
- Afghanistan
- Africa
- Budget Management
- Defense
- Economy
- Education
- Energy
- Environment
- Global Diplomacy
- Health Care
- Homeland Security
- Immigration
- International Trade
- Iraq
- Judicial Nominations
- Middle East
- National Security
- Veterans
- President's Cabinet
- USA Freedom Corps
- Faith-Based & Community Initiatives
- Office of Management and Budget
- National Security Council
- USA.gov
National Strategy for Pandemic Influenza Implementation Plan One Year Summary

Table of Contents
- Executive Summary
- Introduction
- Limiting the International Spread of a Pandemic
- Limiting the Domestic Spread of a Pandemic and Mitigating Disease, Suffering, and Death
- Sustaining Infrastructure and Mitigating Impact to the Economy and the Functioning of Society During a Pandemic
- Looking Ahead: What Have We Learned Through These Efforts and What Gaps Still Need to Be Addressed?
- Acknowledgements
Executive Summary
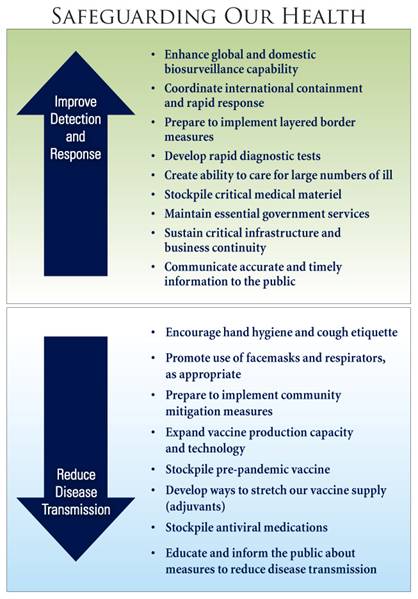
Since the release of the National Strategy for Pandemic Influenza Implementation Plan one year ago, much has been accomplished to realize the U.S. Government's pandemic preparedness and response goals of: (1) stopping, slowing, or otherwise limiting the spread of a pandemic to the United States; (2) limiting the domestic spread of a pandemic and mitigating disease, suffering, and death; and (3) sustaining infrastructure and mitigating impact to the economy and the functioning of society.
Although the visibility of avian influenza and pandemic preparedness has waned in the media, the threat of avian influenza and the potential for an influenza pandemic has not. A pandemic occurs when a novel strain of influenza virus emerges that has the ability to infect humans and to cause severe disease, and where efficient and sustained transmission between humans occurs. Though we cannot be certain that highly pathogenic avian influenza A H5N1 (H5N1) will spark a pandemic, we can be sure that a pandemic will occur at some point in the future. It is everyone's responsibility to remain vigilant. We cannot become complacent and must continue to take the threat of a pandemic very seriously.
Over the past year, through the International Partnership on Avian and Pandemic Influenza, the United States and the international community have mobilized to confront the threat of an influenza pandemic at its source, by containing H5N1 poultry outbreaks and rapidly identifying associated cases of human disease. The United States is supporting efforts to improve laboratory diagnosis and early warning networks in more than 75 countries and is working with its partners to expand on-the-ground surveillance capacity, enhance national and regional laboratories, and improve knowledge about the movement and changes in H5N1 on a global scale to ensure that countries are able to quickly confirm outbreaks in animals or people. In cooperation with the World Health Organization (WHO) and the United Nations Food and Agriculture Organization (FAO), U.S. experts have investigated outbreaks of H5N1 in countries on three continents and provided technical assistance, commodities, and logistical or financial support to 39 of the 60 countries and jurisdictions affected by H5N1. As a result of the efforts of the United States and the support of our international partners, the international community becomes aware of outbreaks sooner and is able to launch more effective and timely responses.
Although a pandemic virus has not yet emerged, the appearance of limited human clusters of H5N1 cases has tested our international surveillance and response capabilities. Should a pandemic emerge, whether from the current H5N1 subtype of concern or from another influenza virus with pandemic potential, the United States is better positioned today to detect an outbreak earlier, to support an international effort to contain the pandemic in its earliest stages, to limit the spread of the pandemic, and to save lives.
 The United States has developed protocols and trained personnel to support an international effort to contain the pandemic in its earliest stages. The U.S. Government procured and pre-positioned overseas stockpiles of personal protective equipment, decontamination kits, and antiviral medications to complement global efforts to contain pandemic outbreaks. Today, our Federal and State stockpiles contain enough antiviral medications to treat nearly 50 million people, with up to 6 million courses now reserved for containment efforts. If a pandemic begins outside the United States, and our international containment efforts fail, the U.S. Government is planning to implement border measures during a severe pandemic to slow the entry of a pandemic virus into the United States while allowing the flow of goods and people.
The United States has developed protocols and trained personnel to support an international effort to contain the pandemic in its earliest stages. The U.S. Government procured and pre-positioned overseas stockpiles of personal protective equipment, decontamination kits, and antiviral medications to complement global efforts to contain pandemic outbreaks. Today, our Federal and State stockpiles contain enough antiviral medications to treat nearly 50 million people, with up to 6 million courses now reserved for containment efforts. If a pandemic begins outside the United States, and our international containment efforts fail, the U.S. Government is planning to implement border measures during a severe pandemic to slow the entry of a pandemic virus into the United States while allowing the flow of goods and people.
Once an influenza pandemic reaches the United States, the primary focus is safeguarding the health of Americans. The U.S. Government is working to enhance the Nation's ability to detect and respond early and effectively to a pandemic. To better identify the first cases of pandemic influenza in a community, the U.S. Government has provided resources to State and local health departments to increase the number of sentinel providers and improve laboratory detection at public health laboratories. The U.S. Laboratory Response Network (LRN), which includes State public health laboratories, is prepared to conduct initial testing of suspected human infection with H5N1 within 24 hours of receipt. To ensure that suspected cases can be promptly confirmed and treated, the Federal Government is working with industry partners to develop rapid diagnostic tests to quickly discriminate pandemic influenza from seasonal influenza or other illnesses.
The Federal Government is investing in the expansion of vaccine manufacturing capacity, the advanced development of new cell-based vaccines, antigen-sparing technologies to stretch our vaccine supply, and the establishment and maintenance of pre-pandemic vaccine stockpiles. In April 2007, the Federal Government approved the first pre-pandemic vaccine for humans against the H5N1 virus. We currently have enough of this pre-pandemic H5N1 vaccine for approximately 6 million people, with plans to stockpile enough pre-pandemic vaccine for 20 million people. In addition, antiviral medications are an important element of pandemic influenza preparedness. As of June 2007, the Strategic National Stockpile contains more than 35 million regimens of antiviral drugs with an additional 2 million regimens on order. So far, individual States have stockpiled more than 13 million regimens of antiviral drugs. The Government's antiviral strategy includes not only stockpiling existing antiviral drugs, but also developing new antiviral medications to further broaden our capabilities to treat and prevent influenza.
In February 2007, the U.S. Government released groundbreaking Federal guidance for non-pharmaceutical interventions for mitigating the impact of a pandemic. This community mitigation strategy is important because the best protection against pandemic influenza, a matched pandemic vaccine, is not likely to be available at the outset of a pandemic. Recent scientific modeling and historical reviews of the 1918 pandemic suggest that non-pharmaceutical interventions (such as school closures, social distancing, and cancellation of large public gatherings) could be very effective at slowing the spread of disease and mitigating the outbreak, but only if they are implemented early and maintained consistently across communities affected by a pandemic. These interventions, coupled with the use of antiviral medications, could dramatically reduce the number of people who become infected, potentially preventing illness and death in millions of Americans.
The U.S. Government has invested in health system preparedness of hospitals and medical facilities across the country, has produced tools to assist in planning for expansion in hospital capacity during a pandemic, and is stockpiling medical supplies for distribution to individual States in the event of a pandemic.
Each Federal department and agency is developing its own department- or agency-specific pandemic preparedness plan to ensure the continuation of Federal Government essential functions. Over the past year, the Federal Government has produced tools for businesses and other employers to assist them in pandemic planning and has conducted an extensive outreach effort to the private sector. Through these efforts, businesses operating at home and abroad have been provided practical action-oriented information to identify essential functions and critical planning elements, to protect the health of employees, to maintain continuity of business operations, and to sustain community function during a pandemic.
Preparing the Nation for the threat of an influenza pandemic has provided a platform to address issues and concerns common to all types of mass casualty disasters. Promoting a culture of individual, family, and community preparedness is the foundation for all emergency planning efforts.
