapters of the FIRM shall be followed when preparing and issuing citations for hazards related to TB. | |||||||||||||||||||||||||||||||||||||||||
| 1. | The following requirements apply when
citing hazards found in target workplaces. Employers must
comply with the provisions of these requirements whenever
an employee may be occupationally exposed to TB: Section 5(a)(1) -- General Duty Clause and Executive Order 12196, Section 1-201(a) for Federal facilities. 29 CFR 1910.134* -- Respiratory Protection 29 CFR 1910.145 -- Accident Prevention Signs and Tags 29 CFR 1910.20 -- Access to Employee Exposure and Medical Records 29 CFR 1904 -- Recording and Reporting Occupational Injuries & Illness *29 CFR 1910.134 is now codified for protection against TB at 29 CFR 1910.139. |
|||||||||||||||||||||||||||||||||||||||||
| L. | Violations. All elements in this section must be addressed to ensure adequate protection of employees from TB hazards. Violations of these OSHA requirements will normally be classified as serious. | |||||||||||||||||||||||||||||||||||||||||
| 1. | General Duty Clause - Section 5(a) (1). Section 5(a)(1) provides: "Each employer shall furnish to each of his employees employment and a place of employment which are free from recognized hazards that are causing or are likely to cause death or serious physical harm to his employees." | |||||||||||||||||||||||||||||||||||||||||
| a. | Section 5(a)(1) citations must meet the requirements outlined in the FIRM, and shall be issued only when there is no standard that applies to the particular hazard. The hazard, not the absence of a particular means of abatement, is the basis for a general duty clause citation. All applicable abatement methods identified as correcting the same hazard shall be issued under a single 5(a)(1) citation. | |||||||||||||||||||||||||||||||||||||||||
| b. | Recognition, for purposes of citing section 5(a)(1), is shown by the CDC Guidelinesfor the types of exposures detailed below because the CDC is an acknowledged body of experts familiar with the hazard. | |||||||||||||||||||||||||||||||||||||||||
| c. | Citations shall be issued to employers with employees working in one of the workplaces where the CDC has identified workers as having a higher incidence of TB infection than the general population, when the employees are not provided appropriate protection and who have exposure as defined below: | |||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
| d. | If a citation under 5(a)(1) is justified, the
citation, after setting forth the SAVE for section
5(a)(1), shall state: Section 5(a)(1) of the Occupational Safety and Health Act of 1970: The employer did not furnish employment and a place of employment which were free from recognized hazards that were causing or likely to cause death or serious physical harm to employees exposed to the hazard of being infected with Mycobacterium tuberculosis through unprotected contact with [specify group such as patients, inmates, clients, etc.] who was/were infectious or suspected to be infectious with tuberculosis in that: [list deficiencies] Feasible and useful abatement methods for reducing this hazard, as recommended by the CDC, include, but are not limited to: [list abatement methods]. |
|||||||||||||||||||||||||||||||||||||||||
| e. | The following are examples of feasible and useful abatement methods, which must be implemented to abate the hazard. Deficiencies found in any category can result in the continued existence of a serious hazard and may, therefore, allow citation under 5(a)(1). | |||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
| 3. | Case Management of Infected Employees shall include the following: | |||||||||||||||||||||||||||||||||||||||||
| a. | Protocol for New Converters. Conversion to a positive TB skin test shall be followed as soon as possible, by appropriate physical, laboratory, and radiographic evaluations to determine whether the employee has infectious TB disease. (see Appendix A, pg. 65). | |||||||||||||||||||||||||||||||||||||||||
| b. | Work Restrictions for Infectious Employees. See Appendix A, page41. | |||||||||||||||||||||||||||||||||||||||||
| 4. | Worker Education and Training.
Training and information to ensure employee knowledge of
such issues as the mode of TB transmission, its signs and
symptoms, medical surveillance and therapy, and site
specific protocols including the purpose and proper use
of controls shall be provided to all current employees
and to new workers upon hiring. (See Appendix A, pgs.
36-37) Training should be repeated as needed. Workers shall be trained to recognize, and report to a designated person, any patients or clients with symptoms suggestive of infectious TB and instructed on the post exposure protocols to be followed in the event of an exposure incident. (see Appendix A, pg. 23) |
|||||||||||||||||||||||||||||||||||||||||
| 5. | Engineering Controls. The use of each control measure must be based on its ability to abate the hazard. | |||||||||||||||||||||||||||||||||||||||||
| a. | Individuals with suspected or confirmed infectious TB disease must be placed in a respiratory acid-fast bacilli (AFB) isolation room. High hazard procedures on individuals with suspected or confirmed infectious TB disease must be performed in AFB treatment rooms, AFB isolation rooms, booths, and/or hoods. AFB isolation refers to a negative pressure room or an area that exhausts room air directly outside or through HEPA filters if recirculation is unavoidable. | |||||||||||||||||||||||||||||||||||||||||
| b. | Isolation and treatment rooms in use by individuals
with suspected or confirmed infectious TB disease shall
be kept under negative pressure to induce airflow into
the room from all surrounding areas (e.g., corridors,
ceiling plenums, plumbing chases, etc.). (See Appendix A,
Supplement No. 3, page 76) Note: The employer must assure that AFB isolation rooms are maintained under negative pressure. At a minimum, the employer must use nonirritating smoke trails or some other indicator to demonstrate that direction of airflow is from the corridor into the isolation/treatment room with the door closed. If an anteroom exists, direction of airflow must be demonstrated at the inner door between the isolation/treatment room and the anteroom. (See Appendix B) |
|||||||||||||||||||||||||||||||||||||||||
| c. | Air exhausted from AFB isolation or treatment rooms
must be safely exhausted directly outside and not
recirculated into the general ventilation system. (See
Appendix A, Supplement No. 3, page 87). In circumstances where recirculation is unavoidable, HEPA filters must be installed in the duct system from the room to the general ventilation system. (See Appendix A, Supplement No. 3, page 82). For these HEPA filters, a regularly scheduled monitoring programto demonstrate as-installed effectiveness should include; 1) recognized field test method, 2) acceptance criteria, and 3) testing frequencies (see Appendix A, Supplement No. 3, page 85). The air handling system should be appropriately marked with a TB warning where maintenance personnel would have access to the duct work, fans, or filters for maintenance or repair activities. |
|||||||||||||||||||||||||||||||||||||||||
| d. | In order to avoid leakage, all potentially contaminated air which is ducted through the facility must be kept under negative pressure until it is discharged safely outside (i.e., away from occupied areas and air intakes), or | |||||||||||||||||||||||||||||||||||||||||
| e. | The air from isolation and treatment rooms must be
decontaminated by a recognized process (e.g., HEPA
filter) before being recirculated back to the
isolation/treatment room. The use of UV radiation as the
sole means of decontamination shall not be used. The CDC
Guidelines allow the use of UV in waiting rooms,
emergency rooms, corridors, and the like where patients
with undiagnosed TB could potentially contaminate the
air. (See appendix A, pg 90) Note: The opening and closing of doors in an isolation or treatment room which is not equipped with an anteroom compromises the ability to maintain negative pressure in the room. For these rooms, the employer should utilize a combination of controls and practices to minimize spillage of contaminated air into the corridor. Recognized controls and practices include, but are not limited to: minimizing entry to the room; adjusting the hydraulic closer to slow the door movement and reduce displacement effects; adjusting doors to swing into the room where fire codes permit; avoiding placement of room exhaust intake near the door; etc. |
|||||||||||||||||||||||||||||||||||||||||
| f. | If high-hazard procedures are performed within AFB isolation or treatment rooms without benefit of source control ventilation or local exhaust ventilation (e.g., hood, booth, tent, etc.), and droplets are released into the environment (e.g., coughing), then a purge time interval must be imposed during which personnel must use a respirator when entering the room. (See Appendix A, pg. 35 and Suppl. 3, Table S3-1) | |||||||||||||||||||||||||||||||||||||||||
| g. | Interim or supplemental ventilation units equipped with HEPA filters as described in Appendix A pgs. 70-73 are acceptable. | |||||||||||||||||||||||||||||||||||||||||
| 2. | Respiratory Protection - 29 CFR
1910.134 (a) (2) and (b).* The standard provides in part: "Respirators shall be provided by the employer when such equipment is necessary to protect the health of the employee. The employer shall provide the respirators which are applicable and suitable for the purpose intended. The employer shall be responsible for the establishment and maintenance of a respiratory protective program which shall include the requirement outlined in paragraph (b) of this section." *29 CFR 1910.134 is now codified for protection against TB at 29 CFR 1910.139. |
|||||||||||||||||||||||||||||||||||||||||
| a. | Requirements for a minimal acceptable
program. The 1994 CDC Guidelines specify standard performance criteria for respirators for exposure to TB. These criteria include (see appendix A pg 97): |
|||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
| b. | Under the new NIOSH criteria, filter materials would
be tested at a flow rate of 85 L/minute for penetration
by particles with a median aerodynamic diameter of 0.3 uµ and, if certified would be
placed in one of the following categories: Type 100
(99.7% efficient), Type 99 (99% efficient), and Type 95
(95% efficient). NIOSH has determined that these
categories of respirators are effective against TB. Based
upon these criteria, the minimally acceptable level of
respiratory protection for TB is the Type 95 Respirator.
The classes of these air-purifying, particulate
respirators to be certified are described under 42 CFR
Part 84 Subpart K. See Volume 60 of the Federal Register,
page 30338 (June 8, 1995). Until these classes of
respirators are commercially available the minimal
acceptable respiratory protection meeting the criteria
will remain the HEPA respirator (see Appendix A, pg 98).
The following respiratory protection measures must be
addressed:
|
|||||||||||||||||||||||||||||||||||||||||
| 3. | Access to employee medical and exposure records: 29 CFR 1910.20. | |||||||||||||||||||||||||||||||||||||||||
| a. | A record concerning employee exposure to TB is an employee exposure record within the meaning of 29 CFR 1910.20. | |||||||||||||||||||||||||||||||||||||||||
| b. | A record of TB skin test results and medical evaluations and treatment are employee medical records within the meaning of 29 CFR 1910.20. Where known, the workers exposure record should contain a notation of the type of TB, to which the employee was exposed to (e.g., multidrug resistant TB). | |||||||||||||||||||||||||||||||||||||||||
| c. | These records shall be handled according to 29 CFR 1913.10 in order for the CSHO to determine compliance with 29 CFR 1910.20. | |||||||||||||||||||||||||||||||||||||||||
| 4. | Accident prevention signs and tags: 29 CFR 1910.145. | |||||||||||||||||||||||||||||||||||||||||
| a. | In accordance with 1910.145(f)(8), a warning shall be posted outside the Respiratory isolation or treatment room. 1910.145(f)(4) requires that a signal word (i.e.; "STOP," "HALT," or "NO ADMITTANCE") or biological hazard symbol be presented as well as a major message (e.g.; "special respiratory isolation," "Respiratory isolation," or AFB isolation). A description of the necessary precautions, e.g., respirators must be donned before entering. Respiratory isolation rooms in an emergency department or a message referring one to the nursing station for instruction must also be posted. | |||||||||||||||||||||||||||||||||||||||||
| b. | The employer shall also use biological hazard tags on air transport components (e.g., fans, ducts, filters) which identify TB hazards to employees associated with working on air systems that transport contaminated air (See Appendix A, page 85). | |||||||||||||||||||||||||||||||||||||||||
| c. | The standard provides in part: 29 CFR 1910.145(e)(4): Biological hazard warning signs were not used to signify the actual or potential presence of a biohazard and to identify equipment, containers, rooms, materials, experimental animals, or combinations thereof, which contain, or are contaminated with viable hazardous agents: Sample violation language:
Abatement Note: Warning signs must be posted on respiratory isolation or treatment rooms stating "pulmonary isolation," "respiratory isolation," or "AFB isolation." The sign must state specifically the precautions required to interact with those patients. Indicators on patient records or tags on corpses, printed in language or symbols easily recognized by employees are additional methods to achieve this purpose. |
|||||||||||||||||||||||||||||||||||||||||
| 5. | OSHA 200 log - 29 CFR 1904: | |||||||||||||||||||||||||||||||||||||||||
| a. | For OSHA Form 200 record keeping purposes, both
tuberculosis infections (positive TB skin test) and
tuberculosis disease are recordable in the high risk
setting referenced in section H.1. A positive skin test
for tuberculosis, even on initial testing (except
pre-assignment screening) is recordable on the OSHA 200
log because there is a presumption of work-relatedness in
these settings unless there is clear documentation that
an outside exposure occurred. Note: In this case pre-assignment means the same as pre employment and initial testing is the same as baseline testing. |
|||||||||||||||||||||||||||||||||||||||||
| b. | If the employee's tuberculosis infection which was entered on the OSHA 200 log progresses to tuberculosis disease during the five-year maintenance period, the original entry for the infection shall be updated to reflect the new information. Because it is difficult to determine if tuberculosis disease resulted from the source indicated by the skin test conversion or from subsequent exposures, only one case should be entered to avoid double counting. | |||||||||||||||||||||||||||||||||||||||||
| c. | A positive TB skin test provided within two weeks of employment does not have to be recorded on the OSHA 200 forms. However, the initial test must be performed prior to any potential workplace exposure within the initial two weeks of employment. | |||||||||||||||||||||||||||||||||||||||||
| M. | Expert Witness. The Directorate of Technical Support will assist Regional Offices and the States in locating expert witnesses. Expert witnesses must be contacted before issuance of citations. | |||||||||||||||||||||||||||||||||||||||||
| 1. | In the event that a 5(a)(1) citation is
contested, proper expert witness support will be
required. Issues which the expert must be prepared to
address include:
|
|||||||||||||||||||||||||||||||||||||||||
| 2. | Expert witnesses may also be necessary in other cases, particularly those involving 29 CFR 1910.134.* | |||||||||||||||||||||||||||||||||||||||||
*29 CFR 1910.134 is now codified for protection against TB at 29 CFR 1910.139. |
||||||||||||||||||||||||||||||||||||||||||
| N. | Recording in the IMIS. A TB-related inspection is any health inspection conducted to investigate the presence or alleged presence of TB disease (i.e., a referral or complaint inspection). | |||||||||||||||||||||||||||||||||||||||||
| 1. | When a TB-related inspection is
conducted, complete the OSHA-1 as for any inspection and
enter the code "N 02 TB" in Item 42, Optional
Information. EXAMPLE:
|
|||||||||||||||||||||||||||||||||||||||||
| 2. | When an OSHA-7 is completed and the complaint alleges the presence of TB hazards, enter the code "N 02 TB" in Item 46, Optional Information. | |||||||||||||||||||||||||||||||||||||||||
| 3. | When an OSHA-90 is completed and the referral alleges the presence of TB hazards, enter the code "N 02 TB" in Item, 26, Optional Information. | |||||||||||||||||||||||||||||||||||||||||
| 4. | All IMIS case file data for TB-related inspections conducted since October 1, 1990, shall be modified to include the appropriate TB code. | |||||||||||||||||||||||||||||||||||||||||
| O. | Referrals | |||||||||||||||||||||||||||||||||||||||||
| 1. | When a complaint or inquiry is received from a source in a state plan regarding occupational exposure to TB, the Area Office shall refer it to the state plan designee for action. | |||||||||||||||||||||||||||||||||||||||||
| 2. | When a complaint or inquiry regarding occupational exposure to TB in a state or local government health care facility is received in a state without an OSHA-approved state plan, the Regional Administrator shall refer it to the appropriate State public health agency or local health agency. | |||||||||||||||||||||||||||||||||||||||||
| P. | Pre-citation Review. Citations proposed pursuant to this program shall be reviewed prior to issuance, by the Regional Administrator and Regional Office Solicitor for consistency with these procedures. The Directorate of Technical Support shall be contacted to establish expert witness support. The Office of Health Compliance Assistance shall be provided with a copy of all citations issued related to TB during the first 6 months of this directive. | |||||||||||||||||||||||||||||||||||||||||
| Joseph A. Dear | |
| Assistant Secretary | |
| Distribution: | National, Regional, and Area Offices All Compliance Officers State Designees NIOSH Regional Program Directors 7(c)(1) Consultation Project Managers |
Appendix A
"Guidelines for preventing the Transmission of
Mycobacterium Tuberculosis in Health care Facilities, 1994"
Appendix B
Smoke-Trail Testing Method for Negative pressure Isolation Room
Test Method Description:
One of the purposes of a negative pressure TB isolation room is
to prevent TB droplet nuclei from escaping the isolation room and
entering the corridor or other surrounding uncontaminated spaces.
