 |
|
Understanding Xenobiotic Transport David S. Miller, Ph.D.
Principal Investigator and Acting Chief Tel (919) 541-3235 Fax (919) 541-5737 miller28@niehs.nih.gov P.O. Box 12233 Mail Drop F2-03 Research Triangle Park, North Carolina 27709 Delivery Instructions Research SummaryOur long-term research focus has been on defining basic cellular mechanisms that drive xenobiotic transport in specialized barrier and excretory tissues, e.g., blood brain barrier, kidney and liver, and the signals that modulate transport activity. For a large number of therapeutic drugs and environmental pollutants, drug and pollutant metabolites and waste products of cellular metabolism, these tissues and the transporters they express govern uptake, distribution and excretion. They are important determinants of drug efficacy on the one hand and drug and pollutant toxicity on the other hand. To design better therapeutic protocols and to be able predict toxic interactions, we must have a thorough understanding of these transport mechanisms and the signals that modulate them. Work over the past several years has been directed at the blood-brain and blood-cerebrospinal fluid (CSF) barriers. For these projects, we have developed confocal- and multiphoton-imaging based techniques to follow the transport of fluorescent drugs across barrier tissues in vitro and in vivo. 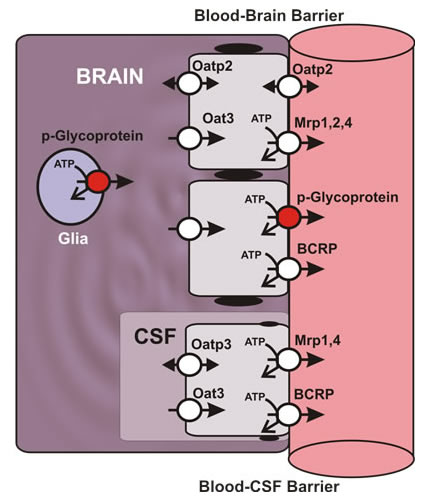
The blood-brain and blood-cerebrospinal fluid (CSF) barriers.
Recent efforts have been directed at understanding the regulation of ATP-driven, xenobiotic efflux pumps (ABC transporters) at the blood-brain barrier. This barrier resides within the brain capillary endothelium and is a limiting factor in treatment of CNS disorders, e.g., neurodegenerative diseases, epilepsy, brain cancer, and neuro-AIDS. P-glycoprotein, a drug efflux transporter, is a critical element of that barrier. High level of expression, luminal membrane location, broad specificity and high transport potency make P-glycoprotein a primary obstacle to drug delivery to the brain and thus to CNS pharmacotherapy. Current projects involve mapping the signals that modulate activity and expression of P-glycoprotein and other ABC transporters (MRPs and BCRP) in brain capillaries with a view towards
Major area of research:
Current projects:
David S. Miller, Ph.D., heads the Intracellular Regulation Group within the Laboratory of Pharmacology. He received his Ph.D. in biochemistry from the University of Maine in 1973. He has published over 150 peer-reviewed articles in leading biomedical journals, as well as several book chapters. He was a Group Leader at the Michigan Cancer Foundation before joining NIEHS in 1985. Over the past ten years his research focus has shifted from renal excretory transport to brain barrier tissues. |
|