Though we have made significant progress over the past year to prepare the Nation and the international community for the threat of an influenza pandemic, much important work lies ahead. One of the most tangible benefits of planning and preparing at a Federal level has been the forging of close working relationships and unity of effort among Federal departments and agencies. It is important that we enhance connectivity and collaboration between all levels of government and all segments of society. Now is the time, before a pandemic emerges, to establish and test these relationships and partnerships. The Federal Government must continue playing a leadership role in fostering an environment of collaboration and public engagement.
Although we have realized progress in enhancing disease surveillance, critical gaps remain with respect to "real-time" disease detection and clinical surveillance in the United States. The Federal Government must redouble its efforts for developing "real-time" surveillance to ensure that we are not "blind" during the next pandemic. Real-time surveillance is needed to provide broad situational awareness, including the ability to detect, integrate, analyze, and operationally respond to pandemic influenza. The Federal Government must accelerate the development of rapid diagnostic tests and screening tests to ensure that our efforts to detect disease, treat ill people, and limit disease transmission can be appropriately and effectively implemented.
Many hospitals and emergency departments nationwide are already operating at or near capacity and may not have the capability to treat the large numbers of patients who may need care during a severe pandemic. Healthcare workers and first responders will be on the front lines during an influenza pandemic. Ensuring the availability of protective measures for these critical workers will be essential to our efforts to protect the health and safety of the public. Community planning efforts will require the coordination of many providers and organizations and must address how healthcare facilities can best share medical response assets of personnel, materiel, and infrastructure in order to assure the greatest benefit for the largest number of people in the most ethical manner with the highest standard of medical care. The Federal Government has incorporated both funding and guidance to assist in planning for the strengthening of mass casualty care capacity. The stockpiling of critical medical materiel, including a reassessment of antiviral medication stockpile goals, is one area the Federal Government needs to address in the coming year. However, medical countermeasures, such as antiviral medications, have little utility if they cannot be delivered quickly to those in need. The Federal Government is working with State and local public health officials to strengthen plans to swiftly distribute needed medical countermeasures.
Although the community mitigation strategy may significantly reduce illness and death, implementing this strategy will not be easy. To enhance individual and community adherence to these community mitigation measures, the Federal Government must continue to work with non-Federal stakeholders to address practical implementation considerations, including legal and feasibility concerns, at the State, local, and tribal levels and minimize any adverse consequences associated with implementation.
Our Nation's investment in pandemic preparedness could translate to a reduction in the number of deaths each year related to seasonal influenza. Improving disease detection and surveillance, utilizing antiviral medications, and promoting healthy behaviors such as hand washing and cough etiquette could help reduce the spread of seasonal influenza and other respiratory diseases.
The unprecedented efforts to prepare for and respond to the threat of a pandemic underscore our resolve to protect human life and safeguard our Nation. No prior generation has ever anticipated and prepared for a pandemic. We have the opportunity to be the first generation to use our collective knowledge, determination, and resources to take on this task. The stakes are high, and our greatest enemy is complacency. We remain committed to this effort, not only for generations of Americans alive today, but also for the sake of generations to come.
Introduction
Although the visibility of avian influenza and pandemic preparedness has waned in the media, the threat of avian influenza and the potential for an influenza pandemic has not. A pandemic occurs when a novel strain of influenza virus emerges that has the ability to infect humans and to cause severe disease, and where efficient and sustained transmission between humans occurs. If humans have little or no immunity to a new virus, a worldwide epidemic, or pandemic, could ensue. Three influenza pandemics occurred during the 20th century. The most lethal of these was the 1918 pandemic, which killed more than 500,000 Americans and 20 million people worldwide. A 1918-like pandemic today would exact a terrible toll. In the United States alone, we could face 90 million persons ill and nearly two million deaths.
Scientists believe that avian (bird) influenza viruses played a role in all three of the pandemics of the 20th century. This is the reason for the concern over the recent spread of the highly pathogenic avian influenza A H5N1 (H5N1) virus. Since the beginning of 2006, the number of countries with documented outbreaks of H5N1 in birds has increased from 16 to 60. Although H5N1 avian influenza is rarely transmitted to humans, it produces severe illness when such infections occur. As of June 2007, there have been more than 300 confirmed human cases and almost 200 deaths. Every human case represents an opportunity for the H5N1 virus to adapt itself to the human host or to exchange genetic material with a human influenza virus in a way that may increase its transmissibility among humans. If this occurs, the H5N1 virus could lead to the first pandemic of the 21st century.
In November 2005, President Bush mobilized the Nation to prepare for an influenza pandemic with the announcement of the National Strategy for Pandemic Influenza (National Strategy). The National Strategy outlines the Federal Government's pandemic preparedness and response goals:(1) stopping, slowing, or otherwise limiting the spread of a pandemic to the United States; (2) limiting the domestic spread of a pandemic and mitigating disease, suffering, and death; and (3) sustaining infrastructure and mitigating impact to the economy and the functioning of society.
One year ago, in May 2006, the U.S. Government issued theNational Strategy for Pandemic Influenza Implementation Plan (National Plan). The National Plan provides a roadmap to achieve the pandemic preparedness and response goals laid out in the National Strategy. To accomplish these goals, the National Plan contains more than 300 specific actions for Federal departments and agencies. Every one of the Federal actions included in the National Plan includes a measure of performance and a timeline for implementation of that action. The U.S. Government committed to this level of transparency and accountability in order to demonstrate to the rest of the world, our international partners, State and local governments, businesses, and the general public, just how seriously we take this threat.
One year after the release of the National Plan, we have an opportunity to review the tremendous progress that has been made, redirect our efforts as necessary, and focus on the highest priority gaps in our capabilities that remain. Approximately two-thirds of the 324 actions in the National Plan were targeted for completion within one year of the National Plan's release. Of these "one year" actions, nearly 90 percent have been completed. While the completion of these actions attests to the level of effort dedicated to pandemic preparedness, it is important to examine how much progress we have made in addressing the three goals of our National Strategy.
It is everyone's responsibility to remain vigilant. Though we cannot be certain that highly pathogenic avian influenza A H5N1 will spark a pandemic, we can be sure that at some point in the future a pandemic will occur. We cannot become complacent and must continue to take the threat of a pandemic very seriously. The ongoing efforts to plan and prepare for a pandemic will serve us well in the future, irrespective of the manner in which the current H5N1 outbreak unfolds.
Limiting the International Spread of a Pandemic
At present, scientists believe that the likelihood of an avian virus changing into a virus that ultimately leads to the next influenza pandemic is greatest in places with widespread outbreaks in birds and with significant contact between infected animals and people. Reducing the opportunities for the virus to mutate, improving global disease monitoring, and helping other nations to prepare for and respond to outbreaks are key components of our risk mitigation strategy and a shared global responsibility. The United States has made pivotal contributions to control the international spread of H5N1 by working with affected countries and international partners to detect, contain, and prevent animal outbreaks, reduce human exposure to the virus, and enhance planning and preparedness for future outbreaks.
Leading the International Effort
In September 2005, President Bush announced the International Partnership on Avian and Pandemic Influenza (IPAPI) at the United Nations General Assembly. The Partnership was developed in concert with the World Health Organization (WHO) to align nations around a series of key goals including:elevating the issue of avian influenza on national agendas; coordinating efforts among donors and affected nations; mobilizing and leveraging resources; increasing transparency in disease reporting; improving surveillance; and building local capacity to identify, contain, and respond to an influenza pandemic. The challenge of avian influenza and the threat of a pandemic have required, and have produced, a coordinated international response, and the Partnership has mobilized the international community. The first meeting of the Partnership took place in Washington, DC in October 2005. In June 2006, 93 countries and 20 international organizations attended the IPAPI meeting in Vienna, Austria. Major international conferences have also taken place in Beijing, China in January 2006 and in Bamako, Mali in December 2006.
The United States is working on avian influenza issues in more than 100 countries, in collaboration with WHO, UNICEF, the United Nations Food and Agriculture Organization (FAO), the World Organization for Animal Health (OIE), and regional organizations, as well as with other international and in-country partners, including national governments and non-governmental organizations. The U.S. Government is supporting communications and public education activities in more than 50 countries to generate awareness about avian influenza and promote healthy behaviors and practices to reduce the risk of disease transmission. To help achieve this goal, the United States has supported the training of more than 113,000 people in the delivery of avian influenza communications messages.