To check for negative room pressure, use smoke-trails to
demonstrate that the pressure differential is inducing airflow
from the corridor, through the crack at the bottom of the door
(undercut) and into the isolation room. When performing a
smoke-trail test follow these recommendations where applicable:
| 1. | Test only with the isolation room door shut. If not equipped with an anteroom, it is assumed that there will be a loss of space pressure control when the isolation door is opened and closed. It is not necessary to demonstrate direction of airflow when the door is open. | ||||||
| 2. | If there is an anteroom, release smoke at the inner door undercut, with both anteroom doors shut. | ||||||
| 3. | In addition to a pedestrian entry, some isolation rooms are also accessed through a wider wheeled-bed stretcher door. Release smoke at all door entrances to isolation rooms. | ||||||
| 4. | So that the smoke is not blown into the isolation room, hold the smoke bottle/tube parallel to the door so the smoke is released perpendicular to the direction of airflow through the door undercut. | ||||||
| 5. | Position the smoke bottle/tube tight to the floor, centered in the middle of the door jamb and approximately two inches out in front of the door. | ||||||
| 6. | Release a puff of smoke and observe the resulting direction of airflow. Repeat the test at least once or until consistent results are obtained. | ||||||
| 7. | Minimize momentum imparted to the smoke by squeezing the bulb or bottle slowly. This will also help minimize the volume of smoke released. | ||||||
| 8. | Depending on the velocity of the air through the door
undercut, the smoke plume will either stay disorganized
or it will form a distinct streamline. In either case,
the smoke will directionally behave in one of three ways.
It will:
Compliance with the intent of the CDC Guidelines for negative pressure requires that the smoke be drawn into the isolation room through the door undercut. |
||||||
| 9. | Release smoke from the corridor side of the door only for occupied TB isolation rooms. If the room is unoccupied, also release smoke inside the isolation room (same position as in Step No. 5) to verify that released smoke remains contained in the isolation room (i.e., smoke as a surrogate for TB droplet nuclei). | ||||||
| 10. | If photography is performed or videotaping, it is recommended that a dark surface be placed on the floor to maximize contrast. Be aware that most auto focusing cameras cannot focus on smoke. |
Testing "As Used" Conditions:
Testing of negative pressure isolation rooms requires that the test reflect "as-used" conditions. Consider the following use variables which may affect space pressurization and the performance of the negative pressure isolation room:
| 1. | Patient toilet rooms are mechanically exhausted to control odors. The position of the toilet room door may affect the pressure differential between the isolation room and the corridor. Smoke-trail tests should be performed with the toilet room door open and the toilet room door closed. This will not be necessary if the toilet room door is normally closed and controlled to that position by a mechanical door closer. |
| 2. | An open window will adversely affect the performance of a negative pressure isolation room. If the isolation room is equipped with an operable window, perform smoke-trail tests with thewindow open and the window closed. |
| 3. | There may be corridor doors that isolate the respiratory ward or wing from the rest of the facility. These corridor doors are provided in the initial design to facilitate space pressurization schemes and/or building life safety codes. Direct communication with the rest of the facility may cause pressure transients in the corridor (e.g., proximity to an elevator lobby) and affect the performance of the isolation room. Perform isolation room smoke-trail testing with these corridor doors in their "as-used" position which is either normally open or normally closed. |
| 4. | Isolation rooms may be equipped with auxiliary, fan-powered, recirculating, stand alone HEPA filtration or UV units. These units must be running when smoke-trail tests are performed |
| 5. | Do not restrict corridor foot traffic while performing smoke-trail tests. |
| 6. | Negative pressure is accomplished by exhausting more air than is supplied to the isolation room. Some HVAC systems employ variable air volume (VAV) supply air and sometimes VAV exhaust air. By varying the supply air delivered to the space to satisfy thermal requirements, these VAV systems can adversely impact the performance of a negative pressure isolation room. If the isolation room or the corridor is served by a VAV system you should perform the smoke test twice. Perform the smoke test with the zone thermostat thermally satisfied and again with the zone thermostat thermally unsatisfied thus stimulating the full volumetric flow rate range of the VAV system serving the area being tested. |
Smoke:
Most smoke tubes, bottles and sticks use titanium chloride (TiCl4) to produce a visible fume. There is no OSHA PEL or ACGIH TLV for this chemical although it is a recognized inhalation irritant. Health care professionals are concerned about releasing TiCl4 around pulmonary patients. The smoke released at the door undercut makes only one pass through the isolation room and is exhausted directly outside. Isolation room air is typically not "recirculated."
The CDC in the supplementary information to the 1994 TB Guidelines has indicated that "The concern over the use of smoke is unfounded." Controlled tests by NIOSH have shown that the quantity of smoke that is released is so minute that it is not measurable in the air. Nonirritating smoke tubes are available and should be utilized whenever possible.
Go back to the Table of Contents1910.1020 - Access To Employee Exposure And Medical Records.
| (a) | Purpose. The purpose of this section is to provide employees and their designated representatives a right of access to relevant exposure and medical records; and to provide representatives of the Assistant Secretary a right of access to these records in order to fulfill responsibilities under the Occupational Safety and Health Act. Access by employees, their representatives, and the Assistant Secretary is necessary to yield both direct and indirect improvements in the detection, treatment, and prevention of occupational disease. Each employer is responsible for assuring compliance with this section, but the activities involved in complying with the access to medical records provisions can be carried out, on behalf of the employer, by the physician or other health care personnel in charge of employee medical records. Except as expressly provided, nothing in this section is intended to affect existing legal and ethical obligations concerning the maintenance and confidentiality of employee medical information, the duty to disclose information to a patient/employee or any other aspect of the medical-care relationship, or affect existing legal obligations concerning the protection of trade secret information. |
||||||||
| (b) | Scope and application. (1) This section applies to each general industry, maritime, and construction employer who makes, maintains, contracts for, or has access to employee exposure or medical records, or analyses thereof, pertaining to employees exposed to toxic substances or harmful physical agents. (2) This section applies to all employee exposure and medical records, and analyses thereof, of such employees, whether or not the records are mandated by specific occupational safety and health standards. (3) This section applies to all employee exposure and medical records, and analyses thereof, made or maintained in any manner, including on an in-house or contractual (e.g., fee-for-service) basis. Each employer shall assure that the preservation and access requirements of this section are complied with regardless of the manner in which records are made or maintained. |
||||||||
| (c) | Definitions. (1) Access means the right and opportunity to examine and copy. (2) Analysis using exposure or medical records means any compilation of data or any statistical study based at least in part on information collected from individual employee exposure or medical records or information collected from health insurance claims records, provided that either the analysis has been reported to the employer or no further work is currently being done by the person responsible for preparing the analysis. (3) Designated representative means any individual or organization to whom an employee gives written authorization to exercise a right of access. For the purposes of access to employee exposure records and analyses using exposure or medical records, a recognized or certified collective bargaining agent shall be treated automatically as a designated representative without regard to written employee authorization. (4) Employee means a current employee, a former employee, or an employee being assigned or transferred to work where there will be exposure to toxic substances or harmful physical agents. In the case of a deceased or legally incapacitated employee, the employee's legal representative may directly exercise all the employee's rights under this section. (5) Employee exposure record means a record containing any of the following kinds of information: |
||||||||
| (i) Environmental (workplace) monitoring
or measuring of a toxic substance or harmful physical
agent, including personal, area, grab, wipe, or other
form of sampling, as well as related collection and
analytical methodologies, calculations, and other
background data relevant to interpretation of the results
obtained; (ii) Biological monitoring results which directly assess the absorption of a toxic substance or harmful physical agent by body systems (e.g., the level of a chemical in the blood, urine, breath, hair, fingernails, etc.) but not including results which assess the biological effect of a substance or agent or which assess an employee's use of alcohol or drugs; (iii) Material safety data sheets indicating that the material may pose a hazard to human health; or (iv) In the absence of the above, a chemical inventory or any other record which reveals where and when used and the identity (e.g., chemical, common, or trade name) of a toxic substance or harmful physical agent. |
|||||||||
| (6) | |||||||||
| (i) Employee medical record means a record concerning the health status of an employee which is made or maintained by a physician, nurse, or other health care personnel, or technician, including: | |||||||||
| (A) Medical and employment questionnaires or
histories (including job description and occupational
exposures), (B) The results of medical examinations (pre-employment, pre-assignment, periodic, or episodic) and laboratory tests (including chest and other X-ray examinations taken for the purpose of establishing a base-line or detecting occupational illnesses and all biological monitoring not defined as an "employee exposure record"), (C) Medical opinions, diagnoses, progress notes, and recommendations, (D) First aid records, (E) Descriptions of treatments and prescriptions, and (F) Employee medical complaints. |
|||||||||
| (ii) "Employee medical record" does not include medical information in the form of: | |||||||||
| (A) Physical specimens (e.g., blood or urine samples)
which are routinely discarded as apart of normal medical
practice,; or (B) Records concerning health insurance claims if maintained separately from the employer's medical program and its records, and not accessible to the employer by employee name or other direct personal identifier (e.g., social security number, payroll number, etc.); or (C) Records created solely in preparation for litigation which are privileged from discovery under the applicable rules of procedure or evidence; or (D) Records concerning voluntary employee assistance programs (alcohol, drug abuse, or personal counseling programs) if maintained separately from the employer's medical program and its records. |
|||||||||
| (7) Employer means a current
employer, a former employer, or a successor employer. (8) Exposure or exposed means that an employee is subjected to a toxic substance or harmful physical agent in the course of employment through any route of entry (inhalation, ingestion, skin contact or absorption, etc.), and includes past exposure and potential (e.g., accidental or possible) exposure, but does not include situations where the employer can demonstrate that the toxic substance or harmful physical agent is not used, handled, stored, generated, or present in the workplace in any manner different from typical non-occupational situations. (9) Health Professional means a physician, occupational health nurse, industrial hygienist, toxicologist, or epidemiologist, providing medical or other occupational health services to exposed employees. (10) Record means any item, collection, or grouping of information regardless of the form or process by which it is maintained (e.g., paper document, microfiche, microfilm, X-ray film, or automated data processing). (11) Specific chemical identity means a chemical name, Chemical Abstracts Service (CAS) Registry Number, or any other information that reveals the precise chemical designation of the substance. |
|||||||||
| (12) | |||||||||
| (i) Specific written consent means a written authorization containing the following: | |||||||||
| (A) The name and signature of the employee
authorizing the release of medical information, (B) The date of the written authorization, (C) The name of the individual or organization that is authorized to release the medical information, (D) The name of the designated representative (individual or organization) that is authorized to receive the released information, (E) A general description of the medical information that is authorized to be released, (F) A general description of the purpose for the release of the medical information, and (G) A date or condition upon which the written authorization will expire (if less than one year).
|
|||||||||
| (13) Toxic substance or harmful physical agent means any chemical substance, biological agent (bacteria, virus, fungus, etc.), or physical stress (noise, heat, cold, vibration, repetitive motion, ionizing and non-ionizing radiation, hypo - or hyperbaric pressure, etc.) which: | |||||||||
| (i) Is listed in the latest printed
edition of the National Institute for Occupational Safety
and Health (NIOSH) Registry of Toxic Effects of Chemical
Substances (RTECS) which is incorporated by reference as
specified in Sec. 1910.6; or (ii) Has yielded positive evidence of an acute or chronic health hazard in testing conducted by, or known to, the employer; or (iii) Is the subject of a material safety data sheet kept by or known to the employer indicating that the material may pose a hazard to human health. |
|||||||||
| (14) Trade secret means any confidential formula, pattern, process, device, or information or compilation of information that is used in an employer's business and that gives the employer an opportunity to obtain an advantage over competitors who do not know or use it. | |||||||||
| (d) Preservation of records. | |||||||||
| (1) Unless a specific occupational safety and health standard provides a different period of time, each employer shall assure the preservation and retention of records as follows: | |||||||||
| (i) Employee medical records. The medical record for each employee shall be preserved and maintained for at least the duration of employment plus thirty (30) years, except that the following types of records need not be retained for any specified period: | |||||||||
| (A) Health insurance claims records maintained
separately from the employer's medical program and its
records, (B) First aid records (not including medical histories) of one-time treatment and subsequent observation of minor scratches, cuts, burns, splinters, and the like which do not involve medical treatment, loss of consciousness, restriction of work or motion, or transfer to another job, if made on-site by a non-physician and if maintained separately from the employer's medical program and its records, and (C) The medical records of employees who have worked for less than (1) year for the employer need not be retained beyond the term of employment if they are provided to the employee upon the termination of employment. |
|||||||||
| (ii) Employee exposure records. Each employee exposure record shall be preserved and maintained for at least thirty (30) years, except that: | |||||||||
| (A) Background data to environmental (workplace)
monitoring or measuring, such as laboratory reports and
worksheets, need only be retained for one (1) year so
long as the sampling results, the collection methodology
(sampling plan), a description of the analytical and
mathematical methods used, and a summary of other
background data relevant to interpretation of the results
obtained, are retained for at least thirty (30) years1; and (B) Material safety data sheets and paragraph (c)(5)(iv) records concerning the identity of a substance or agent need not be retained for any specified period as long as some record of the identity (chemical name if known) of the substance or agent, where it was used, and when it was used is retained for at least thirty (30) years1 ; and (C) Biological monitoring results designated as exposure records by specific occupational safety and health standards shall be preserved and maintained as required by the specific standard. 1 Material safety data sheets must be kept for those chemicals currently in use that are effected by the Hazard Communication Standard in accordance with 29 CFR 1910.1200(g). |
|||||||||
| (iii) Analyses using exposure or medical records. Each analysis using exposure or medical records shall be preserved and maintained for at least thirty (30) years. | |||||||||
| (2) Nothing in this section is intended to mandate the form, manner, or process by which an employer preserves a record so long as the information contained in the record is preserved and retrievable, except that chest X-ray films shall be preserved in their original state. | |||||||||
| (e) Access to records | |||||||||
| (1) General. | |||||||||
| (i) Whenever an employee or designated
representative requests access to a record, the employer
shall assure that access is provided in a reasonable
time, place, and manner. If the employer cannot
reasonably provide access to the record within fifteen
(15) working days, the employer shall within the fifteen
(15) working days apprise the employee or designated
representative requesting the record of the reason for
the delay and the earliest date when the record can be
made available. (ii) The employer may require of the requester only such information as should be readily known to the requester and which may be necessary to locate or identify the records being requested (e.g. dates and locations where the employee worked during the time period in question). (iii) Whenever an employee or designated representative requests a copy of a record, the employer shall assure that either: |
|||||||||
| (A) A copy of the record is provided without cost to
the employee or representative, (B) The necessary mechanical copying facilities (e.g., photocopying) are made available without cost to the employee or representative for copying the record, or (C) The record is loaned to the employee or representative for a reasonable time to enable a copy to be made. |
|||||||||
| (iv) In the case of an original X-ray,
the employer may restrict access to on-site examination
or make other suitable arrangements for the temporary
loan of the X-ray. (v) Whenever a record has been previously provided without cost to an employee or designated representative, the employer may charge reasonable, non-discriminatory administrative costs (i.e., search and copying expenses but not including overhead expenses) for a request by the employee or designated representative for additional copies of the record, except that |
|||||||||
| (A) An employer shall not charge for an initial
request for a copy of new information that has been added
to a record which was previously provided; and (B) An employer shall not charge for an initial request by a recognized or certified collective bargaining agent for a copy of an employee exposure record or an analysis using exposure or medical records. |
|||||||||
| (vi) Nothing in this section is intended to preclude employees and collective bargaining agents from collectively bargaining to obtain access to information in addition to that available under this section. | |||||||||
| (2) Employee and designated representative access | |||||||||
| (i) Employee exposure records. | |||||||||
(A) Except as limited by paragraph (f) of this
section, each employer shall, upon request, assure the
access to each employee and designated representative to
employee exposure records relevant to the employee. For
the purpose of this section, an exposure record relevant
to the employee consists of:
(B) Requests by designated representatives for unconsented access to employee exposure records shall be in writing and shall specify with reasonable particularity:
|
|||||||||
| (ii) Employee medical records. | |||||||||
| (A) Each employer shall, upon request, assure the
access of each employee to employee medical records of
which the employee is the subject, except as provided in
paragraph (e)(2)(ii)(D) of this section. (B) Each employer shall, upon request, assure the access of each designated representative to the employee medical records of any employee who has given the designated representative specific written consent. Appendix A to this section contains a sample form which may be used to establish specific written consent for access to employee medical records. (C) Whenever access to employee medical records is requested, a physician representing the employer may recommend that the employee or designated representative:
(D) Whenever an employee requests access to his or her employee medical records, and a physician representing the employer believes that direct employee access to information contained in the records regarding a specific diagnosis of a terminal illness or a psychiatric condition could be detrimental to the employee's health, the employer may inform the employee that access will only be provided to a designated representative of the employee having specific written consent, and deny the employee's request for direct access to this information only. Where a designated representative with specific written consent requests access to information so withheld, the employer shall assure the access of the designated representative to this information, even when it is known that the designated representative will give the information to the employee. (E) A physician, nurse, or other responsible health care personnel maintaining employee medical records may delete from requested medical records the identity of a family member, personal friend, or fellow employee who has provided confidential information concerning an employee's health status. |
|||||||||
| (iii) Analyses using exposure or medical records. | |||||||||
| (A) Each employer shall, upon request, assure the
access of each employee and designated representative to
each analysis using exposure or medical records
concerning the employee's working conditions or
workplace. (B) Whenever access is requested to an analysis which reports the contents of employee medical records by either direct identifier (name, address, social security number, payroll number, etc.) or by information which could reasonably be used under the circumstances indirectly to identify specific employees (exact age, height, weight, race, sex, date of initial employment, job title, etc.), the employer shall assure that personal identifiers are removed before access is provided. If the employer can demonstrate that removal of personal identifiers from an analysis is not feasible, access to the personally identifiable portions of the analysis need not be provided. |
|||||||||
| (3) OSHA access. | |||||||||
| (i) Each employer shall, upon request,
and without derogation of any rights under the
Constitution or the Occupational Safety and Health Act of
1970, 29 U.S.C. 651 et seq., that the employer
chooses to exercise, assure the prompt access of
representatives of the Assistant Secretary of Labor for
Occupational Safety and Health to employee exposure and
medical records and to analyses using exposure or medical
records. Rules of agency practice and procedure governing
OSHA access to employee medical records are contained in
29 CFR 1913.10. (ii) Whenever OSHA seeks access to personally identifiable employee medical information by presenting to the employer a written access order pursuant to 29 CFR 1913.10(d), the employer shall prominently post a copy of the written access order and its accompanying cover letter for at least fifteen (15) working days. |
|||||||||
| (f) Trade secrets. | |||||||||
| (1) Except as provided in paragraph
(f)(2) of this section, nothing in this section precludes
an employer from deleting from records requested by a
health professional, employee, or designated
representative any trade secret data which discloses
manufacturing processes, or discloses the percentage of a
chemical substance in mixture, as long as the health
professional, employee, or designated representative is
notified that information has been deleted. Whenever
deletion of trade secret information substantially
impairs evaluation of the place where or the time when
exposure to a toxic substance or harmful physical agent
occurred, the employer shall provide alternative
information which is sufficient to permit the requesting
party to identify where and when exposure occurred. (2) The employer may withhold the specific chemical identity, including the chemical name and other specific identification of a toxic substance from a disclosable record provided that: |
|||||||||
| (i) The claim that the information
withheld is a trade secret can be supported; (ii) All other available information on the properties and effects of the toxic substance is disclosed; (iii) The employer informs the requesting party that the specific chemical identity is being withheld as a trade secret; and (iv) The specific chemical identity is made available to health professionals, employees and designated representatives in accordance with the specific applicable provisions of this paragraph. |
|||||||||
| (3) Where a treating physician or nurse
determines that a medical emergency exists and the
specific chemical identity of a toxic substance is
necessary for emergency or first-aid treatment, the
employer shall immediately disclose the specific chemical
identity of a trade secret chemical to the treating
physician or nurse, regardless of the existence of a
written statement of need or a confidentiality agreement.
The employer may require a written statement of need and
confidentiality agreement, in accordance with the
provisions of paragraphs (f)(4) and (f)(5), as soon as
circumstances permit. (4) In non-emergency situations, an employer shall, upon request, disclose a specific chemical identity, otherwise permitted to be withheld under paragraph (f)(2) of this section, to a healthprofessional, employee, or designated representative if: |
|||||||||
| (i) The request is in writing; (ii) The request describes with reasonable detail one or more of the following occupational health needs for the information: |
|||||||||
| (A) To assess the hazards of the chemicals to which
employees will be exposed; (B) To conduct or assess sampling of the workplace atmosphere to determine employee exposure levels; (C) To conduct pre-assignment or periodic medical surveillance of exposed employees; (D) To provide medical treatment to exposed employees; (E) To select or assess appropriate personal protective equipment for exposed employees; (F) To design or assess engineering controls or other protective measures for exposed employees; and (G) To conduct studies to determine the health effects of exposure. |
|||||||||
| (iii) The request explains in detail why the disclosure of the specific chemical identity is essential and that, in lieu thereof, the disclosure of the following information would not enable the health professional, employee or designated representative to provide the occupational health services described in paragraph (f)(4)(ii) of this section: | |||||||||
| (A) The properties and effects of the chemical; (B) Measures for controlling workers' exposure to the chemical; (C) Methods of monitoring and analyzing worker exposure to the chemical; and (D) Methods of diagnosing and treating harmful exposures to the chemical; |
|||||||||
| (iv) The request includes a description
of the procedures to be used to maintain the
confidentiality of the disclosed information; and (v) The health professional, employee, or designated representative and the employer or contractor of the services of the health professional or designated representative agree in a written confidentiality agreement that the health professional, employee or designated representative will not use the trade secret information for any purpose other than the health need(s) asserted and agree not to release the information under any circumstances other than to OSHA, as provided in paragraph (f)(9) of this section, except as authorized by the terms of the agreement or by the employer. |
|||||||||
| (5) The confidentiality agreement authorized by paragraph (f)(4)(iv) of this section: | |||||||||
| (i) May restrict the use of the
information to the health purposes indicated in the
written statement of need; (ii) May provide for appropriate legal remedies in the event of a breach of the agreement, including stipulation of a reasonable pre-estimate of likely damages; and, (iii) May not include requirements for the posting of a penalty bond. |
|||||||||
| (6) Nothing in this section is meant to
preclude the parties from pursuing non-contractual
remedies to the extent permitted by law. (7) If the health professional, employee or designated representative receiving the trade secret information decides that there is a need to disclose it to OSHA, the employer who provided the information shall be informed by the health professional prior to, or at the same time as, such disclosure. (8) If the employer denies a written request for disclosure of a specific chemical identity, the denial must: |
|||||||||
| (i) Be provided to the health
professional, employee, or designated representative
within thirty days of the request; (ii) Be in writing; (iii) Include evidence to support the claim that the specific chemical identity is a trade secret; (iv) State the specific reasons why the request is being denied; and (v) Explain in detail how alternative information may satisfy the specific medical or occupational health need without revealing the specific chemical identity. |
|||||||||
| (9) The health professional, employee, or
designated representative whose request for information
is denied under paragraph (f)(4) of this section may
refer the request and the written denial of the request
to OSHA for consideration. (10) When a health professional, employee, or designated representative refers a denial to OSHA under paragraph (f)(9) of this section, OSHA shall consider the evidence to determine if: |
|||||||||
| (i) The employer has supported the claim
that the specific chemical identity is a trade secret; (ii) The health professional employee, or designated representative has supported the claim that there is a medical or occupational health need for the information; and (iii) The health professional, employee, or designated representative has demonstrated adequate means to protect the confidentiality. |
|||||||||
| (11) | |||||||||
| (i) If OSHA determines that the specific
chemical identity requested under paragraph (f)(4) of
this section is not a bona fide trade secret, or
that it is a trade secret but the requesting health
professional, employee, or designated representatives has
a legitimate medical or occupational health need for the
information, has executed a written confidentiality
agreement, and has shown adequate means forcomplying with
the terms of such agreement, the employer will be subject
to citation by OSHA. (ii) If an employer demonstrates to OSHA that the execution of a confidentiality agreement would not provide sufficient protection against the potential harm from the unauthorized disclosure of a trade secret specific chemical identity, the Assistant Secretary may issue such orders or impose such additional limitations or conditions upon the disclosure of the requested chemical information as may be appropriate to assure that the occupational health needs are met without an undue risk of harm to the employer. |
|||||||||
| (12) Notwithstanding the existence of a
trade secret claim, an employer shall, upon request,
disclose to the Assistant Secretary any information which
this section requires the employer to make available.
Where there is a trade secret claim, such claim shall be
made no later than at the time the information is
provided to the Assistant Secretary so that suitable
determinations of trade secret status can be made and the
necessary protections can be implemented. (13) Nothing in this paragraph shall be construed as requiring the disclosure under any circumstances of process or percentage of mixture information which is a trade secret. |
|||||||||
| (g) Employee information. | |||||||||
| (1) Upon an employee's first entering into employment, and at least annually thereafter, each employer shall inform current employees covered by this section of the following: | |||||||||
| (i) The existence, location, and
availability of any records covered by this section; (ii) The person responsible for maintaining and providing access to records; and (iii) Each employee's rights of access to these records. |
|||||||||
| (2) Each employer shall keep a copy of this section and its appendices, and make copies readily available, upon request, to employees. The employer shall also distribute to current employees any informational materials concerning this section which are made available to the employer by the Assistant Secretary of Labor for Occupational Safety and Health. | |||||||||
| (h) Transfer of records. | |||||||||
| (1) Whenever an employer is ceasing to do
business, the employer shall transfer all records subject
to this section to the successor employer. The successor
employer shall receive and maintain these records. (2) Whenever an employer is ceasing to do business and there is no successor employer to receive and maintain the records subject to this standard, the employer shall notify affected current employees of their rights of access to records at least three (3) months prior to the cessation of the employer's business. (3) Whenever an employer either is ceasing to do business and there is no successor employer to receive and maintain the records, or intends to dispose of any records required to be preserved for at least thirty (30) years, the employer shall: |
|||||||||
| (i) Transfer the records to the Director
of the National Institute for Occupational Safety and
Health (NIOSH) if so required by a specific occupational
safety and health standard; or (ii) Notify the Director of NIOSH in writing of the impending disposal of records at least three (3) months prior to the disposal of the records. |
|||||||||
| (4) Where an employer regularly disposes of records required to be preserved for at least thirty (30) years, the employer may, with at least (3) months notice, notify the Director of NIOSH on an annual basis of the records intended to be disposed of in the coming year. | |||||||||
| (i) Appendices. The information contained in appendices A and B to this section is not intended, by itself, to create any additional obligations not otherwise imposed by this section nor detract from any existing obligation. |
|||||||||
APPENDIX A TO 1910.1020 - SAMPLE AUTHORIZATION LETTER FOR THE RELEASE OF EMPLOYEE MEDICAL RECORD INFORMATION TO A DESIGNATED REPRESENTATIVE (NON-MANDATORY)
I, _______, (full name of worker/patient) hereby authorize
__________ (individual or organization holding the medical
records) to release to _________ (individual or organization
authorized to receive the medical information), the following
medical information from my personal medical records:
__________________________________________________________________________
__________________________________________________________________________
(Describe generally the information desired to be released).
I give my permission for this medical information to be used
for the following purpose:
___________________________________________________________________________
___________________________________________________________________________
but I do not give permission for any other use or re-disclosure of this information.
(Note: Several extra lines are provided below so that you can place additional restrictions on this authorization letter if you want to. You may, however, leave these lines blank. On the other hand, you may want to (1) specify a particular expiration date for this letter (if less than one year); (2) describe medical information to be created in the future that you intend to be covered by this authorization letter; or (3) describe portions of the medical information in your records which you do not intend to be released as a result of this letter.)
__________________________________________________________________________
__________________________________________________________________________
Full name of Employee or Legal Representative
___________________________________________________________________________
Signature of Employee or Legal Representative
____________________________________________________________________________
Date of Signature
APPENDIX B TO §1910.1020 - AVAILABILITY OF NIOSH REGISTRY OF TOXIC EFFECTS OF CHEMICAL SUBSTANCES (RTECS)(NON-MANDATORY)
The final regulation, 29 CFR 1910.1020, applies to all employee exposure and medical records, and analyses thereof, of employees exposed to toxic substances or harmful physical agents (paragraph (b)(2)). The term toxic substance or harmful physical agent is defined by paragraph (c)(13) to encompass chemical substances, biological agents, and physical stresses for which there is evidence of harmful health effects. The regulation uses the latest printed edition of the National Institute for Occupational Safety and Health (NIOSH) Registry of Toxic Effects of Chemical Substances (RTECS) as one of the chief sources of information as to whether evidence of harmful health effects exists. If a substance is listed in the latest printed RTECS, the regulation applies to exposure and medical records (and analyses of these records) relevant to employees exposed to the substance.