U.S. Government agencies have deployed scientists, veterinarians, public health and communications experts, physicians, and emergency response teams to affected and high-risk countries to assist in the development and implementation of emergency preparedness plans and procedures for response to avian and pandemic influenza. As a result of these efforts, the United States and its global partners have made much progress across the spectrum of animal health protection, public health preparedness, information sharing, and public communication and awareness.
The United States Provides Assistance for Laboratories Overseas
The Lao People's Democratic Republic (Laos), a high-risk Southeast Asian country, has become a full participant in the Global Influenza Surveillance Network. The new Lao National Influenza Laboratory was established in 2006 with on-site U.S. Government assistance. In March 2007, the Lao laboratory identified a human case of H5N1 and sent the specimen to a WHO Collaborating Center for confirmatory testing. In 2006, the U.S. Naval Medical Research Unit in Egypt (NAMRU-3) assisted the Ukrainian Ministry of Health in establishing a national influenza laboratory that conducts molecular diagnostic testing for both seasonal and pandemic influenza. The United States provided equipment and reagents and provides ongoing confirmatory diagnostic testing. With technical and financial support from the United States, the Pacific Public Health Surveillance Network laboratory consortium in New Caledonia began routine testing of influenza viruses in 2006, and identified a new influenza A (H1N1) strain from the Solomon Islands that was recommended by WHO as a vaccine strain for vaccines to be used in the 2007-8 season. These examples represent major milestones in the development of capacity for pandemic influenza detection and response in priority countries.
Establishing Surveillance Capability Worldwide
Constant vigilance is the key to combating avian influenza and preventing pandemic influenza. By detecting avian influenza outbreaks early, with improved surveillance and laboratory diagnostic capacity, the world community has the opportunity to contain outbreaks in birds and intensify surveillance for human H5N1 cases that may accompany those outbreaks. When human cases occur, U.S. scientists are working with international partners to help determine whether the infecting virus is developing the capacity for efficient, sustained human-to-human transmission. The United States is supporting efforts to improve laboratory diagnosis and early warning networks in 75 countries and is working with its partners to expand on-the-ground surveillance capacity and improve knowledge about the movement and changes in H5N1 on a global scale. This includes support for improving national and regional laboratories to ensure that countries are able to quickly confirm the presence of H5N1 virus in people or animals so that a timely response is possible.
In 2004, the United States launched the Influenza Genome Sequencing Project to track genetic changes in viral strains. As a result, genome sequences of more than 2,250 human and avian influenza isolates have been made publicly available. Scientists around the world can now use the public sequence data to compare different strains of the virus, identify the genetic factors that determine their virulence, and develop new countermeasures. In 2006, the U.S. Government supported the creation of the Wild Bird Global Avian Influenza Network for Surveillance project in order to share information, increase the availability of scientific information for detection and containment, and track changes in virus isolates in wild birds.
The United States Helps Enhance Surveillance in High-Risk Areas
Due in large part to support from the United States, countries particularly vulnerable to avian influenza outbreaks have made significant developments in surveillance and response capacity at national and local levels. In Indonesia, an increasing number of high-risk areas are now able to provide weekly outbreak updates and launch outbreak response efforts within 24 hours. Contributing to this improvement are U.S. Government efforts to work with FAO and the Ministries of Agriculture and Health to develop participatory disease surveillance and response teams in all 154 avian influenza-endemic districts. The United States is also helping to expand local capacity for active surveillance across 27,000 villages in Java, Bali, and Sumatra through two Indonesian non-governmental organizations. In addition, increased surveillance and detection capacity in Laos allowed for rapid and accurate diagnosis of the July 2006 outbreak in poultry as an H5 avian influenza virus. These enhancements in surveillance resulted in more rapid response to outbreaks and improved containment to limit the spread of the virus.
The U.S. Department of Defense's Global Emerging Infections Surveillance and Response System (GEIS) supports surveillance activities in the military health system worldwide. Overseas, there are five laboratories located in Peru, Egypt, Kenya, Thailand, and Indonesia. These laboratories represent a global network of expertise in emerging infectious disease detection. The U.S. Navy's laboratory in Lima, Peru, expanded the number of sites from which it receives specimens from 12 sites in three countries to a total of 35 clinic/hospital sentinel sites in six countries of Central and South America, with plans for expansion to another four countries in 2007. The U.S. Naval Medical Research Unit in Cairo, Egypt (NAMRU-3) provides influenza surveillance including human and animal sampling in 19 countries in Africa, East Europe, the Middle East, and the former Soviet Union. Additional surveillance sites are being established in 2007 in six countries. Previously there was no consistent surveillance in sub-Saharan Africa. The U.S. Army Medical Research Unit (USAMRU) in Kenya now provides ongoing human influenza surveillance in the region. The U.S. Naval Medical Research Unit in Jakarta, Indonesia (NAMRU-2) has probably had the largest expansion in influenza surveillance efforts this past year because it is located in the epicenter of the H5N1 epidemic in the region. Syndromic surveillance has been expanded in 2006 to ten hospitals in the Lao People's Democratic Republic (Laos) and two in Cambodia.
Supporting a Coordinated International Response to H5N1 Avian (Bird) Influenza
Once an influenza virus with pandemic potential, such as highly pathogenic avian influenza A H5N1, is found in domestic birds, swine, or other domestic animals, it is necessary to eradicate the disease as quickly as possible and to take public health measures to minimize human exposure to the virus. If such a virus is found in wild birds or other wildlife, efforts should be directed at preventing it from being introduced into domestic birds or other susceptible animals.
The United States Helps Reduce H5N1 Outbreaks in Southeast Asia
Between 2003 and 2005, Thailand and Vietnam accounted for 88 percent of the world's H5N1 avian influenza outbreaks in animals. In 2005, there were at least 1,500 reported poultry outbreaks; however, the number fell to just 209 in 2006, and human cases fell from 75 to 3 over the same period of time. While many factors likely contributed to this shift, a substantial portion of this dramatic reduction in outbreaks can be attributed to the aggressive measures these countries, with U.S. Government support, have taken to combat avian influenza. As a result, countries across the region are more prepared and better able to mount effective outbreak response. In Cambodia and Laos, for instance, the time between onset of outbreaks and reporting has shortened from up to 5 weeks to 48 hours, significantly improving the opportunity for an effective outbreak response and containment.
The U.S. Government provided funding to strengthen WHO's Global Outbreak Alert and Response Network (GOARN), which supports public health surveillance and response in nations worldwide. The United States, with international partners, is providing training for thousands of individuals who will lead efforts to detect, contain, and mitigate the impact of animal outbreaks and take steps to prevent future outbreaks from occurring. Over the past year, the U.S. Government has supported the training of more than 129,000 animal health workers and 17,000 human health workers in H5N1 surveillance and outbreak response. Since January 2006, the United States has deployed more than 300,000 personal protective equipment kits to over 70 countries for use by surveillance workers and outbreak-response teams. In cooperation with WHO and the United Nations Food and Agriculture Organization (FAO), U.S. experts have provided vital technical expertise to national investigations of actual outbreaks of H5N1 in countries on three continents and provided technical assistance, commodities, and logistical or financial support to 39 of the 60 countries and jurisdictions affected by H5N1 to date. As a result of the efforts of the United States and the support of our international partners, the international community is aware of outbreaks sooner and is able to launch more effective and timely responses.
The U.S. Government has expanded the HHS/CDC network of Global Disease Detection (GDD) Centers, which works closely with WHO-Geneva and WHO Regional and Country Offices. This program is a network of international centers of excellence dedicated to the surveillance of emerging infectious diseases, outbreak detection, identification, tracking, and response, as well as the provision of training programs for field epidemiology and laboratory scientists. New GDD Centers have been established in China, Egypt, and Guatemala, and GDD Centers in Thailand and Kenya have been strengthened. The GDD Centers in Thailand and Kenya have fully trained rapid response teams and stockpiles of protective equipment available for deployment internationally within 24 hours. Work is underway to establish regional rapid response teams and stockpiles at GDD Centers in China and Egypt.

The United States Prepares for H5N1 Avian Influenza Spread to North America
Although the H5N1 avian influenza virus has not yet spread to North America, the United States is collaborating closely with Canada and Mexico through the Security and Prosperity Partnership to coordinate surveillance efforts for the early detection of the virus in wild birds migrating within and across North America. In the United States, a joint Federal/State program for the early detection of highly pathogenic avian influenza virus in wild birds has collected more than 145,000 samples from birds in all 50 States and territories since April 1, 2006. None of these U.S. samples tested positive for highly pathogenic avian influenza A H5N1.