It is appropriate to note that the final regulation does not require that employers purchase a copy of RTECS, and many employers need not consult RTECS to ascertain whether their employee exposure or medical records are subject to the rule. Employers who do not currently have the latest printed edition of the NIOSH RTECS, however, may desire to obtain a copy. The RTECS is issued in an annual printed edition as mandated by section 20(a)(6) of the Occupational Safety and Health Act (29 U.S.C. 669(a)(6)).
The introduction to the 1980 printed edition describes the
RTECS as follows:
"The 1980 edition of the Registry of Toxic Effects of
Chemical Substances, formerly known as the Toxic Substances list,
is the ninth revision prepared in compliance with the
requirements of Section 20(a)(6) of the Occupational Safety and
Health Act of 1970 (Public Law 91-596). The original list was
completed on June 28, 1971, and has been updated annually in book
format. Beginning in October 1977, quarterly revisions have been
provided in microfiche. This edition of the Registry contains
168,096 listings of chemical substances; 45,156 are names of
different chemicals with their associated toxicity data and
122,940 are synonyms. This edition includes approximately 5,900
new chemical compounds that did not appear in the 1979
Registry.(p. xi)
"The Registry's purposes are many, and it serves a variety of users. It is a single source document for basic toxicity information and for other data, such as chemical identifiers and information necessary for the preparation of safety directives and hazard evaluations for chemical substances. The various types of toxic effects linked to literature citations provide researchers and occupational health scientists with an introduction to the toxicological literature, making their own review of the toxic hazards of a given substance easier. By presenting data on the lowest reported doses that produce effects by several routes of entry in various species, the Registry furnishes valuable information to those responsible for preparing safety data sheets for chemical substances in the workplace. Chemical and production engineers can use the Registry to identify the hazards which may be associated with chemical intermediates in the development of final products, and thus can more readily select substitutes or alternate processes which may be less hazardous. Some organizations, including health agencies and chemical companies, have included the NIOSH Registry accession numbers with the listing of chemicals in their files to reference toxicity information associated with those chemicals. By including foreign language chemical names, a start has been made toward providing rapid identification of substances produced in other countries. (p xi)
"In this edition of the Registry, the editors intend to identify "all known toxic substances" which may exist in the environment and to provide pertinent data on the toxic effects from known doses entering an organism by any route described. (p xi)
"It must be reemphasized that the entry of a substance in the Registry does not automatically mean that it must be avoided. A listing does mean, however, that the substance has the documented potential of being harmful if misused, and care must be exercised to prevent tragic consequences. Thus the Registry lists many substances that are common in everyday life and are in nearly every household in the United States. One can name a variety of such dangerous substances: prescription and non-prescription drugs; food additives; pesticide concentrates, sprays, and dusts; fungicides; herbicides, paints; glazes, dyes; bleaches and other household cleaning agents; alkalis; and various solvents and diluents. The list is extensive because chemicals have become an integral part of our existence."
The RTECS printed edition may be purchased from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402 (202-783-3238).
Some employers may desire to subscribe to the quarterly update to the RTECS which is published in a microfiche edition. An annual subscription to the quarterly microfiche may be purchased from the GPO (Order the "Microfiche Edition, Registry of Toxic Effects of Chemical Substances"). Both the printed edition and the microfiche edition of RTECS are available for review at many university and public libraries throughout the country. The latest RTECS editions may also be examined at the OSHA Technical Data Center, Room N2439 - Rear, United States Department of Labor, 200 Constitution Avenue, N.W., Washington, DC 20210 (202-523-9700), or at any OSHA Regional or Area Office (See, major city telephone directories under United States Government - Labor Department).
Go back to the Table of ContentsNames And Addresses Of Respirator Manufacturers And Distributors |
||||
|
|
|||
Respiratory Protection Checklist
Modified from the New Jersey Department of Health
Public Employees Occupational Safety and Health Program
| Facility_________________________ | Contact Person___________________________ |
| Date___________________________ | Phone___________________________________ |
| (month/day/year) | (area code) |
| Data Collected By_________________________________________________________ | |
1. ASSESSMENT OF RESPIRATORY PROTECTION USE
| A. | Is respiratory protection used: | |
| 1. | By persons entering rooms where patients with known or suspected infectious TB are isolated? | Y/N/*Sometimes |
| 2. | During cough inducing procedures with known or suspect TB patients? | Y/N/*Sometimes |
| 3. | During administration of aerosolized medications with known or suspect TB patients? | Y/N/*Sometimes |
| 4. | During surgical procedures with a known or suspect TB patient? | Y/N/*Sometimes |
| 5. | During bronchoscopy on a known or suspect TB patient? | Y/N/*Sometimes |
| 6. | During autopsy of a deceased person suspected or known to have had active TB? | Y/N/*Sometimes |
| 7. | By visitors of patients with known or suspect TB? Y/N/*Sometimes | Y/N/*Sometimes |
| 8. | On patients with known or suspect TB while transporting patient within the hospital? | Y/N/*Sometimes |
| 9. | During urgent dental treatment on a known or suspect TB patient? | Y/N/*Sometimes |
| 10. | Where administrative and engineering controls may not provide adequate protection? | Y/N/*Sometimes |
| Specify any such areas:________________________________________________________ |
*Clarify all responses noted as "sometimes" on back of this page; noting the number of the question with each clarification
Respiratory Protection Checklist
II. SELECTION OF RESPIRATORY PROTECTION
A. Do respirators issued to HCWs meet the following performance for protection against Mycobacterium tuberculosis:
| 1. | Are all respirators used approved by NIOSH? | Y / N |
| 2. | Does the respirator have the ability to
filter particles 1 micrometer in size in the unloaded
state with a filter efficiency greater than or equal to
95% (i.e., filter leakage of less than or equal to 5%),
given flow rates of up to 50 liters (L) per minute? (If "yes" attach any documentation attesting to this) |
Y / N |
| 3. | Does the respirator have the ability to
be qualitatively or quantitatively fit tested in a
reliable way to obtain face-seal leakage of less than or
equal to 10%? (If "yes" attach any documentation attesting to this) |
Y / N |
| 4. | Does the respirator have the ability to
fit different facial sizes and characteristics of HCWs
(i.e., is it available in at least three sizes)? (If "yes" attach any documentation attesting to this) |
Y / N |
| 5. | Does the respirator have the ability to
be checked for facepiece fit, in accordance with OSHA
standards and good industrial hygiene practice, by HCWs
each time they put on their respirator? (If "yes" attach any documentation attesting to this) |
Y / N |
| 6. | Are different levels of respiratory
protection (such as powered air purifying or positive
pressure airline respirators), which exceed the above performance criteria, available for selected high-risk procedures on patients known or suspected to have TB (e.g., bronchoscopy, autopsy). (If "yes" clarify what respirators are used for high-risk procedures) |
Y / N |
| 7. | Are the respirators selected appropriate for procedures requiring a sterile field? (if respirators have exhalation valves or are positive pressure they do not protect the sterile field) | Y / N |
Respiratory Protection Checklist
III. FIT TESTING OF RESPIRATORY PROTECTION DEVICES
|
All / *Some / *None | |||
|
All / *Some / *None | |||
|
All / *Some / *None | |||
|
All / *Some / *None | |||
|
All / *Some / *None |
IV. STORAGE, REUSE AND DISPOSAL
|
All / *Some / *None | |||
|
Y / N | |||
|
Y / N | |||
|
Y / N | |||
|
Y / N | |||
|
Y / N | |||
|
Y / N |
V. RESPIRATORY PROGRAM
* Clarify "some" and "none" responses by number on the back of this page. |
Y / N | |||||||
| Respiratory Protection Checklist _______________________________________________________________ |
||||||||
| 2. | Who is responsible for the respiratory
protection program? Who is responsible for the respiratory protection program?
|
|||||||
| 3. | Does the written program provide written procedures for: | |||||||
| Respirator selection? | Y / N | |||||||
| Assessing the need for respirators (i.e., exposure monitoring data)? | Y / N | |||||||
| Employee training? | Y / N | |||||||
| Cleaning, inspection and disinfecting? | Y / N | |||||||
| Storage? | Y / N | |||||||
| Medical surveillance? | Y / N | |||||||
| Prohibiting facial hair which prevents adequate facepiece-to-face seal? | Y / N | |||||||
| Accommodating employees who must wear corrective lenses? | Y / N | |||||||
| Program evaluation on a yearly basis? (Inclusive of employee input) | Y / N |
VI. MEDICAL SURVEILLANCE
|
Y / N | |||
|
Y / N | |||
|
Y / N | |||
|
Y / N | |||
|
Y / N |
VII. TRAINING
|
||||
|
Y / N | |||
|
Y / N | |||
|
Y / N | |||
|
Y / N | |||
|
Y / N | |||
|
Y / N | |||
|
Y / N | |||
|
Y / N | |||
|
||||
|
||||
|
FIT TESTING RECORD FOR RESPIRATOR USERS
Employee:________________________________
Job Title:_______________________________
SS#:_____________________
Date of Birth:___________
Employer:_______________________________
Employer Phone Number: ______________________________
Age:______________Height:______________Weight:_______________
Description of condition requiring RPE use:
_____________________________________
______________________________________________________________________
FIT TESTING RECORD
PE Manufacturer__________________________________
Model Number_________________________
Facepiece Type and Size_________________________________________________________________
NIOSH Approval Number ______________________________________
Cartridge Type________________________________________________
NIOSH Approval Number _______________________________________
| Medical Restriction Noted By Physician? | Yes No |
| Odor Detection Adequate? | Yes No |
Date Fit Tested______________________________
Test Atmosphere___________________________
Pass/Fail______________________Comments:_________________________________
________________________________________________________________________
_____________________________
SIGNATURE OF FIT TESTER
____________________________
DATE
MEDICAL QUESTIONNAIRE FOR RESPIRATOR USERS
Employee:_______________________________________
Job Title:________________________________
SS#: _______________Date of Birth:
Employer:__________________________________
Employer Phone Number: ____________________________________
Age: ________ Height: ________ Weight: _____________
| Have you worn a respirator before? | Yes No | |
| If Yes, describe any
difficulties noted with respirator
use:__________________________________________________________________ _____________________________________________________________________ |
||
| Will you be wearing any other personal protective equipment? | Yes No | |
| If Yes, please describe: ___________________________________________________ | ||
| Have you had or do you currently have any of the following: | ||
|
Yes No | |
|
Yes No | |
|
Yes No | |
|
Yes No | |
|
Yes No | |
|
Yes No | |
|
Yes No | |
|
Yes No | |
|
Yes No | |
|
Yes No | |
|
Yes No | |
|
Yes No | |
|
Yes No | |
|
Yes No | |
|
Yes No | |
|
Yes No | |
|
Yes No | |
| Please explain YES answers (use back of form if necessary)_________________________________________ | ||
| Are you currently taking any medications? | Yes No | |
| If YES, please list:________________________________________________________________________ | ||
| Are you currently taking any medications? | Yes No | |
| If YES, please list:________________________________________________________________________ | ||
| Do you now or have you ever smoked? | Yes No | |
| At what age did you start
smoking?_________________________________________ How long ago did you quit smoking?________________________________________ How many packs per day did or do you smoke? _______________________________ |
||
| ____________________ PHYSICIAN SIGNATURE ____________________ DATE |
____________________ EMPLOYEE SIGNATURE ____________________ DATE |
REQUEST FOR MEDICAL CLEARANCE FOR RESPIRATOR USE
Employee:
_______________________________________________________________
Job Title:
________________________________________________________________
SS#: _______________Date of Birth:_________________Employer:_________________
Employer Phone Number:___________________________________________________
Age: _________________Height: __________________
Weight:______________________
Describe the job or work assignment for which respiratory protection will be used:________________
What hazardous material will respiratory
protection be used for?
________________________________________________________________________
________________________________________________________________________
Circle Type or Types of Respirator(s) to be used?
Powered-air purifying
Supplied-air (pressure demand) Air-purifying (non-powered)
Other
Circle extent of use:
| Full Shift (Daily) | Task Dependent (Occasionally) | Rarely Emergency Use Only |
Length of time respiratory protection will be required (hours per day):________________________________
Will there be elevated temperatures? Yes No
Supervisor___________________________________Date__________________
PHYSICIAN'S EVALUATION
Employee Name__________________________________________________________
May __________May not ___________Wear the above noted respirtor(s)_____________
The restrictions for respirator use by this employee are:______________________________
Examining Physician _________________________________ Date___________________
Go back to the Table of Contents| Section B of Introduction to CDC Guidelines Pages 4-6 |
B. Epidemiology, Transmission, and Pathogenesis of TB
The prevalence of TB is not distributed evenly throughout all segments of the U.S. population. Some subgroups or persons have a higher risk for TB either because they are more likely than other persons in the general population to have been exposed to and infected with M. tuberculosis or because their infection is more likely to progress to active TB after they have been infected (5). In some cases, both of these factors may be present. Groups of persons known to have a higher prevalence of TB infection include contacts of persons who have active TB, foreign-born persons from areas of the world with a high prevalence of TB (e.g., Asia, Africa, the Caribbean, and Latin America), medically underserved populations (e.g., some African-Americans, Hispanics, Asians and Pacific Islanders, American Indians, and Alaskan Natives), homeless persons, current or former correctional-facility inmates, alcoholics, injecting-drug users, and the elderly. Groups with a higher risk for progression from latent TB infection to active disease include persons who have been infected recently (i.e., within the previous 2 years), children less than 4 years of age, persons with fibrotic lesions on chest radiographs, and persons with certain medical conditions (i.e., human immunodeficiency virus {HIV} infection, silicosis, gastrectomy or jejuno-ileal bypass, being greater than or equal to 10% below ideal body weight, chronic renal failure with renal dialysis, diabetes mellitus, immunosuppression resulting fro receipt of high-dose corticosteroid or other immunosuppressive therapy, and some malignancies) (5). M. tuberculosis is carried in airborne particles, or droplet nuclei, that can be generated when persons who have pulmonary or laryngeal TB sneeze, cough, speak, or sing (6). The particles are an estimated 1-5 mm in size, and normal air currents can keep them airborne for prolonged time periods and spread them throughout a room or building (7). Infection occurs when a susceptible person inhales droplet nuclei containing M. tuberculosis, and these droplet nuclei traverse the mouth or nasal passages, upper respiratory tract, and bronchi to reach the alveoli of the lungs. Once in the alveoli, the organisms are taken up by alveolar macrophages and spread throughout the body. Usually within 2-10 weeks after initial infection with M. tuberculosis, the immune response limits further multiplication and spread of the tubercle bacilli; however, some of the bacilli remain dormant and viable for many years. This condition is referred to as latent TB infection. Persons with latent TB infection usually have positive purified protein derivative (PPD)-tuberculin skin-test results, but they do not have symptoms of active TB, and they are not infectious.
In general, persons who become infected with M. tuberculosis have approximately a 10% risk for developing active TB during their lifetimes. This risk is greatest during the first 2 years after infection. Immunocompromised persons have a greater risk for the progression of latent TB infection to active TB disease; HIV infection is the strongest known risk factor for this progression. Persons with latent TB infection who become coinfected with HIV have approximately an 8%-10% risk per year for developing active TB (8). HIV- infected persons who are already severely immunosuppressed and who become newly infected with M. tuberculosis have an even greater risk for developing active TB (9-12).
The probability that a person who is exposed to M. tuberculosis will become infected depends primarily on the concentration of infectious droplet nuclei in the air and the duration of exposure. Characteristics of the TB patient that enhance transmission include a) disease in the lungs, airways, or larynx; b) presence of cough or other forceful expiratory measures; c) presence of acid-fast bacilli (AFB) in the sputum; d) failure of the patient to cover the mouth and nose when coughing or sneezing ; e) presence of cavitation on chest radiograph; f) inappropriate or short duration of chemotherapy; and g) administration of procedures that can induce coughing or cause aerosolization of M. tuberculosis (e.g., sputum induction). Environmental factors that enhance the likelihood of transmission include a) exposure in relatively small, enclosed spaces; b) inadequate local or general ventilation that results in insufficient dilution and/or removal of infectious droplet nuclei; and c) recirculation of air containing infectious droplet nuclei. Characteristics of the persons exposed to M. tuberculosis that may affect the risk for becoming infected are not as well defined. In general, persons who have been infected previously with M. tuberculosis may be less susceptible to subsequent infection. However, reinfection can occur among previously infected persons, especially if they are severely immunocompromised. Vaccination with Bacille of Calmette and Guérin (BCG) probably does not affect the risk for infection; rather, it decreases the risk for progressing from latent TB infection to active TB (13). Finally, although it is well established that HIV infection increases the likelihood of progressing from latent TB infection to active TB, it is unknown whether HIV infection increases the risk for becoming infected if exposed to M. tuberculosis.
C. Risk for Nosocomial Transmission of M. tuberculosis
Transmission of M. tuberculosis is a recognized risk in health care facilities (14-22). The magnitude of the risk varies considerably by the type of health care facility, the prevalence of TB in the community, the patient population served, the HCW's occupational group, the area of the health care facility in which the HCW works, and the effectiveness of TB infection-control interventions. The risk may be higher in areas where patients with TB are provided care before diagnosis and initiation of TB treatment and isolation precautions (e.g., in clinic waiting areas and emergency departments) or where diagnostic or treatment procedures that stimulate coughing are performed. Nosocomial transmission of M. tuberculosis has been associated with close contact with persons who have infectious TB and with the performance of certain procedures (e.g., bronchoscopy [17], endotracheal intubation and suctioning [18], open abscess irrigation [20], and autopsy [21,22]). Sputum induction and aerosol treatments that induce coughing may also increase the potential for transmission of M. tuberculosis (23,24). Personnel of health care facilities should be particularly alert to the need for preventing transmission of M. tuberculosis in those facilities in which immunocompromised persons (e.g., HIV-infected persons) work or receive care-especially if cough-inducing procedures, such as sputum induction and aerosolized pentamidine treatments, are being performed.
Several TB outbreaks among persons in health care facilities have been reported recently (11,24-28; CDC unpublished data). Many of these outbreaks involved transmission of multidrug-resistant strains of M. tuberculosis to both patients and HCWs. Most of the patients and some of the HCWs were HIV-infected persons in whom new infection progressed rapidly to active disease. Mortality associated with those outbreaks was high (range: 43%-93%). Furthermore, the interval between diagnosis and death was brief (range of median intervals: 4-16 weeks). Factors contributing to these outbreaks included delayed diagnosis of TB, delayed recognition of drug resistance, and delayed initiation of effective therapy-all of which resulted in prolonged infectiousness, delayed initiation and inadequate duration of TB isolation, inadequate ventilation in TB isolation rooms, lapses in TB isolation practices and inadequate precautions for cough-inducing procedures, and lack of adequate respiratory protection. Analysis of data collected from three of the health care facilities involved in the outbreaks indicates that transmission of M. tuberculosis decreased significantly or ceased entirely in areas where measures similar to those in the 1990 TB Guidelines were implemented (2,29-32). However, several interventions were implemented simultaneously, and the effectiveness of the separate interventions could not be determined.
References
| 2. | CDC. Guidelines for preventing the transmission of tuberculosis in health care settings, with special focus on HIV-related issues. MMWR 1990;39(No. RR-17). |
| 5. | CDC. Screening for tuberculosis and tuberculous infection in high-risk populations, and the use of preventive therapy for tuberculous infection in the United States: recommendations of the Advisory Committee for Elimination of Tuberculosis. MMWR 1990;39(No. RR-8). |
| 6. | American Thoracic Society/CDC. Diagnostic standards and classification of tuberculosis. Am Rev Respir Dis 1990;142:725-35. |
| 7. | Wells WF. Aerodynamics of droplet nuclei. In: Airborne contagion and air hygiene. Cambridge: Harvard University Press, 1955:13-9. |
| 8. | Selwyn PA, Hartel D, Lewis VA, et al. A prospective study of the risk of tuberculosis among intravenous drug users with human immunodeficiency virus infection. N Engl J Med 1989;320:545-50. |
| 9. | Di Perri G, Cruciani M, Danzi MC, et al. Nosocomial epidemic of active tuberculosis among HIV-infected patients. Lancet 1989;2:1502-4. |
| 10. | Daley CL, Small PM, Schecter GF, et al. An outbreak of tuberculosis with accelerated progression among persons infected with the human immunodeficiency virus: an analysis using restriction-fragment-length polymorphisms. N Engl J Med 1992;326:231-5. |
| 11. | Edlin BR, Tokars JI, Grieco MH, et al. An outbreak of multidrug-resistant tuberculosis among hospitalized patients with the acquired immunodeficiency syndrome. N Engl J Med 1992;326:1514-21. |
| 12. | Dooley SW, Villarino E, Lawrence M, et al. Nosocomial transmission of tuberculosis in a hospital unit for HIV-infected patients. JAMA 1992;267:2632-4. |
| 13. | Ten Dam HG. Research on BCG vaccination. Adv Tuberc Res 1984;21:79-106. |
| 14. | Barrett-Connor E. The epidemiology of tuberculosis in physicians. JAMA 1979;241:33-8. |
| 15. | Brennen C, Muder RR, Muraca PW. Occult endemic tuberculosis in a chronic care facility. Infect Control Hosp Epidemiol 1988;9:548-52. |
| 16. | Goldman KP. Tuberculosis in hospital doctors. Tubercle 1988;69:237-40. |
| 17. | Catanzaro A. Nosocomial tuberculosis. Am Rev Respir Dis 1982;125:559-62. |
| 18. | Ehrenkranz NJ, Kicklighter JL. Tuberculosis outbreak in a general hospital: evidence of air-borne spread of infection. Ann Intern Med 1972;77:377-82. |
| 19. | Haley CE, McDonald RC, Rossi L, et al. Tuberculosis epidemic among hospital personnel. Infect Control Hosp Epidemiol 1989;10:204-10. |
| 20. | Hutton MD, Stead WW, Cauthen GM, et al. Nosocomial Transmission of tuberculosis associated with a draining tuberculosis abscess. J Infect Dis 1990;161:286-95. |
| 21. | Kantor HS, Poblete R, Pusateri SL. Nosocomial transmission of tuberculosis from unsuspected disease. Am J Med 1988;84:833-8. |
| 22. | Lundgren R, Norrman E, Asberg I. Tuberculosis infection transmitted at autopsy. Tubercle 1987;68:147-50. |
| 23. | CDC, Mycobacterium tuberculosis transmission in a health clinic-Florida, 1988. MMWR 1989;38:256-8,263-4. |
| 24. | Beck-Sagué C, Dooley SW, Hutton MD, et al. Outbreak of multidrug-resistant Mycobacterium tuberculosis infections in a hospital: transmission to patients with HIV infection and staff. JAMA 1992;268:1280-6. |
| 25. | CDC. Nosocomial transmission of multidrug-resistant tuberculosis to health care workers and HIV-infected patients in an urban hospital-Florida. MMWR 1990;39:718-22. |
| 26. | CDC. Nosocomial transmission of multidrug-resistant tuberculosis among HIV-infected persons-Florida and New York, 1988-1991. MMWR 1991;40:585-91. |
| 27. | Pearson ML, Jereb JA, Frieden TR, et al. Nosocomial transmission of multidrug-resistant Mycobacterium tuberculosis: a risk to patients and health care workers. Ann Intern Med 1992;117:191-6. |
| 28. | Dooley SW, Jarvis WR, Martone WJ, Snider DE Jr. Multidrug-resistant tuberculosis [Editorial]. Ann Intern Med 1992;117:257-8. |
| 29. | Wenger P, Beck-Sagué C, Otten J, et al. Efficacy of control measures in preventing nosocomial transmission of multidrug-resistant tuberculosis among patient and health care workers [Abstract 53A]. In: Program and abstracts of the World Congress on Tuberculosis. Bethesda, MD: National Institutes of Health, Fogarty International Center, 1992. |
| 30. | Otten J, Chen J, Cleary T. Successful control of an outbreak of multidrug-resistant tuberculosis in an urban teaching hospital [Abstract 51D]. In: Program and abstracts of the World Congress on Tuberculosis. Bethesda, MD: National Institutes of Health, Fogarty International Center, 1992. |
| 31. | Maloney S, Pearson M, Gordon M, et al. The efficacy of recommended infection control measures in preventing nosocomial transmission of multidrug-resistant TB [Abstract 51C]. In: Program and abstracts of the World Congress on Tuberculosis. Bethesda, MD: National Institutes of Health, Fogarty International Center, 1992. |
| 32. | Stroud L, Tokars J, Grieco M, Gilligan M, Jarvis W. Interruption of nosocomial transmission of multidrug-resistant Mycobacterium tuberculosis (MDR-TB) among AIDS patients in a New York City Hospital [Abstract A1-3]. In: Third Annual Meeting of the Society for Hospital Epidemiologists of America. Chicago: Society for Hospital Epidemiologists of America, 1993. |
| MEMORANDUM FOR: | REGIONAL ADMINISTRATORS |
| FROM: | JOHN B. MILES, JR., DIRECTOR DIRECTORATE OF COMPLIANCE PROGRAMS |
| SUBJECT: | RESPIRATORY FIT TESTING AND FIT CHECKING PROCEDURES |
This memorandum replaces the February 26 memorandum on the same subject. As you are aware, OSHA and NIOSH during the last year have been involved in a number respirator related issues. OSHA is in the process of issuing a final respiratory protection standard that revises 29 CFR 1910.134,* while NIOSH issued new certification guidelines (42 CFR Part 84) last summer for particulate respirators. The new NIOSH certification procedures directly affected our requirements for respiratory protection for exposure to tuberculosis and particulates. The new NIOSH certification procedures coupled with OSHA's proposed respiratory protection standard has generated numerous questions to the Office of Health Compliance Assistance. Respirator related questions have centered around OSHA requirements for fit-testing, fit checking, and reuse of the new respirators certified under 42 CFR Part 84. The most commonly asked questions include:
| 1. What does OSHA expect for a fit-test? |
| 2. What is the difference between a fit-test and user seal check? |
| 3. What does OSHA expect for an acceptable fit-test and user seal check? |
In response to these questions, this memorandum is being distributed to provide information and guidance on current Agency respirator requirements.
* CFR 1910.134 is now codified for protection against TB as 29 CFR 1910.139.
Respirator Fit-Testing and User seal checking Requirements:
The procedures and requirements for fit-testing the new classes of particulate respirators have not changed from those OSHA currently requires. While the respirator standard (29 CFR 1910.134)* does not specify what fit tests and fit-testing protocols to be used, OSHA would accept either a quantitative fit test (QNFT) or qualitative fit test (QLFT) as meeting the fit test requirement.
A QNFT consists of wearing the respirator in a stable test atmosphere that contains a suitable challenge agent (e.g., corn oil). The adequacy of the fit is determined by measuring and establishing a ratio of the actual levels of the challenge agent both inside and outside of the respirator. Among QNFT methods, OSHA allows the use of ambient-particulate measurement technology (Portacount) and the controlled-negative pressure technology (Dynatech Fit-Tester 3000). [Note: the controlled negative-pressure technology is only applicable to respirators with replaceable filters. It is not applicable to filtration facepiece respirators.] A QLFT involves the introduction of an aerosol challenge agent into the area around the face of the respirator wearer. OSHA requirements for fit-testing of respiratory protection are spelled out under the third sentence of 29 CFR 1910.134(e)(5):*
"Training shall provide... an opportunity to handle the respirator, have it fitted properly, test its face-piece-to-face seal, wear it in normal air for a long familiarity period, and, finally, to wear it in a test atmosphere."
* CFR 1910.134 is now codified for protection against TB as 29 CFR 1910.139.
Protection Factors and QNFT/QLFT:
Half-mask respirators (including disposable or filtering facepiece and replaceable filters) have typically received an assigned protection factor (APF) of 10 by ANSI and NIOSH. For a QNFT, one uses the APF plus an assigned minimum safety factor of 10 to establish the "fit factor (FF)." The FF is what the wearer of that respirator is assigned for the respirator with which he/she was tested. For a disposable respirator or half-mask respirator, based upon the APF times the safety factor, one must achieve a minimum FF of 100 in a QNFT. [Note: the safety factor of 10 was chosen based upon standard practice to help ensure adequate protection during field use; ANSI Z88.2-1992 also recommends a safety factor of 10]. OSHA has not formally assigned an APF to the Type 95, 99, or 100 respirators certified under 42 CFR Part 84. As you know, OSHA issued a proposed respiratory protection standard in the Federal Register on November 15th, 1994 (Vol. 59, No. 219). When the final respiratory protection standard is issued, the Agency will take a formal position on the APFs. In the interim, when the Type 95, 99, or 100 respirators are quantitatively fit tested, OSHA will continue to require a minimum FF of 100 in order for the employer to conclude that they fit the worker well enough to satisfy the respiratory protection standard.
In the 1994 CDC Tuberculosis Guidelines where respiratory protection for occupational exposure to Tuberculosis is addressed, the CDC spelled out standard performance criteria for half mask respirators. One point under the criteria stated the following:
"The ability to be qualitatively or quantitatively fit tested in a reliable way to obtain a face-seal leakage of < 10%."
This statement apparently has been misinterpreted by some to imply that the new classes of half mask respirators must only meet an individual FF of 10 rather than a FF of 100. Until assigned protection factors are determined for these respirators, for those facilities conducting QNFT, OSHA continues to require minimum fit factors of 100 to be obtained.
Qualitative Fit-Testing Procedures:
For facilities electing to conduct QLFTs, a determination of adequate fit is based upon whether or not the individual can smell, taste or detect the challenge agent. OSHA will accept those tests using the following challenge agents currently available: irritant fume, saccharin or Bitrex™ aerosol. When an individual passes a QLFT, a minimum FF of 100 will be assumed to have been achieved.