The U.S. Government has completed acquisition of 140 million doses of avian vaccine. The National Veterinary Stockpile has entered into a contract for delivery of up to 500 million doses of an additional vaccine that can be used in young poultry. These avian vaccines can be acquired more rapidly than human vaccines because they, or similar vaccines, have already been tested and approved for use in poultry, in some cases long before highly pathogenic avian influenza H5N1 was a subtype of concern. Since May 2006, Federal veterinary specialists have participated in more than 50 State- and county-level exercises for highly contagious animal diseases, including avian influenza. The U.S. Government also held a national exercise that focused on the roles of Federal, State, and local government agencies, industry (primarily poultry), and consumer groups in collectively responding to an outbreak. More exercises are planned for 2007, with a focus on State- and tribal-level response planning and on mobilizing the National Veterinary Stockpile.
In addition, the U.S. Government has in place a process that provides real-time technical information and policy guidance to prevent the spread of avian influenza in commercial and other domestic birds and animals during a disease outbreak. This guidance includes biosecurity practices to help prevent disease from being introduced into a bird or flock, including restricting access to outside persons; preventing contact with wild birds; cleaning equipment, footwear, and vehicles; and separating new or returning birds from others in the flock for a period of time. Nearly one million copies of "Biosecurity for Birds" campaign materials have been distributed to all 50 States and more than 50 countries, and bilingual biosecurity information has been placed on more than 1.7 million poultry feed sacks. This guidance has also reached nearly 30 million readers through newspaper and magazine ads and 23 million listeners in 29 States through 30-second radio ads on national and regional agricultural radio networks.
International Response to Pandemic Influenza
By its very nature, a pandemic respects no borders. An outbreak of pandemic influenza anywhere poses a risk to populations everywhere. Our international effort to contain and mitigate the effects of an outbreak of pandemic influenza beyond our borders is a central component of our strategy.
The United States Responds to Human H5N1 Cluster
In May 2006, a cluster of human cases in Indonesia raised alarms that the disease may have spread between humans. Through prior preparedness planning and through coordination centered in the State Department with the U.S. Naval Medical Research Unit (NAMRU-2), the CDC, and the U.S. Agency for International Development, U.S. scientists, as part of a WHO team, were able to provide emergency support to the Indonesian Government. The international team investigated the outbreak, quickly analyzed samples through NAMRU-2 in Jakarta, and performed additional analyses and H5N1 confirmation through the CDC in Atlanta. This coordinated international response allowed WHO to quickly provide updates and reduce alarm as the cases were confirmed to be isolated incidents of inefficient human-to-human transmission and that the virus was not widely circulating among humans. The outbreak of avian influenza was not the start of a human pandemic, but the coordinated international response was an opportunity to practice what we intend to do if a pandemic arises.
The United States is working to enhance the ability of international organizations and individual countries to detect and respond to a pandemic. We are supporting many countries in their efforts to strengthen their laboratory diagnostic capacity, improve public communication, and develop national preparedness plans.
Although a pandemic virus has not yet emerged, the appearance of limited human clusters of H5N1 cases has tested our international surveillance and response capabilities. Since January 2006, U.S. Government scientists have assisted with on-site H5N1 investigations in Turkey, Nigeria, Romania, Djibouti, Indonesia, China, Laos, Vietnam, and southern Sudan. Investigative assistance included laboratory diagnosis, identification of disease risk factors, and analysis of clusters of disease to establish whether human-to-human (second generation) or human-to-human-to-human (third generation) transmission was occurring. U.S. Government scientists helped investigate several clusters of suspected human-to-human transmission including:a three-person family cluster in Thailand in 2004, in which limited second generation transmission was documented; three family clusters of H5N1 cases in South Sumatra, Indonesia, in 2005, in which the possibility of limited second generation H5N1 transmission could not be excluded for two of the clusters; and a large family cluster of H5N1 cases in North Sumatra, Indonesia (eight cases, seven deaths) in 2006, in which limited, non-sustained third generation transmission of H5N1 viruses likely occurred.
If, despite our efforts, a pandemic virus does emerge (i.e., the virus develops easy and ongoing transmission among humans), the United States and our global partners are preparing to mount an international rapid containment effort wherever the virus emerges in order to contain it or to slow its spread.
The United States has developed protocols and trained personnel to support an international effort to contain the pandemic in its earliest stages. The U.S. Government procured and pre-positioned overseas stockpiles of personal protective equipment, decontamination kits, and antiviral medications to complement global containment efforts.
National Border Measures: One Layer of Protection against the Pandemic Virus
If a pandemic begins outside the United States, and international containment efforts fail, the U.S. Government has planned a series of layered border measures that may be implemented incrementally during a severe pandemic to slow the entry of a pandemic virus into the United States while allowing the flow of goods and people. These border measures during the early stages of a severe pandemic may include flight restrictions from affected regions, issuance of health guidance to travelers intending to enter the United States, health screening of travelers before departure, en route, and on arrival to the United States, as well as public health measures to limit onward transmission of the disease.
We are working closely with our neighbors Canada and Mexico to establish a common North American approach to delay the arrival and impact of a pandemic. One of the objectives of the pandemic planning efforts in the Security and Prosperity Partnership is the development of the North American Plan for Avian and Pandemic Influenza. This trilateral plan, now being finalized, establishes a framework for coordinated, trilateral actions regarding communication, responses to avian and pandemic influenza, border monitoring, and critical infrastructure protection. Developed as part of the Plan is a concept of operations for responding to aircraft inbound to North America that are carrying passengers potentially infected with the pandemic virus. This approach is currently being shared with other aviation partners around the world. U.S. Quarantine Stations, located at ports of entry and land-border crossings where international travelers arrive, will play an important role in delaying the introduction of pandemic influenza into the United States and helping to limit its spread. The number of quarantine stations in the United States has more than doubled since 2004, expanding from 8 to 20 locations, with quarantine stations in Dallas and Philadelphia added this past year.
Limiting the Domestic Spread of a Pandemic and Mitigating Disease, Suffering, and Death
Should an influenza pandemic reach the United States, our primary focus will be to safeguard the health of the U.S. population. Our ability to accomplish this goal will require:(1) effective surveillance for influenza outbreaks, including improved diagnostic tests; (2) the rapid development, production, and distribution of vaccine; (3) the targeted and effective use of antiviral medications; (4) the application of community mitigationmeasures; (5) the expansion of hospital and medical care capability; and (6) effective communication of risk reduction strategies. Although we still have much to do in these areas, progress has been made on each of these fronts over the past year.
Enhancing Clinical Surveillance and Response
The U.S. Government is working to enhance the Nation's ability to detect and respond early and effectively to a pandemic, particularly through partnerships with State and local governments.
To better detect first cases of pandemic influenza in a community, the U.S. Government has provided resources to State and local health departments to increase the number of sentinel providers, to improve laboratory detection at public health laboratories, to support an influenza coordinator in each jurisdiction, and to educate clinicians to increase testing for and detection of influenza infection. The Sentinel Provider Network includes approximately 2,300 healthcare providers nationwide who report the number of weekly outpatient visits for influenza-like illness and submit specimens to State public health laboratories for influenza virus testing. This information helps identify emerging influenza strains and monitor disease patterns.
Since 2003, the Federal Government had designated $5 million per year for the States bordering Canada and Mexico to create and maintain an Early Warning Infectious Disease Surveillance System. This system is a unique collaboration of State, Federal, and international partners who work together to provide rapid and effective laboratory confirmation of urgent infectious disease case reports in the border regions of the United States, Canada, and Mexico. More recently, the border surveillance system has expanded to include, among other activities, 11 border pandemic influenza tabletop exercises, which included testing response by local personnel to the arrival of inbound flights with ill passengers.
In partnership with the Council of State and Territorial Epidemiologists (CSTE), the Federal Government operates the National Notifiable Disease Surveillance System. Through this system, public health practitioners at local, State, and national levels provide weekly information about specific diseases occurring in the 50 States, 5 territories, New York City, and the District of Columbia. In January 2007, the CSTE adopted an interim position statement that added "novel influenza A virus infections" to its list of reportable diseases. This action will help activate timely and appropriate health responses to human infections that might have pandemic potential and meets WHO's International Health Regulations 2005 revision that member states report human infections with new human influenza viruses.
The U.S. Laboratory Response Network (LRN), which includes State public health laboratories, has the capacity to perform tests using the real-time reverse transcriptase-polymerase chain reaction (RT-PCR) technique. All 50 States are prepared to conduct initial testing of suspectedhuman infection with H5N1 within 24 hours of receipt, using RT-PCR primers and probes developed and validated at the CDC. Reagents and protocols for testing for H5 influenza have been distributed to 99 LRN laboratories throughout the country.