A number of queries to OSHA have involved concerns regarding the use of irritant smoke or saccharin aerosol QLFT protocols. Recently, a peer reviewed article discussed the validation of an alternative qualitative fit testing media. That test media used is a substance called Bitrex™ (denatonium benzoate). This test and the testing protocols are described in:
| Mullins, H.E.; Danisch, S.G.; and Johnston, A.R.: Development of a New Qualitative Test for Fit Testing Respirators. American Industrial Hygiene Journal. Vol. 56: 1068-1073 (November 1995). |
Based upon the information presented in this peer-reviewed article, OSHA will accept for an interim period the common Bitrex™ aerosol QLFT procedure when performed using the protocol described in the article, as a valid alternative to the other QLFT protocols. All of the QLFT protocols will be reviewed as part of the 29 CFR 1910.134* revision.
* CFR 1910.134 is now codified for protection against TB as 29 CFR 1910.139.
User seal checking Procedures:
In contrast to a QNFT or QLFT fit-test, a fit check is a quick determination of respirator fit by the wearer each time a respirator is donned to assure that a proper face-to-respirator seal has been achieved. The requirements for fit checking are spelled out under the last two sentences of 29 CFR 1910.134(5)(I):*
* CFR 1910.134 is now codified for protection against TB as 29 CFR 1910.139.
"To assure proper protection, the facepiece fit shall be checked by the wearer each time he puts on the respirator. This may be done by following the manufacturer's facepiece fitting instruction."
Concerns have been raised from the field about user seal checking procedures and whether or not there is a valid procedure for user seal checking disposable respirators. The user seal check on a disposable respirator is difficult and often less reliable than fit checks done on an elastomeric half mask respirator. However, a recently published article does provide valid information on how to conduct a user seal check with a disposable respirator. The article also provides a validation and comparison data with the saccharin-aerosol protocol. The reference for the article is:
| Myers, W.A.; Jaraiedi, M; and Hendricks, L.: Effectiveness of Fit Check Methods on Half Mask Respirators. Applied Occupational and Environmental Hygiene Journal. Vol. 10 (11): 934-942 (November 1995). |
One of the main conclusions of the article was that when employees donned a respirator and followed the manufacturers' recommended fit check procedures, the wearer was better able to detect and thus prevent a poorer-quality fit of the respirator. Hence, the fit check provided an added assurance that the respirator was correctly being worn. This is currently under review by OSHA as part of the 29 CFR 1910.134* revision.
* CFR 1910.134 is now codified for protection against TB as 29 CFR 1910.139.
Reuse of Disposable Respirators:
According to NIOSH, the reuse of the Part 84 particulate respirators is permitted for tuberculosis provided the respirators have not been damaged, soiled, or the breathing resistance becomes great enough to cause discomfort to the wearer (overloaded) or the integrity of the mask has not been compromised. OSHA accepts this view. Apparently some suppliers of the new disposable respirators are informing clients that the respirators can only be used one time and then must be replaced.
We suggest individuals who have any additional questions or require any further assistance should contact either Richard Fairfax of my staff at (202) 219-8036 or the local OSHA Regional Office (see attachment).
OSHA Regional Offices |
|
| Region I - Boston: | 617-565-7164 |
| Region II - New York: | 212-337-2326 |
| Region III - Philadelphia: | 215-596-1201 |
| Region IV - Atlanta: | 404-347-3573 |
| Region V - Chicago: | 312-353-2220 |
| Region VI - Dallas: | 214-767-4731 |
| Region VII - Kansas City: | 816-426-5861 |
| Region VIII - Denver: | 303-391-5858 |
| Region IX - San Francisco: | 415-975-4310 |
| Region X - Seattle: | 206-553-5930 |
Appendix A to 1910.134: Fit Testing Procedure
Part I. OSHA-Accepted Fit Test Protocols
A. Fit Testing Procedures-General Requirements
The employer shall conduct fit testing using the following procedures. The requirements in this appendix apply to all OSHA-accepted fit test methods, both QLFT and QNFT.
1. The test subject shall be allowed to pick the most acceptable respirator from a sufficient number of respirator models and sizes so that the respirator is acceptable to, and correctly fits, the user.
2. Prior to the selection process, the test subject shall be shown how to put on a respirator, how it should be positioned on the face, how to set strap tension and how to determine an acceptable fit. A mirror shall be available to assist the subject in evaluating the fit and positioning of the respirator. This instruction may not constitute the subject's formal training on respirator use, because it is only a review.
3. The test subject shall be informed that he/she is being asked to select the respirator that provides the most acceptable fit. Each respirator represents a different size and shape, and if fitted and used properly, will provide adequate protection.
4. The test subject shall be instructed to hold each chosen facepiece up to the face and eliminate those that obviously do not give an acceptable fit.
5. The more acceptable facepieces are noted in case the one selected proves unacceptable; the most comfortable mask is donned and worn at least five minutes to assess comfort. Assistance in assessing comfort can be given by discussing the points in the following item A.6. If the test subject is not familiar with using a particular respirator, the test subject shall be directed to don the mask several times and to adjust the straps each time to become adept at setting proper tension on the straps.
6. Assessment of comfort shall include a review of the following points with the test subject and allowing the test subject adequate time to determine the comfort of the respirator:
(a) Position of the mask on the nose
(b) Room for eye protection
(c) Room to talk
(d) Position of mask on face and cheeks
7. The following criteria shall be used to help determine the adequacy of the respirator fit:
(a) Chin properly placed;
(b) Adequate strap tension, not overly tightened;
(c) Fit across nose bridge;
(d) Respirator of proper size to span distance from nose to chin;
(e) Tendency of respirator to slip;
(f) Self-observation in mirror to evaluate fit and respirator position.
8. The test subject shall conduct a user seal check, either the negative and positive pressure seal checks described in Appendix B-1 of this section or those recommended by the respirator manufacturer which provide equivalent protection to the procedures in Appendix B- 1. Before conducting the negative and positive pressure checks, the subject shall be told to seat the mask on the face by moving the head from side-to-side and up and down slowly while taking in a few slow deep breaths. Another facepiece shall be selected and retested if the test subject fails the user seal check tests.
9. The test shall not be conducted if there is any hair growth between the skin and the facepiece sealing surface, such as stubble beard growth, beard, mustache or sideburns which cross the respirator sealing surface. Any type of apparel which interferes with a satisfactory fit shall be altered or removed.
10. If a test subject exhibits difficulty in breathing during the tests, she or he shall be referred to a physician or other licensed health care professional, as appropriate, to determine whether the test subject can wear a respirator while performing her or his duties.
11. If the employee finds the fit of the respirator unacceptable, the test subject shall be given the opportunity to select a different respirator and to be retested.
12. Exercise regimen. Prior to the commencement of the fit test, the test subject shall be given a description of the fit test and the test subject's responsibilities during the test procedure. The description of the process shall include a description of the test exercises that the subject will be performing. The respirator to be tested shall be worn for at least 5 minutes before the start of the fit test.
13. The fit test shall be performed while the test subject is wearing any applicable safety equipment that may be worn during actual respirator use which could interfere with respirator fit.
14. Test Exercises. (a) The following test exercises are to be performed for all fit testing methods prescribed in this appendix, except for the CNP method. A separate fit testing exercise regimen is contained in the CNP protocol. The test subject shall perform exercises, in the test environment, in the following manner:
(1) Normal breathing. In a normal standing position, without talking, the subject shall breathe normally.
(2) Deep breathing. In a normal standing position, the subject shall breathe slowly and deeply, taking caution so as not to hyperventilate.
(3) Turning head side to side. Standing in place, the subject shall slowly turn his/her head from side to side between the extreme positions on each side. The head shall be held at each extreme momentarily so the subject can inhale at each side.
(4) Moving head up and down. Standing in place, the subject shall slowly move his/her head up and down. The subject shall be instructed to inhale in the up position (i.e., when looking toward the ceiling).
(5) Talking. The subject shall talk out loud slowly and loud enough so as to be heard clearly by the test conductor. The subject can read from a prepared text such as the Rainbow Passage, count backward from 100, or recite a memorized poem or song.
Rainbow Passage
When the sunlight strikes raindrops in the air, they act like a prism and form a rainbow. The rainbow is a division of white light into many beautiful colors. These take the shape of a long round arch, with its path high above, and its two ends apparently beyond the horizon. There is, according to legend, a boiling pot of gold at one end. People look, but no one ever finds it. When a man looks for something beyond reach, his friends say he is looking for the pot of gold at the end of the rainbow.
(6) Grimace. The test subject shall grimace by smiling or frowning. (This applies only to QNFT testing; it is not performed for QLFT)
(7) Bending over. The test subject shall bend at the waist as if he/she were to touch his/her toes. Jogging in place shall be substituted for this exercise in those test environments such as shroud type QNFT or QLFT units that do not permit bending over at the waist.
(8) Normal breathing. Same as exercise (1).
(b) Each test exercise shall be performed for one minute except for the grimace exercise which shall be performed for 15 seconds. The test subject shall be questioned by the test conductor regarding the comfort of the respirator upon completion of the protocol. If it has become unacceptable, another model of respirator shall be tried. The respirator shall not be adjusted once the fit test exercises begin. Any adjustment voids the test, and the fit test must be repeated.
B. Qualitative Fit Test (QLFT) Protocols
1. General
(a) The employer shall ensure that persons administering QLFT are able to prepare test solutions, calibrate equipment and perform tests properly, recognize invalid tests, and ensure that test equipment is in proper working order.
(b) The employer shall ensure that QLFT equipment is kept clean and well maintained so as to operate within the parameters for which it was designed.
2. Isoamyl Acetate Protocol
Note: This protocol is not appropriate to use for the fit testing of particulate respirators. If used to fit test particulate respirators, the respirator must be equipped with an organic vapor filter.
(a) Odor Threshold Screening
Odor threshold screening, performed without wearing a respirator, is intended to determine if the individual tested can detect the odor of isoamyl acetate at low levels.
(1) Three 1 liter glass jars with metal lids are required.
(2) Odor-free water (e.g., distilled or spring water) at approximately 25 deg. C (77 deg. F) shall be used for the solutions.
(3) The isoamyl acetate (IAA) (also known at isopentyl acetate) stock solution is prepared by adding 1 ml of pure IAA to 800 ml of odor-free water in a 1 liter jar, closing the lid and shaking for 30 seconds. A new solution shall be prepared at least weekly.
(4) The screening test shall be conducted in a room separate from the room used for actual fit testing. The two rooms shall be well-ventilated to prevent the odor of IAA from becoming evident in the general room air where testing takes place.
(5) The odor test solution is prepared in a second jar by placing 0.4 ml of the stock solution into 500 ml of odor-free water using a clean dropper or pipette. The solution shall be shaken for 30 seconds and allowed to stand for two to three minutes so that the IAA concentration above the liquid may reach equilibrium. This solution shall be used for only one day.
(6) A test blank shall be prepared in a third jar by adding 500 cc of odor-free water.
(7) The odor test and test blank jar lids shall be labeled (e.g., 1 and 2) for jar identification. Labels shall be placed on the lids so that they can be peeled off periodically and switched to maintain the integrity of the test.
(8) The following instruction shall be typed on a card and placed on the table in front of the two test jars (i.e., 1 and 2): "The purpose of this test is to determine if you can smell banana oil at a low concentration. The two bottles in front of you contain water. One of these bottles also contains a small amount of banana oil. Be sure the covers are on tight, then shake each bottle for two seconds. Unscrew the lid of each bottle, one at a time, and sniff at the mouth of the bottle. Indicate to the test conductor which bottle contains banana oil."
(9) The mixtures used in the IAA odor detection test shall be prepared in an area separate from where the test is performed, in order to prevent olfactory fatigue in the subject.
(10) If the test subject is unable to correctly identify the jar containing the odor test solution, the IAA qualitative fit test shall not be performed.
(11) If the test subject correctly identifies the jar containing the odor test solution, the test subject may proceed to respirator selection and fit testing.
(b) Isoamyl Acetate Fit Test
(1) The fit test chamber shall be a clear 55-gallon drum liner suspended inverted over a 2-foot diameter frame so that the top of the chamber is about 6 inches above the test subject's head. If no drum liner is available, a similar chamber shall be constructed using plastic sheeting. The inside top center of the chamber shall have a small hook attached.
(2) Each respirator used for the fitting and fit testing shall be equipped with organic vapor cartridges or offer protection against organic vapors.
(3) After selecting, donning, and properly adjusting a respirator, the test subject shall wear it to the fit testing room. This room shall be separate from the room used for odor threshold screening and respirator selection, and shall be well-ventilated, as by an exhaust fan or lab hood, to prevent general room contamination.
(4) A copy of the test exercises and any prepared text from which the subject is to read shall be taped to the inside of the test chamber.
(5) Upon entering the test chamber, the test subject shall be given a 6-inch by 5-inch piece of paper towel, or other porous, absorbent, single-ply material, folded in half and wetted with 0.75 ml of pure IAA. The test subject shall hang the wet towel on the hook at the top of the chamber. An IAA test swab or ampule may be substituted for the IAA wetted paper towel provided it has been demonstrated that the alternative IAA source will generate an IAA test atmosphere with a concentration equivalent to that generated by the paper towel method.
(6) Allow two minutes for the IAA test concentration to stabilize before starting the fit test exercises. This would be an appropriate time to talk with the test subject; to explain the fit test, the importance of his/her cooperation, and the purpose for the test exercises; or to demonstrate some of the exercises.
(7) If at any time during the test, the subject detects the banana-like odor of IAA, the test is failed. The subject shall quickly exit from the test chamber and leave the test area to avoid olfactory fatigue.
(8) If the test is failed, the subject shall return to the selection room and remove the respirator. The test subject shall repeat the odor sensitivity test, select and put on another respirator, return to the test area and again begin the fit test procedure described in (b) (1) through (7) above. The process continues until a respirator that fits well has been found. Should the odor sensitivity test be failed, the subject shall wait at least 5 minutes before retesting. Odor sensitivity will usually have returned by this time.
(9) If the subject passes the test, the efficiency of the test procedure shall be demonstrated by having the subject break the respirator face seal and take a breath before exiting the chamber.
(10) When the test subject leaves the chamber, the subject shall remove the saturated towel and return it to the person conducting the test, so that there is no significant IAA concentration buildup in the chamber during subsequent tests. The used towels shall be kept in a self-sealing plastic bag to keep the test area from being contaminated.
3. Saccharin Solution Aerosol Protocol
The entire screening and testing procedure shall be explained to the test subject prior to the conduct of the screening test.
(a) Taste threshold screening. The saccharin taste threshold screening, performed without wearing a respirator, is intended to determine whether the individual being tested can detect the taste of saccharin.
(1) During threshold screening as well as during fit testing, subjects shall wear an enclosure about the head and shoulders that is approximately 12 inches in diameter by 14 inches tall with at least the front portion clear and that allows free movements of the head when a respirator is worn. An enclosure substantially similar to the 3M hood assembly, parts # FT 14 and # FT 15 combined, is adequate.
(2) The test enclosure shall have a 3/4-inch (1.9 cm) hole in front of the test subject's nose and mouth area to accommodate the nebulizer nozzle.