Online Training for Public Health Response
To improve domestic response efforts, the CDC has released an online version of its three-day training course that provides a standardized curriculum to State and local public health responders about how to identify and control human infections and illness associated with avian influenza A H5N1. The course, entitled "CDC/CSTE Rapid Response Training: The Role of Public Health in a Multi-Agency Response to Avian Influenza in the United States" is the result of a partnership between the Centers for Disease Control and Prevention (CDC) and the Council of State and Territorial Epidemiologists (CSTE). The course is available at http://www. Cste.org/influenza/avian. Asp.
The U.S. Government is also working to create a test that would, within 30 minutes, detect and differentiate seasonal influenza from highly pathogenic avian influenza H5N1. In November 2006, the Federal Government awarded contracts to four companies to develop new diagnostic tests that doctors and field epidemiologists could eventually use to quickly and accurately test patients for avian-origin H5N1 and other emerging influenza viruses, as well as more common influenza viruses.
The Federal Government has been working to ensure that both Emergency Medical Services (EMS) and 9-1-1 public safety answering points are well integrated into the Nation's pandemic influenza planning and response, as both are essential to the Nation's health and safety in the event of a pandemic. To accomplish this integration new guidelines and protocols for both EMS and 9-1-1 have been developed. Representatives from Federal agencies and national EMS, 9-1-1, and public health organizations jointly developed these new guidelines. Taken together, the two documents provide general guidance, considerations, and ideas that can enhance the optimal delivery of emergency care and 9-1-1 services during an influenza pandemic.
Expanding Vaccine Supply
If our containment efforts fail, a well-matched pandemic vaccine will be our most effective countermeasure during an influenza pandemic. However, the current global capacity to produce a vaccine to respond to an influenza pandemic is insufficient to meet global needs. Developing improved vaccines and novel vaccine technologies and enhancing vaccine production is a top priority. The U.S. Government is providing direct financial support to WHO to expand the development and manufacturing infrastructure for influenza vaccine in six key developing countries, which will give them the capability to manufacture safe and effective influenza vaccines for seasonal influenza, and for their own, and possibly regional, needs in the event of a pandemic.
It is the goal of the Federal Government to be able to vaccinate every American as rapidly as possible during a pandemic. To help accomplish this, the Federal Government is investing in the expansion of both egg- and cell-based vaccine manufacturing capacity, the advanced development of new cell-based vaccines, antigen-sparing technologies, and the establishment and maintenance of pre-pandemic vaccine stockpiles.
DARPA Challenge -- Accelerated Manufacture of Vaccines
Our best scientists are racing to develop new vaccine production technologies now. The Department of Defense's Defense Advanced Research Projects Agency (DARPA) is working with scientists on radical technologies to create new vaccines, at a fraction of the cost of traditional vaccines, in much less time. The DARPA Challenge: to create a scalable system that makes 3 million doses of vaccine within 12 weeks of an outbreak of a new disease -- enough to protect our fighting men and women. Because of the goal, scientists are exploring novel and innovative production techniques. The principal aim of the DARPA projects is to remove technological barriers to imaginative solutions. This project, like other DARPA Challenges, taps the talent and ingenuity of our Nation's scientists. If any of the technologies under development prove feasible, they will further revolutionize our vaccine production capability.
Licensed influenza vaccines are currently produced in special chicken eggs, in a technique that has changed little in over 50 years. In contrast, cell-based vaccine manufacturing, a technology that is used to produce other modern vaccines, holds the promise of a reliable, flexible, and scalable method of producing influenza vaccines. To help achieve this goal, the U.S. Government awarded over $1 billion in May 2006 to major influenza vaccine manufacturers to enable them, over the next 5 years, to produce at least 300 million courses of egg- and cell-based pandemic vaccine within 6 months of the emergence of a pandemic virus. This year, the U.S. Government will request proposals from commercial manufacturers for the advanced development of other technologies such as recombinant DNA vaccines that may be made more rapidly at the time of a pandemic. Not only will such vaccines further diversify our vaccine arsenal, these vaccine technologies have the potential to be manufactured faster than traditional vaccines.
In April 2007, the Federal Government approved the first vaccine for humans against the H5N1 avian-origin influenza virus. We currently have enough of this pre-pandemic H5N1 vaccine for approximately 6 million people, with plans to stockpile enough pre-pandemic vaccine for 20 million people. The approval and availability of this vaccine will enhance national readiness and the Nation's ability to protect those at increased risk of exposure. This vaccine could be used if the current H5N1 avian virus were to develop the capability for efficient, sustained spread among humans, resulting in the spread of the disease across the globe. Should the H5N1 virus lead to a pandemic, this vaccine may provide early limited protection in the months before a vaccine tailored to the pandemic strain of the virus could be developed and produced.
We are also investing heavily in the evaluation of adjuvants, which could allow us to greatly increase the number of people vaccinated with a limited supply of vaccine material. An adjuvant is a substance that may be added to a vaccine to increase the body's immune response to the vaccine's active ingredient, called antigen. Adjuvants are one of the most promising technologies, given that they could dramatically extend the reach of a limited supply of the pre-pandemic vaccine and expand the production of a well-matched pandemic vaccine when it becomes available. Contracts totaling $132.5 million were awarded in January 2007 to three vaccine makers for the advanced development of H5N1 influenza vaccines for humans using antigen-sparing techniques. Initial clinical studies from two of the vaccine manufacturers have shown that the addition of their adjuvants to H5N1 vaccines may increase the vaccine's efficacy 10- to 20-fold and may provide cross protection against variants of virus subtypes. Additional clinical studies are planned in 2007 for the H5N1 vaccine products with each of the three new adjuvants. If these results are confirmed, then these adjuvants offer hope that we will be able to stretch our pre-pandemic vaccine supplies and, in effect, increase our pandemic vaccine manufacturing capacity 10- to 20-fold.
U.S. Scientists Identify Harmful Genes in 1918 Virus
Groundbreaking research efforts are helping us understand what makes an influenza virus into a virulent pandemic virus. U.S. scientists have reconstructed the influenza virus strain responsible for the 1918 pandemic, a project that greatly advances preparedness efforts for the next pandemic. U.S. Government researchers have also analyzed one of the proteins that covers the surface of the 1918 influenza virus and discovered a molecular property that may help to explain the virus's ability to spread easily from human to human. They found that the hemagglutinin protein, which is found on the surface of influenza viruses and binds to host cells, plays an important role in the transmission efficiency of the virus. By changing two amino acids (the basic building blocks of proteins) in the hemagglutinin of the 1918 virus, the researchers were able to create a version of the 1918 virus that was incapable of transmission in an animal model. This knowledge will help scientists develop new drugs and vaccines that act on the proteins encoded by these genes.
At the beginning of a pandemic, the scarcity of vaccine will require the limited supply to be prioritized for distribution and administration. The Federal Government has begun a process to revise previous interim guidance for Federal, State, local, tribal, and territorial planners on groups to target for earlier access to pandemic vaccines. The U.S. Government has sought input from influenza experts, State and local public health officials, homeland security experts, ethicists, private sector stakeholders, and the public in developing this guidance.
Stockpiling Antiviral Medications
Antiviral medications are an important element of pandemic influenza preparedness. Our current national goal is to have 81 million courses of antiviral drugs in Federal and State stockpiles by 2008, of which 50 million regimens will be purchased and managed in Federal stockpiles and 31 million in State stockpiles. As of June 2007, the Strategic National Stockpile contains more than 35 million regimens of antiviral drugs with an additional 2 million regimens on order. Thus far, States have stockpiled over 13 million regimens of antiviral drugs. At this time all State and Federal stockpiles of antiviral medications should be reserved for treatment of symptomatic patients. As stockpiles of antiviral drugs increase, the strategies for use may be expanded to include prophylaxis. Additional guidance on prophylaxis strategies is being developed.
The Government's antiviral strategy includes not only stockpiling existing antiviral drugs, but also developing new antiviral medications to further broaden our capabilities to treat and prevent influenza. In January 2007, the Federal Government awarded a 4-year contract of over $100 million for advanced development of a new influenza antiviral drug that can be given by injection to rapidly treat persons with severe influenza.
Limiting the Spread and Impact of a Pandemic:Community Mitigation
There is no question that the United States and the world stand to benefit from the unprecedented investments in influenza vaccine production, but the reality is that it will be years before we have enough capacity to quickly produce enough pandemic vaccine for the entire population. Even then, the time required to produce a vaccine may exceed the duration of the first wave of a pandemic. This means that we must have a plan to deal with a pandemic that does not rely upon the immediate availability of a vaccine.