(3) The test subject shall don the test enclosure. Throughout the threshold screening test, the test subject shall breathe through his/her slightly open mouth with tongue extended. The subject is instructed to report when he/she detects a sweet taste.
(4) Using a DeVilbiss Model 40 Inhalation Medication Nebulizer or equivalent, the test conductor shall spray the threshold check solution into the enclosure. The nozzle is directed away from the nose and mouth of the person. This nebulizer shall be clearly marked to distinguish it from the fit test solution nebulizer.
(5) The threshold check solution is prepared by dissolving 0.83 gram of sodium saccharin USP in 100 ml of warm water. It can be prepared by putting 1 ml of the fit test solution (see (b)(5) below) in 100 ml of distilled water.
(6) To produce the aerosol, the nebulizer bulb is firmly squeezed so that it collapses completely, then released and allowed to fully expand.
(7) Ten squeezes are repeated rapidly and then the test subject is asked whether the saccharin can be tasted. If the test subject reports tasting the sweet taste during the ten squeezes, the screening test is completed. The taste threshold is noted as ten regardless of the number of squeezes actually completed.
(8) If the first response is negative, ten more squeezes are repeated rapidly and the test subject is again asked whether the saccharin is tasted. If the test subject reports tasting the sweet taste during the second ten squeezes, the screening test is completed. The taste threshold is noted as twenty regardless of the number of squeezes actually completed.
(9) If the second response is negative, ten more squeezes are repeated rapidly and the test subject is again asked whether the saccharin is tasted. If the test subject reports tasting the sweet taste during the third set of ten squeezes, the screening test is completed. The taste threshold is noted as thirty regardless of the number of squeezes actually completed.
(10) The test conductor will take note of the number of squeezes required to solicit a taste response.
(11) If the saccharin is not tasted after 30 squeezes (step 10), the test subject is unable to taste saccharin and may not perform the saccharin fit test.
Note to paragraph 3. (a): If the test subject eats or drinks something sweet before the screening test, he/she may be unable to taste the weak saccharin solution.
(12) If a taste response is elicited, the test subject shall be asked to take note of the taste for reference in the fit test.
(13) Correct use of the nebulizer means that approximately 1 ml of liquid is used at a time in the nebulizer body.
(14) The nebulizer shall be thoroughly rinsed in water, shaken dry, and refilled at least each morning and afternoon or at least every four hours.
(b) Saccharin solution aerosol fit test procedure.
(1) The test subject may not eat, drink (except plain water), smoke, or chew gum for 15 minutes before the test.
(2) The fit test uses the same enclosure described in 3. (a) above.
(3) The test subject shall don the enclosure while wearing the respirator selected in section I. A. of this appendix. The respirator shall be properly adjusted and equipped with a particulate filter(s).
(4) A second DeVilbiss Model 40 Inhalation Medication Nebulizer or equivalent is used to spray the fit test solution into the enclosure. This nebulizer shall be clearly marked to distinguish it from the screening test solution nebulizer.
(5) The fit test solution is prepared by adding 83 grams of sodium saccharin to 100 ml of warm water.
(6) As before, the test subject shall breathe through the slightly open mouth with tongue extended, and report if he/she tastes the sweet taste of saccharin.
(7) The nebulizer is inserted into the hole in the front of the enclosure and an initial concentration of saccharin fit test solution is sprayed into the enclosure using the same number of squeezes (either 10, 20 or 30 squeezes) based on the number of squeezes required to elicit a taste response as noted during the screening test. A minimum of 10 squeezes is required.
(8) After generating the aerosol, the test subject shall be instructed to perform the exercises in section I. A. 14. of this appendix.
(9) Every 30 seconds the aerosol concentration shall be replenished using one half the original number of squeezes used initially (e.g., 5, 10 or 15).
(10) The test subject shall indicate to the test conductor if at any time during the fit test the taste of saccharin is detected. If the test subject does not report tasting the saccharin, the test is passed.
(11) If the taste of saccharin is detected, the fit is deemed unsatisfactory and the test is failed. A different respirator shall be tried and the entire test procedure is repeated (taste threshold screening and fit testing).
(12) Since the nebulizer has a tendency to clog during use, the test operator must make periodic checks of the nebulizer to ensure that it is not clogged. If clogging is found at the end of the test session, the test is invalid.
4. Bitrex™ (Denatonium Benzoate) Solution Aerosol Qualitative Fit Test Protocol
The Bitrex™ (Denatonium benzoate) solution aerosol QLFT protocol uses the published saccharin test protocol because that protocol is widely accepted. Bitrex is routinely used as a taste aversion agent in household liquids which children should not be drinking and is endorsed by the American Medical Association, the National Safety Council, and the American Association of Poison Control Centers. The entire screening and testing procedure shall be explained to the test subject prior to the conduct of the screening test.
(a) Taste Threshold Screening.
The Bitrex taste threshold screening, performed without wearing a respirator, is intended to determine whether the individual being tested can detect the taste of Bitrex.
(1) During threshold screening as well as during fit testing, subjects shall wear an enclosure about the head and shoulders that is approximately 12 inches (30.5 cm) in diameter by 14 inches (35.6 cm) tall. The front portion of the enclosure shall be clear from the respirator and allow free movement of the head when a respirator is worn. An enclosure substantially similar to the 3M hood assembly, parts # FT 14 and # FT 15 combined, is adequate.
(2) The test enclosure shall have a 3/4 inch (1.9 cm) hole in front of the test subject's nose and mouth area to accommodate the nebulizer nozzle.
(3) The test subject shall don the test enclosure. Throughout the threshold screening test, the test subject shall breathe through his or her slightly open mouth with tongue extended. The subject is instructed to report when he/she detects a bitter taste.
(4) Using a DeVilbiss Model 40 Inhalation Medication Nebulizer or equivalent, the test conductor shall spray the Threshold Check Solution into the enclosure. This Nebulizer shall be clearly marked to distinguish it from the fit test solution nebulizer.
(5) The Threshold Check Solution is prepared by adding 13.5 milligrams of Bitrex to 100 ml of 5% salt (NaCl) solution in distilled water.
(6) To produce the aerosol, the nebulizer bulb is firmly squeezed so that the bulb collapses completely, and is then released and allowed to fully expand.
(7) An initial ten squeezes are repeated rapidly and then the test subject is asked whether the Bitrex can be tasted. If the test subject reports tasting the bitter taste during the ten squeezes, the screening test is completed. The taste threshold is noted as ten regardless of the number of squeezes actually completed.
(8) If the first response is negative, ten more squeezes are repeated rapidly and the test subject is again asked whether the Bitrex is tasted. If the test subject reports tasting the bitter taste during the second ten squeezes, the screening test is completed. The taste threshold is noted as twenty regardless of the number of squeezes actually completed.
(9) If the second response is negative, ten more squeezes are repeated rapidly and the test subject is again asked whether the Bitrex is tasted. If the test subject reports tasting the bitter taste during the third set of ten squeezes, the screening test is completed. The taste threshold is noted as thirty regardless of the number of squeezes actually completed.
(10) The test conductor will take note of the number of squeezes required to solicit a taste response.
(11) If the Bitrex is not tasted after 30 squeezes (step 10), the test subject is unable to taste Bitrex and may not perform the Bitrex fit test.
(12) If a taste response is elicited, the test subject shall be asked to take note of the taste for reference in the fit test.
(13) Correct use of the nebulizer means that approximately 1 ml of liquid is used at a time in the nebulizer body.
(14) The nebulizer shall be thoroughly rinsed in water, shaken to dry, and refilled at least each morning and afternoon or at least every four hours.
(b) Bitrex Solution Aerosol Fit Test Procedure.
(1) The test subject may not eat, drink (except plain water), smoke, or chew gum for 15 minutes before the test.
(2) The fit test uses the same enclosure as that described in 4. (a) above.
(3) The test subject shall don the enclosure while wearing the respirator selected according to section I. A. of this appendix. The respirator shall be properly adjusted and equipped with any type particulate filter(s).
(4) A second DeVilbiss Model 40 Inhalation Medication Nebulizer or equivalent is used to spray the fit test solution into the enclosure. This nebulizer shall be clearly marked to distinguish it from the screening test solution nebulizer.
(5) The fit test solution is prepared by adding 337.5 mg of Bitrex to 200 ml of a 5% salt (NaCl) solution in warm water.
(6) As before, the test subject shall breathe through his or her slightly open mouth with tongue extended, and be instructed to report if he/she tastes the bitter taste of Bitrex.
(7) The nebulizer is inserted into the hole in the front of the enclosure and an initial concentration of the fit test solution is sprayed into the enclosure using the same number of squeezes (either 10, 20 or 30 squeezes) based on the number of squeezes required to elicit a taste response as noted during the screening test.
(8) After generating the aerosol, the test subject shall be instructed to perform the exercises in section I. A. 14. of this appendix.
(9) Every 30 seconds the aerosol concentration shall be replenished using one half the number of squeezes used initially (e.g., 5, 10 or 15).
(10) The test subject shall indicate to the test conductor if at any time during the fit test the taste of Bitrex is detected. If the test subject does not report tasting the Bitrex, the test is passed.
(11) If the taste of Bitrex is detected, the fit is deemed unsatisfactory and the test is failed. A different respirator shall be tried and the entire test procedure is repeated (taste threshold screening and fit testing).
5. Irritant Smoke (Stannic Chloride) Protocol
This qualitative fit test uses a person's response to the irritating chemicals released in the "smoke" produced by a stannic chloride ventilation smoke tube to detect leakage into the respirator.
(a) General Requirements and Precautions
(1) The respirator to be tested shall be equipped with high efficiency particulate air (HEPA) or P100 series filter(s).
(2) Only stannic chloride smoke tubes shall be used for this protocol.
(3) No form of test enclosure or hood for the test subject shall be used.
(4) The smoke can be irritating to the eyes, lungs, and nasal passages. The test conductor shall take precautions to minimize the test subject's exposure to irritant smoke. Sensitivity varies, and certain individuals may respond to a greater degree to irritant smoke. Care shall be taken when performing the sensitivity screening checks that determine whether the test subject can detect irritant smoke to use only the minimum amount of smoke necessary to elicit a response from the test subject.
(5) The fit test shall be performed in an area with adequate ventilation to prevent exposure of the person conducting the fit test or the buildup of irritant smoke in the general atmosphere.
(b) Sensitivity Screening Check
The person to be tested must demonstrate his or her ability to detect a weak concentration of the irritant smoke.
(1) The test operator shall break both ends of a ventilation smoke tube containing stannic chloride, and attach one end of the smoke tube to a low flow air pump set to deliver 200 milliliters per minute, or an aspirator squeeze bulb. The test operator shall cover the other end of the smoke tube with a short piece of tubing to prevent potential injury from the jagged end of the smoke tube.
(2) The test operator shall advise the test subject that the smoke can be irritating to the eyes, lungs, and nasal passages and instruct the subject to keep his/her eyes closed while the test is performed.
(3) The test subject shall be allowed to smell a weak concentration of the irritant smoke before the respirator is donned to become familiar with its irritating properties and to determine if he/she can detect the irritating properties of the smoke. The test operator shall carefully direct a small amount of the irritant smoke in the test subject's direction to determine that he/she can detect it.
(c) Irritant Smoke Fit Test Procedure
(1) The person being fit tested shall don the respirator without assistance, and perform the required user seal check(s).
(2) The test subject shall be instructed to keep his/her eyes closed.
(3) The test operator shall direct the stream of irritant smoke from the smoke tube toward the faceseal area of the test subject, using the low flow pump or the squeeze bulb. The test operator shall begin at least 12 inches from the facepiece and move the smoke stream around the whole perimeter of the mask. The operator shall gradually make two more passes around the perimeter of the mask, moving to within six inches of the respirator.
(4) If the person being tested has not had an involuntary response and/or detected the irritant smoke, proceed with the test exercises.
(5) The exercises identified in section I.A. 14. of this appendix shall be performed by the test subject while the respirator seal is being continually challenged by the smoke, directed around the perimeter of the respirator at a distance of six inches.
(6) If the person being fit tested reports detecting the irritant smoke at any time, the test is failed. The person being retested must repeat the entire sensitivity check and fit test procedure.
(7) Each test subject passing the irritant smoke test without evidence of a response (involuntary cough, irritation) shall be given a second sensitivity screening check, with the smoke from the same smoke tube used during the fit test, once the respirator has been removed, to determine whether he/she still reacts to the smoke. Failure to evoke a response shall void the fit test.
(8) If a response is produced during this second sensitivity check, then the fit test is passed.
C. Quantitative Fit Test (QNFT) Protocols
The following quantitative fit testing procedures have been demonstrated to be acceptable: Quantitative fit testing using a non-hazardous test aerosol (such as corn oil, polyethylene glycol 400 [PEG 400], di-2-ethyl hexyl sebacate [DEHS], or sodium chloride) generated in a test chamber, and employing instrumentation to quantify the fit of the respirator; Quantitative fit testing using ambient aerosol as the test agent and appropriate instrumentation (condensation nuclei counter) to quantify the respirator fit; Quantitative fit testing using controlled negative pressure and appropriate instrumentation to measure the volumetric leak rate of a facepiece to quantify the respirator fit.
1. General
(a) The employer shall ensure that persons administering QNFT are able to calibrate equipment and perform tests properly, recognize invalid tests, calculate fit factors properly and ensure that test equipment is in proper working order.
(b) The employer shall ensure that QNFT equipment is kept clean, and is maintained and calibrated according to the manufacturer's instructions so as to operate at the parameters for which it was designed.
2. Generated Aerosol Quantitative Fit Testing Protocol
(a) Apparatus.
(1) Instrumentation. Aerosol generation, dilution, and measurement systems using particulates (corn oil, polyethylene glycol 400 [PEG 400], di-2-ethyl hexyl sebacate [DEHS] or sodium chloride) as test aerosols shall be used for quantitative fit testing.
(2) Test chamber. The test chamber shall be large enough to permit all test subjects to perform freely all required exercises without disturbing the test agent concentration or the measurement apparatus. The test chamber shall be equipped and constructed so that the test agent is effectively isolated from the ambient air, yet uniform in concentration throughout the chamber.
(3) When testing air-purifying respirators, the normal filter or cartridge element shall be replaced with a high efficiency particulate air (HEPA) or P100 series filter supplied by the same manufacturer.
(4) The sampling instrument shall be selected so that a computer record or strip chart record may be made of the test showing the rise and fall of the test agent concentration with each inspiration and expiration at fit factors of at least 2,000. Integrators or computers that integrate the amount of test agent penetration leakage into the respirator for each exercise may be used provided a record of the readings is made.
(5) The combination of substitute air-purifying elements, test agent and test agent concentration shall be such that the test subject is not exposed in excess of an established exposure limit for the test agent at any time during the testing process, based upon the length of the exposure and the exposure limit duration.
(6) The sampling port on the test specimen respirator shall be placed and constructed so that no leakage occurs around the port (e.g., where the respirator is probed), a free air flow is allowed into the sampling line at all times, and there is no interference with the fit or performance of the respirator. The in-mask sampling device (probe) shall be designed and used so that the air sample is drawn from the breathing zone of the test subject, midway between the nose and mouth and with the probe extending into the facepiece cavity at least 1/4 inch.