In February 2007, the U.S. Government released groundbreaking Federal guidance for non-pharmaceutical interventions for mitigating the impact of a pandemic. This community mitigation strategy is important because the best protection against pandemic influenza, a matched pandemic vaccine, is not likely to be available at the outset of a pandemic.
Lessons from the 1918 Pandemic can Help Communities Today
Researchers studying the outcomes of the 1918 pandemic in dozens of U.S. cities have concluded that the speed of the public health response matters tremendously. Implementing multiple non-pharmaceutical interventions early in a local epidemic can save lives. As an example, the contrast of mortality outcomes in 1918 between Philadelphia and St. Louis is particularly striking. The first cases of disease among civilians in Philadelphia were reported on September 17, 1918, but authorities allowed large public gatherings, most notably a citywide parade on September 28, 1918, to continue. School closures, bans on public gatherings, and other social distancing interventions that reduce contacts between people were not implemented until October 3, when disease spread had already begun to overwhelm the city. In contrast, the first cases of disease among civilians in St. Louis were reported on October 5, and authorities introduced interventions essentially identical to those introduced in Philadelphia on October 7. The difference in response times between the two cities was approximately 14 days, when measured from the first reported cases. The costs of this delay were enormous. Philadelphia ultimately experienced a cumulative excess pneumonia and influenza death rate during the fall of 1918, more than twice that of St. Louis.
The impact of a poorly mitigated 1918-like pandemic today would be staggering. In the United States alone, we could face 90 million ill persons and nearly two million deaths. Recent scientific modeling and historical reviews of the 1918 pandemic suggest that non-pharmaceutical interventions could be very effective at slowing the spread of disease and mitigating the outbreak, but only if they are implemented early and maintained consistently across communities affected by a pandemic. We estimate that these interventions could dramatically reduce the number of people who become infected, potentially preventing illness and death in millions of Americans. Therefore, community mitigation measures will serve as a first line of defense to help delay or mitigate the spread of influenza.
These interventions include:
- Staying home if one is ill;
- Staying home if someone in one's household is ill;
- Dismissing students from school, closing childcare facilities, and keeping children home; and
- Reducing close contacts in the community and at work (i.e., social distancing).
These interventions are strengthened by the combining of these non-pharmaceutical interventions with antiviral medications and the layering of other infection control measures to reduce disease transmission, such as hand hygiene (frequent hand washing), respiratory etiquette (covering coughs and sneezes), and the use of facemasks.
The Community Mitigation Guidance also introduces a Pandemic Severity Index (PSI) for assessing the health threat posed by a pandemic virus. The index will allow us to tailor community mitigation interventions and balance the need to protect the public's health while minimizing societal and economic disruptions. The community mitigationstrategy was designed with input from many Federal agencies, State and local public health officials, researchers and mathematical modelers, healthcare and influenza experts, private sector entities, faith-based and community organizations, labor unions, educational stakeholders, and the public. The development from inception to adoption of a Federal policy on community mitigation within 9 months of the release of the National Plan underscores the level of commitment and coordination among government agencies with responsibility for emergency response.
Expanding Medical Capacity to Care for large Numbers of Ill Patients
A severe influenza pandemic would place a tremendous burden on the U.S. healthcare system. Estimates based on extrapolation of a severe 1918-like pandemic project that 45 million Americans would seek medical care with 10 million of those requiring hospitalization. The projected demand for inpatient and intensive care unit beds and mechanical ventilation services would overwhelm the Nation's healthcare system. Pre-pandemic planning by healthcare facilities is essential to prepare for the surge in medical care associated with a pandemic.
Coordinated community-wide and regional planning is needed for an optimal medical care response to a pandemic. An essential part of community and regional planning is the development and implementation of electronic systems that exchange information among hospitals and public health agencies to provide the needed situational awareness to manage patients, medical supplies, and staff during an influenza pandemic. The Federal Government has produced tools to assist in planning for improvements in hospital capacity during a pandemic. The Community Planning Guide for Providing Mass Medical Care with Scarce Resources offers specific recommendations for providing the highest possible standard of care where resources are limited. This guide addresses ethical and legal issues and offers recommendations across the spectrum of care, including pre-hospital, hospital, alternative care sites, and palliative care. Implementing these recommendations will help prevent degradation of care if large numbers of patients are seeking care in healthcare facilities.
Federal Healthcare Systems Prepare for a Pandemic
The Department of Veterans Affairs, one of the largest integrated national healthcare systems in the United States, consisting of 150 hospitals and more than 700 outpatient clinics, has taken steps to ensure that medical care services can be sustained during a pandemic. The VA healthcare system has a fully functional electronic medical record system that automatically provides critical surveillance data from all sites of care to the CDC. Through its Consolidated Mail Outpatient Pharmacy program, more than three-fourths of all prescriptions are delivered to patients, thus avoiding the need for patient visits to pharmacies, clinics, or hospitals for medications. The organization has made a commitment to protect patient care staff during a pandemic by providing them with the best available personal protective equipment and access to antiviral medications. A system-wide retired clinician volunteer corps is being organized to enhance staffing during a pandemic.
Pre-hospital care and emergency response is a critical component of medical surge planning. EMS responders are a respected, well-trained, mobile healthcare workforce that exists in every community in the country and can be quickly dispatched when the public calls 9-1-1 for assistance. Because the Nation will rely on this workforce during an influenza pandemic, the Federal Government has developed new guidelines and protocols to enhance the delivery of EMS and 9-1-1 services during an influenza pandemic.
The Federal Government has incorporated both funding and guidance to ensure that public health and healthcare delivery partners throughout the country work together to strengthen public health and health system preparedness. Awardees of Federal Pandemic Influenza funding were required to hold exercises in the areas critical to expanding mass casualty care capability and health system preparedness, including:use of the Emergency System for Advanced Registration of Volunteer Health Professionals (ESAR-VHP), mass-fatality plans, communications systems, triage and admission plans, alternate care sites, and healthcare facility-level infection control. These activities support both pandemic and all-hazards preparedness.
As part of the joint effort to strengthen capacity within the healthcare and medical communities, funding has been directed to the purchase and storage of pharmaceutical caches, personal protective equipment, ventilators, and other critical medical materiel. In addition to these stockpiles, the Department of Veterans Affairs has more than 150 emergency pharmaceutical and medical caches around the Nation.
By reducing disease transmission and decreasing the number of Americans becoming ill, and therefore the number requiring hospitalization, community mitigation measures offer an additional strategy to help narrow the gap between existing acute care capacity in the United States and the surge demand for medical care. The community mitigation measures, as well as infection control practices such as hand hygiene and respiratory etiquette, can help lessen the strain on healthcare systems by focusing on the goal of reducing disease transmission.
Risk Communication
Effective risk and crisis communications will help guide the public, the news media, healthcare providers, and other groups to respond appropriately in outbreak situations and to adhere to public health measures. The website www.pandemicflu.gov, available in English, Spanish, Chinese, and Vietnamese, provides one-stop access to all Federal avian and pandemic influenza information. More than 1,700 websites around the world link to the PandemicFlu. Gov homepage.
In early 2007, the U.S. Government sponsored tabletop exercises with key media leaders and senior government officials in Atlanta, Chicago, Los Angeles, Miami, New York, and Washington, DC. These exercises facilitated effective two-way communication between the media and senior government officials about the challenges of delivering timely, accurate information to the public during a pandemic. In addition, the U.S. Government has delivered 10 Crisis and Emergency-Risk Communications courses to State and local officials, presenting and applying proven tools and approaches to the communication of crisis information.
In February 2007, the Federal Government launched a series of television and radio public service announcements (PSAs) to raise awareness of pandemic influenza and to educate members of the public about the steps they can take now to prepare. The PSAs, released under the brand "Know What to do About Pandemic Flu," were distributed to 300 television and 1,000 radio stations across the country. Basic collateral materials were developed to support the PSA campaign and have been posted on PandemicFlu.gov. As of May 21, 2007, the PSAs have been aired more than 20,000 times. Overseas, the U.S. Government has reached out to the estimated 4 to 5 million private U.S. citizens abroad through a variety of methods to provide information on individual pandemic preparedness measures. The Department of State, through its website (www.travel.state.gov), American community newsletters, warden messages, and town hall meetings, has conducted an aggressive outreach campaign to private U.S. citizens traveling and residing abroad.
In the event of a pandemic, the Federal Government will communicate to the public through both traditional and new media channels and will work with partners including the business community, faith-based and civic organizations, and healthcare providers to reach the general public with critical information on how to protect themselves and their loved ones.
Sustaining Infrastructure and Mitigating Impact to the Economy and the Functioning of Society During a Pandemic
Although social distancing is critical for helping to limit the spread of the pandemic, it will be difficult to maintain if people feel that they do not have access to the resources, information, and support systems necessary to keep them safe. A severe pandemic could dramatically reduce the number of available workers and significantly disrupt the movement of people and goods, which could in turn threaten essential services and operations across our Nation.
Strengthening Community Resiliency
A major U.S. university, partnering with leading businesses, public health officials, and public schools, is developing and testing innovative approaches to increase local community and neighborhood resiliency. To provide local and timely information during a pandemic, they have engaged major technology companies to help deliver community-specific information for pandemic preparedness over the web and to increase the capacity for providing local information to the public by phone. Using existing technologies, large numbers of toll-free calls could be routed to informed community volunteers able to answer phones from their homes. Local schools would also play an important role. Through a new classroom curriculum, students will help their families develop pandemic disaster plans. This model program would also build upon and leverage the existing relationships among students attending K-12 schools and their parents to serve as a nucleus for strengthening community cohesion.
The scale and scope of a pandemic necessitate a dedicated effort and investment beyond typical business continuity planning. The effort to sustain our critical infrastructure and the functioning of society will require the engagement of all levels of government and all segments of society. Ultimately, the effectiveness of our response depends upon individual, family, and community preparedness.
Ensuring Continuity of Federal Government Operations
Over the past year, Federal departments and agencies, including Federal healthcare systems, have been developing and exercising preparedness and response plans that take into account the potential impact of a pandemic on the Federal workforce and are configured to support State, local, and private sector efforts where appropriate.
Streamlining Access to Essential Government Services
With the complete conversion of food benefits delivery from paper coupons to electronic benefit transfer (EBT) cards, the Federal Government's Disaster Food Stamp Program is better positioned to reinforce the public health response during a pandemic. This program allows maximum benefits for low-income families with children to help compensate for the loss of free and reduced price school and childcare meals due to school dismissal. This new technology also facilitates the purchasing of food from supermarkets through self-checkout or drive-through and could minimize the risk of face-to-face contact with others. Current participants of the Food Stamp Program would continue to receive benefits automatically as they are deposited into the client's food stamp account on a monthly basis. Because of the EBT card, extra or extended benefits to current participants can be electronically added to the EBT card. Since the certification process is more flexible and can be completed by mail, telephone, or internet, rapid food assistance to newly eligible families experiencing financial hardships would be possible during a pandemic, including the possibility of offering them pre-loaded EBT cards.
Pandemic planning summits were conducted in all States to start the process of community-level planning for a pandemic. Congress appropriated $600 million over the past year for State and local preparedness efforts, including the exercising of community mitigation measures, medical surge plans, and mass inoculation plans. As a follow-on to the 2006 planning summits, the National Governors Association, with Federal funding, has launched a series of 10 regional pandemic influenza workshops to enhance intergovernmental and interstate coordination.
The National Plan highlighted the importance of having in place a comprehensive and effective program to ensure the uninterrupted continuation of Federal Government essential functions. The Federal Government has been refining and strengthening continuity plans to account for the unique challenges associated with a pandemic. All Federal departments and agencies are addressing their own pandemic preparedness by completing all elements of a comprehensive checklist. The "meta-checklist" guiding their efforts is available for any institution to use, at www.pandemicflu.gov. In addition, comprehensive guidance on Human Capital Planning for Pandemic Influenza, available to Federal agencies and employees, includes a series of helpful planning guides for managers and supervisors, frequently asked questions and answers, and a newly revised telework guide to assist the Government in continuing operations.
Building a "Chain of Preparedness"
Businesses realize that their ability to cope with a pandemic depends both on steps that they take and on the preparedness levels of the businesses that make up their critical supply chain. One large business recently invited more than 300 of their top suppliers to a pandemic preparedness workshop, so they could pass along pandemic planning information and encourage each one of the attending companies to start to prepare. By sharing information and encouraging planning along their supply chain, this large business enhances its readiness and mentors other smaller businesses to do the same. This "chain of preparedness" improves overall business preparedness and resiliency and enables communities to be better prepared to face a pandemic.
Preparing and Engaging the Private Sector
The private sector has an important role to play in preparing for, responding to, and recovering from a pandemic. The private sector owns and operates over 85 percent of the critical infrastructure in the United States, and therefore represents an integral part of our society because of the critical goods and services that it provides. Moreover, it touches the majority of our population on a daily basis, through employer-employee or vendor-customer relationships. For these reasons, it is essential that the U.S. private sector be engaged in preparedness and response activities for a pandemic. In the event of an influenza pandemic, businesses and other employers will play a key role in protecting employees' health and safety as well as limiting the negative impact to the community, economy, and society.
Over the past year, the Federal Government has produced numerous tools for businesses of all types and sizes to assist them in planning for a
pandemic. Several checklists have been produced that include information for businesses in general (Business Pandemic Influenza Planning Checklist), as well as Planning for U.S. Businesses with Overseas Operations,Health Insurer Pandemic Influenza Planning Checklist, and Travel Industry Pandemic Influenza Planning Checklist. State governments, local governments, and thousands of businesses and employers in this country and worldwide have used the checklists to improve their pandemic planning efforts. Federal agencies have also developed and distributed other tools for businesses to use, including:
- Guidance on Preparing Workplaces for an Influenza Pandemic: guidance and recommendations on infection control in the workplace, including information on engineering controls, work practices, and personal protective equipment, such as respirators and surgical masks.
- Guidance for Protecting Workers against Avian Flu: information for protecting employees who may have been exposed to avian influenza.
- Cover Your Cough: flyers and posters showing ways to reduce transmission of respiratory illnesses.
- Stopping the Spread of Germs at Work: basic precautions for protecting employee health.
- Quick Cards for Employees to Protect Yourself from Avian Flu: general precautions and specific information for poultry employees, laboratory employees, animal handlers, food handlers, and healthcare workers .
- Pandemic Influenza Preparedness and Response Guidance for Healthcare Workers and Healthcare Employers: information and tools helpful to healthcare planners.
In addition, the recently released Community Mitigation Guidance includes specific planning recommendations for aligning business practices with public health protection interventions. The document provides clear steps an employer can take to potentially slow the spread of pandemic influenza, help keep workplaces safe, and reduce the number of people who become sick. All of these tools are posted on www.pandemicflu.gov.
Protecting and Sustaining Critical Infrastructure
Critical infrastructure entities that provide essential services, such as food, water, healthcare, power, and telecommunications, have a special responsibility to prepare and plan for continued operation during a pandemic. Efforts that focus on both protecting health and maintaining continuity of operations are vital parts of pandemic preparedness for critical infrastructure businesses.
Protecting the Nation's critical infrastructure, and the businesses both large and small within these sectors, requires the full cooperation and coordinated actions of the public and private sectors. In the Fall of 2006, the Federal Government, working collaboratively with partners in the public and private sectors, released its Pandemic Influenza Preparedness, Response, and Recovery Guide for Critical Infrastructure and Key Resources (Guide). Tailored to national goals and capabilities, and to the specific needs identified by the private sector, this business continuity guidance represented an important first step in working with the owners and operators of critical infrastructure to prepare for a potentially severe pandemic outbreak. The Guide has served to support private sector pandemic planning by complementing and enhancing, not replacing, their existing continuity planning efforts. With that in mind, the Federal Government developed the Guide to assist businesses whose existing continuity plans generally do not include strategies to protect human health during emergencies such as those caused by pandemic influenza or other diverse natural and manmade disasters.
Over the last year, the Federal Government has conducted an extensive outreach effort to the private sector, particularly critical infrastructure businesses. In the last year, more than 150 presentations, workshops, and fora have been conducted and attended by thousands of key stakeholders from critical infrastructure entities (e. G., healthcare operations, banking and finance entities, operations centers, retail operations, transportation and trucking operations, supply warehousing operations, grocery and food suppliers, and supply distributors) as well as businesses of all types. These information sharing sessions have provided practical action-oriented information to identify essential functions and critical planning elements and to assist businesses in protecting the health of employees and in maintaining continuity of business operations during a pandemic.