(7) The test setup shall permit the person administering the test to observe the test subject inside the chamber during the test.
(8) The equipment generating the test atmosphere shall maintain the concentration of test agent constant to within a 10 percent variation for the duration of the test.
(9) The time lag (interval between an event and the recording of the event on the strip chart or computer or integrator) shall be kept to a minimum. There shall be a clear association between the occurrence of an event and its being recorded.
(10) The sampling line tubing for the test chamber atmosphere and for the respirator sampling port shall be of equal diameter and of the same material. The length of the two lines shall be equal.
(11) The exhaust flow from the test chamber shall pass through an appropriate filter (i.e., high efficiency particulate filter) before release.
(12) When sodium chloride aerosol is used, the relative humidity inside the test chamber shall not exceed 50 percent.
(13) The limitations of instrument detection shall be taken into account when determining the fit factor.
(14) Test respirators shall be maintained in proper working order and be inspected regularly for deficiencies such as cracks or missing valves and gaskets.
(b) Procedural Requirements.
(1) When performing the initial user seal check using a positive or negative pressure check, the sampling line shall be crimped closed in order to avoid air pressure leakage during either of these pressure checks.
(2) The use of an abbreviated screening QLFT test is optional. Such a test may be utilized in order to quickly identify poor fitting respirators that passed the positive and/or negative pressure test and reduce the amount of QNFT time. The use of the CNC QNFT instrument in the count mode is another optional method to obtain a quick estimate of fit and eliminate poor fitting respirators before going on to perform a full QNFT.
(3) A reasonably stable test agent concentration shall be measured in the test chamber prior to testing. For canopy or shower curtain types of test units, the determination of the test agent's stability may be established after the test subject has entered the test environment.
(4) Immediately after the subject enters the test chamber, the test agent concentration inside the respirator shall be measured to ensure that the peak penetration does not exceed 5 percent for a half mask or 1 percent for a full facepiece respirator.
(5) A stable test agent concentration shall be obtained prior to the actual start of testing.
(6) Respirator restraining straps shall not be over-tightened for testing. The straps shall be adjusted by the wearer without assistance from other persons to give a reasonably comfortable fit typical of normal use. The respirator shall not be adjusted once the fit test exercises begin.
(7) The test shall be terminated whenever any single peak penetration exceeds 5 percent for half masks and 1 percent for full facepiece respirators. The test subject shall be refitted and retested.
(8) Calculation of fit factors.
(i) The fit factor shall be determined for the quantitative fit test by taking the ratio of the average chamber concentration to the concentration measured inside the respirator for each test exercise except the grimace exercise.
(ii) The average test chamber concentration shall be calculated as the arithmetic average of the concentration measured before and after each test (i.e., 7 exercises) or the arithmetic average of the concentration measured before and after each exercise or the true average measured continuously during the respirator sample.
(iii) The concentration of the challenge agent inside the respirator shall be determined by one of the following methods:
(A) Average peak penetration method means the method of determining test agent penetration into the respirator utilizing a strip chart recorder, integrator, or computer. The agent penetration is determined by an average of the peak heights on the graph or by computer integration, for each exercise except the grimace exercise. Integrators or computers that calculate the actual test agent penetration into the respirator for each exercise will also be considered to meet the requirements of the average peak penetration method.
(B) Maximum peak penetration method means the method of determining test agent penetration in the respirator as determined by strip chart recordings of the test. The highest peak penetration for a given exercise is taken to be representative of average penetration into the respirator for that exercise.
(C) Integration by calculation of the area under the individual peak for each exercise except the grimace exercise. This includes computerized integration.
(D) The calculation of the overall fit factor using individual exercise fit factors involves first converting the exercise fit factors to penetration values, determining the average, and then converting that result back to a fit factor. This procedure is described in the following equation:
Overall Fit Factor =
Number of exercises
______________________________________________
1/ff1 + 1/ff2 + 1/ff3 + 1/ff4
+ 1/ff5 + 1/ff6 + 1/ff7 + 1/ff8
Where ff1, ff2, ff3, etc. are the fit factors for exercises 1, 2, 3, etc.
(9) The test subject shall not be permitted to wear a half mask or quarter facepiece respirator unless a minimum fit factor of 100 is obtained, or a full facepiece respirator unless a minimum fit factor of 500 is obtained.
(10) Filters used for quantitative fit testing shall be replaced whenever increased breathing resistance is encountered, or when the test agent has altered the integrity of the filter media.
3. Ambient aerosol condensation nuclei counter (CNC) quantitative fit testing protocol.
The ambient aerosol condensation nuclei counter (CNC) quantitative fit testing (PortacountTM) protocol quantitatively fit tests respirators with the use of a probe. The probed respirator is only used for quantitative fit tests. A probed respirator has a special sampling device, installed on the respirator, that allows the probe to sample the air from inside the mask. A probed respirator is required for each make, style, model, and size that the employer uses and can be obtained from the respirator manufacturer or distributor. The CNC instrument manufacturer, TSI Inc., also provides probe attachments (TSI sampling adapters) that permit fit testing in an employee's own respirator. A minimum fit factor pass level of at least 100 is necessary for a half-mask respirator and a minimum fit factor pass level of at least 500 is required for a full facepiece negative pressure respirator. The entire screening and testing procedure shall be explained to the test subject prior to the conduct of the screening test.
(a) Portacount Fit Test Requirements.
(1) Check the respirator to make sure the sampling probe and line are properly attached to the facepiece and that the respirator is fitted with a particulate filter capable of preventing significant penetration by the ambient particles used for the fit test (e.g., NIOSH 42 CFR 84 series 100, series 99, or series 95 particulate filter) per manufacturer's instruction.
(2) Instruct the person to be tested to don the respirator for five minutes before the fit test starts. This purges the ambient particles trapped inside the respirator and permits the wearer to make certain the respirator is comfortable. This individual shall already have been trained on how to wear the respirator properly.
(3) Check the following conditions for the adequacy of the respirator fit: Chin properly placed; Adequate strap tension, not overly tightened; Fit across nose bridge; Respirator of proper size to span distance from nose to chin; Tendency of the respirator to slip; Self-observation in a mirror to evaluate fit and respirator position.
(4) Have the person wearing the respirator do a user seal check. If leakage is detected, determine the cause. If leakage is from a poorly fitting facepiece, try another size of the same model respirator, or another model of respirator.
(5) Follow the manufacturer's instructions for operating the Portacount and proceed with the test.
(6) The test subject shall be instructed to perform the exercises in section I. A. 14. of this appendix.
(7) After the test exercises, the test subject shall be questioned by the test conductor regarding the comfort of the respirator upon completion of the protocol. If it has become unacceptable, another model of respirator shall be tried.
(b) Portacount Test Instrument.
(1) The Portacount will automatically stop and calculate the overall fit factor for the entire set of exercises. The overall fit factor is what counts. The Pass or Fail message will indicate whether or not the test was successful. If the test was a Pass, the fit test is over.
(2) Since the pass or fail criterion of the Portacount is user programmable, the test operator shall ensure that the pass or fail criterion meet the requirements for minimum respirator performance in this Appendix.
(3) A record of the test needs to be kept on file, assuming the fit test was successful. The record must contain the test subject's name; overall fit factor; make, model, style, and size of respirator used; and date tested.
4. Controlled negative pressure (CNP) quantitative fit testing protocol.
The CNP protocol provides an alternative to aerosol fit test methods. The CNP fit test method technology is based on exhausting air from a temporarily sealed respirator facepiece to generate and then maintain a constant negative pressure inside the facepiece. The rate of air exhaust is controlled so that a constant negative pressure is maintained in the respirator during the fit test. The level of pressure is selected to replicate the mean inspiratory pressure that causes leakage into the respirator under normal use conditions. With pressure held constant, air flow out of the respirator is equal to air flow into the respirator. Therefore, measurement of the exhaust stream that is required to hold the pressure in the temporarily sealed respirator constant yields a direct measure of leakage air flow into the respirator. The CNP fit test method measures leak rates through the facepiece as a method for determining the facepiece fit for negative pressure respirators. The CNP instrument manufacturer Dynatech Nevada also provides attachments (sampling manifolds) that replace the filter cartridges to permit fit testing in an employee's own respirator. To perform the test, the test subject closes his or her mouth and holds his/her breath, after which an air pump removes air from the respirator facepiece at a pre-selected constant pressure. The facepiece fit is expressed as the leak rate through the facepiece, expressed as milliliters per minute. The quality and validity of the CNP fit tests are determined by the degree to which the in-mask pressure tracks the test pressure during the system measurement time of approximately five seconds. Instantaneous feedback in the form of a real-time pressure trace of the in-mask pressure is provided and used to determine test validity and quality. A minimum fit factor pass level of 100 is necessary for a half-mask respirator and a minimum fit factor of at least 500 is required for a full facepiece respirator. The entire screening and testing procedure shall be explained to the test subject prior to the conduct of the screening test.
(a) CNP Fit Test Requirements.
(1) The instrument shall have a non-adjustable test pressure of 15.0 mm water pressure.
(2) The CNP system defaults selected for test pressure shall be set at - 15 mm of water (-0.58 inches of water) and the modeled inspiratory flow rate shall be 53.8 liters per minute for performing fit tests.
(Note: CNP systems have built-in capability to conduct fit testing that is specific to unique work rate, mask, and gender situations that might apply in a specific workplace. Use of system default values, which were selected to represent respirator wear with medium cartridge resistance at a low-moderate work rate, will allow inter- test comparison of the respirator fit.)
(3) The individual who conducts the CNP fit testing shall be thoroughly trained to perform the test.
(4) The respirator filter or cartridge needs to be replaced with the CNP test manifold. The inhalation valve downstream from the manifold either needs to be temporarily removed or propped open.
(5) The test subject shall be trained to hold his or her breath for at least 20 seconds.
(6) The test subject shall don the test respirator without any assistance from the individual who conducts the CNP fit test.
(7) The QNFT protocol shall be followed according to section I. C. 1. of this appendix with an exception for the CNP test exercises.
(b) CNP Test Exercises.
(1) Normal breathing. In a normal standing position, without talking, the subject shall breathe normally for 1 minute. After the normal breathing exercise, the subject needs to hold head straight ahead and hold his or her breath for 10 seconds during the test measurement.
(2) Deep breathing. In a normal standing position, the subject shall breathe slowly and deeply for 1 minute, being careful not to hyperventilate. After the deep breathing exercise, the subject shall hold his or her head straight ahead and hold his or her breath for 10 seconds during test measurement.
(3) Turning head side to side. Standing in place, the subject shall slowly turn his or her head from side to side between the extreme positions on each side for 1 minute. The head shall be held at each extreme momentarily so the subject can inhale at each side. After the turning head side to side exercise, the subject needs to hold head full left and hold his or her breath for 10 seconds during test measurement. Next, the subject needs to hold head full right and hold his or her breath for 10 seconds during test measurement.
(4) Moving head up and down. Standing in place, the subject shall slowly move his or her head up and down for 1 minute. The subject shall be instructed to inhale in the up position (i.e., when looking toward the ceiling). After the moving head up and down exercise, the subject shall hold his or her head full up and hold his or her breath for 10 seconds during test measurement. Next, the subject shall hold his or her head full down and hold his or her breath for 10 seconds during test measurement.
(5) Talking. The subject shall talk out loud slowly and loud enough so as to be heard clearly by the test conductor. The subject can read from a prepared text such as the Rainbow Passage, count backward from 100, or recite a memorized poem or song for 1 minute. After the talking exercise, the subject shall hold his or her head straight ahead and hold his or her breath for 10 seconds during the test measurement.
(6) Grimace. The test subject shall grimace by smiling or frowning for 15 seconds.
(7) Bending Over. The test subject shall bend at the waist as if he or she were to touch his or her toes for 1 minute. Jogging in place shall be substituted for this exercise in those test environments such as shroud-type QNFT units that prohibit bending at the waist. After the bending over exercise, the subject shall hold his or her head straight ahead and hold his or her breath for 10 seconds during the test measurement.
(8) Normal Breathing. The test subject shall remove and re-don the respirator within a one-minute period. Then, in a normal standing position, without talking, the subject shall breathe normally for 1 minute. After the normal breathing exercise, the subject shall hold his or her head straight ahead and hold his or her breath for 10 seconds during the test measurement. After the test exercises, the test subject shall be questioned by the test conductor regarding the comfort of the respirator upon completion of the protocol. If it has become unacceptable, another model of a respirator shall be tried.
(c) CNP Test Instrument.
(1) The test instrument shall have an effective audio warning device when the test subject fails to hold his or her breath during the test. The test shall be terminated whenever the test subject failed to hold his or her breath. The test subject may be refitted and retested.
(2) A record of the test shall be kept on file, assuming the fit test was successful. The record must contain the test subject's name; overall fit factor; make, model, style and size of respirator used; and date tested.
Part II. New Fit Test Protocols
A. Any person may submit to OSHA an application for approval of a new fit test protocol. If the application meets the following criteria, OSHA will initiate a rulemaking proceeding under section 6(b)(7) of the OSH Act to determine whether to list the new protocol as an approved protocol in this Appendix A.
B. The application must include a detailed description of the proposed new fit test protocol. This application must be supported by either:
1. A test report prepared by an independent government research laboratory (e.g., Law-rence Livermore National Laboratory, Los Alamos National Laboratory, the National Institute for Standards and Technology) stating that the laboratory has tested the protocol and had found it to be accurate and reliable; or
2. An article that has been published in a peer-reviewed industrial hygiene journal describing the protocol and explaining how test data support the protocol's accuracy and reliability.
C. If OSHA determines that additional information is required before the Agency commences a rulemaking proceeding under this section, OSHA will so notify the applicant and afford the applicant the opportunity to submit the supplemental information. Initiation of a rulemaking proceeding will be deferred until OSHA has received and evaluated the supplemental information.
Appendix B-1 to 1910.134 user seal check procedures
I. Facepiece Positive and/or Negative Pressure Checks
A. Positive pressure check. Close off the exhalation valve and exhale gently into the facepiece. The face fit is considered satisfactory if a slight positive pressure can be built up inside the facepiece without any evidence of outward leakage of air at the seal. For most respirators this method of leak testing requires the wearer to first remove the exhalation valve cover before closing off the exhalation valve and then carefully replacing it after the test.
B. Negative pressure check. Close off the inlet opening of the canister or cartridge(s) by covering with the palm of the hand(s) or by replacing the filter seal(s), inhale gently so that the facepiece collapses slightly, and hold the breath for ten seconds. The design of the inlet opening of some cartridges cannot be effectively covered with the palm of the hand. The test can be performed by covering the inlet opening of the cartridge with a thin latex or nitrile glove. If the facepiece remains in its slightly collapsed condition and no inward leakage of air is detected, the tightness of the respirator is considered satisfactory.
II. Manufacturer's Recommended User Seal Check Procedures
The respirator manufacturer's recommended procedures for performing a user seal check may be used instead of the positive and/or negative pressure check procedures provided that the employer demonstrates that the manufacturer's procedures are equally effective.
Go back to the Table of Contents