Preserving and Protecting Financial Services
A large retail financial institution has institutionalized a good health awareness program to improve employees' understanding of how to protect themselves during a pandemic. This program includes a website with guidance information by health officials and models to inform employees on the progression of H5N1 globally. They have modified their human resources policies to support employees during a pandemic outbreak whether they are home due to illness, taking care of a loved one, or because of social distancing advice from local authorities. Furthermore, this financial institution is assessing all of its vendors to determine whether or not they have pandemic plans that can support the organization's supply chain during a pandemic.
Sustaining the Economy
Sustaining infrastructure and mitigating the pandemic influenza-related impacts to the economy and society require vigilance that extends beyond the workplace. Policymakers and interagency subject matter experts have developed border policies and protocols that aim to slow the spread of disease while limiting, to the extent possible, disruptions to trade and passenger flows. Minimizing economic impacts while limiting disease entry is the primary goal of this approach.
The Federal Government has conducted a number of pandemic preparedness exercises that include financial institutions, public health officials, and other relevant Federal, State, and local officials. Additionally, both the Financial and Banking Information Infrastructure Committee (FBIIC) and the Financial Services Sector Coordinating Council (FSSCC) have established working groups that identify and address issues related to the resilience of the financial services sector during a pandemic. The FSSCC established the Infectious Disease Forum and convenes regular meetings with the FBICC and other stakeholders to discuss preparations and to identify interdependencies in other critical sectors.
Keeping Courts Open to Ensure Justice for All
Building on lessons learned from existing "all-hazards" emergency planning efforts initiated after 9/11, and incorporating lessons learned from their experience planning for and responding to hurricanes, the Supreme Court in Florida developed a strategy for Pandemic Influenza with the goals of protecting the health and safety of everyone at the court facilities and "keeping the courts open" to ensure justice for the people. A detailed planning template was produced to assist local courts in developing their own pandemic plans. Court Emergency Management Teams, consisting of judges, attorneys, deputy clerks, deputy sheriffs, information technology staff, and others, are exploring a number of innovative ideas including holding court proceedings via videoconference, and assembling and managing juries while adhering to social distancing measures.
Maintaining Society and Civil Order
Hurricane Katrina highlighted the necessity for the uninterrupted continuity of the justice system. States must know what the procedures are for requesting Federal law enforcement assistance in the event of an emergency, such as a pandemic outbreak. On May 31, 2006, the Attorney General sent a letter to each Governor outlining the procedures for obtaining assistance under the Emergency Federal Law Enforcement Assistance provisions of the Justice Assistance Act of 1984. The letter recognized that pre-event collaborative planning would have improved the Katrina response and stressed that the goal was "to ensure that any future response, whether to a natural disaster, a pandemic influenza outbreak, or an act of terrorism occurs as expeditiously as possible."
The Federal Government has also engaged in extensive outreach efforts to criminal justice stakeholder groups stressing the importance of pandemic preparedness and encouraging agencies to begin planning now. In May 2006, the Federal Government convened a forum for more than 200
professionals representing a cross-section of all components of the justice system, including police, probation, corrections, and court personnel. Officials shared information on the challenges these stakeholders may face, and the participants discussed and shared "best practices."Furthermore, representatives from each of the 94 U.S. Attorney's Offices met with Federal court representatives to coordinate continuity planning efforts in the event of a pandemic or other emergency.
An influenza pandemic would also present significant challenges to public service response organizations and the communities they serve. Earlier this year, a 3-day forum brought together partners from Federal, State, local, territorial, and tribal emergency medical services, fire, emergency management, law enforcement, and public works in order to develop best practices and model protocols, to coordinate pandemic preparedness activities, and to standardize all-hazards training and exercises.
In the coming months, the Federal Government will work with the private sector to develop and release sector-specific guidelines that will complement and enhance these activities. These efforts by the Federal Government, in partnership with the private sector, are crucial for sustaining society, and for ensuring the continued availability of essential goods and services such as food, water, healthcare, power, transportation, and communications during a pandemic.
One final question deserves to be asked as we review the progress over the past year.
Looking Ahead:What have we learned through these efforts and what gaps still need to Be addressed?
Building a Foundation for All-Hazards Preparedness
The threats facing our Nation in the 21st century, from pandemic influenza and the emergence of new diseases such as SARS, to terrorist attacks and naturally occurring disasters such as hurricanes and earthquakes, require an all-hazards approach to emergency planning and preparedness. However, pandemic preparedness is often viewed as separate from or competing with other disaster preparedness efforts. Although there are some unique aspects to pandemic planning, preparing the Nation for the threat of an influenza pandemic has provided a platform to address issues and concerns common to mass casualty disasters. Promoting a culture of individual, family, and community preparedness is the foundation for emergency planning efforts. Strengthening our public health infrastructure by enhancing public health agency coordination with partner State and local agencies, enhancing biosurveillance, and accelerating countermeasure development, production, and distribution, sustaining our Nation's critical infrastructure, and developing strategies for expanding mass casualty care capability and for prioritizing scarce medical resources are important goals for preparing for many of the threats we now face. By investing in pandemic preparedness, our Nation is building a foundation for all-hazards preparedness.
Achieving the goals outlined above and preparing the Nation for a pandemic will not be easy. The Federal Government has made a commitment to transparency and accountability in reporting our progress. While we have made progress over the past year to prepare the Nation for the threat of an influenza pandemic, much important work lies ahead. Critical gaps in pandemic preparedness efforts remain and are outlined below. Despite the waning media interest in avian and pandemic influenza, the potential consequences of a severe pandemic demand that we redouble our efforts to address these critical gaps.
Building Connectivity and Unity of Effort
The National Plan recognized that one of our greatest vulnerabilities was the lack of connectivity and coordination of effort between the various communities responsible for pandemic preparedness. Developing effective strategies and policies to meet the challenge of a pandemic require the engagement of all nations, all levels of government, and all segments of society. Internationally, collaboration and transparency are essential. Here at home, a unified effort that engages all levels of government, as well as the public health community, the medical care community, the private sector, faith-based and community-based organizations, and the public, is critical for an effective response.
One of the most tangible benefits of pandemic planning at a Federal level has been the forging of close working relationships among Federal departments and agencies. This began with the writing of the National Plan. Many of the actions in the National Plan required collaboration, coordination, and cooperation between Federal departments. Recognizing the need for a unified effort across the Federal Government, an interagency group with senior managers representing 16 Federal departments and agencies directly engaged in pandemic preparedness has been meeting weekly since the release of the National Plan in May 2006. This interagency group monitors progress on all actions in the National Plan and reviews pandemic policy issues. To ensure that the Federal Government speaks with one voice on pandemic issues, all pandemic-related information developed by departments is posted on www.pandemicflu.gov and reviewed for consistency prior to release by other departments. The establishment of trusted relationships across the Federal Government as a result of the ongoing work of this interagency group has enhanced connectivity and has improved communication among Federal departments and agencies.
The benefits of a unified effort at the Federal level can also be realized at a State and local levels. It is critical that we establish connectivity and build relationships between various layers of the government, the private sector, and faith-based and community-based organizations. Now is the time, before a pandemic emerges, to establish and test these relationships and partnerships. The Federal Government must take a leadership role in fostering an environment of collaboration and public engagement that will serve our Nation well for any hazard we might face.
Strengthening Disease Detection and Biosurveillance
The strategies associated with pandemic preparedness serve to strengthen the public health infrastructure in the United States and the world. One of the most important areas is disease detection and surveillance.
Although we have realized progress in expanding disease surveillance abroad, critical gaps remain with respect to "real-time" disease detection and clinical surveillance in the United States. As part of its national influenza surveillance effort, the CDC currently receives weekly mortality reports from 122 cities and metropolitan areas in the United States. This information helps the CDC track trends in disease spread, identify severely affected populations, and monitor the impact of influenza on health. One of the limitations of this system, however, is an approximately 2-week lag in obtaining data. BioSense is a national program intended to improve the Nation's capabilities by conducting nearly real-time clinical disease surveillance. Of the nearly 6,000 hospitals in the United States, only 700 hospitals are currently engaged in some stage of implementation for sharing data with the BioSense program.
Given how rapidly a pandemic could spread, it is crucial that we establish this "real-time" surveillance capability at a national, State, and community level. It is imperative to identify outbreaks and implement interventions as quickly as possible, in addition to monitoring the outbreak and evaluating the effectiveness of our interventions.
Another critical gap related to biosurveillance is the availability of rapid diagnostic tests that can quickly discriminate pandemic influenza from non-influenza illnesses. Use of these tests in outpatient clinics and in ambulatory settings will greatly enhance the Nation's capacity to detect pandemic influenza more rapidly. Also, to effectively implement our border measures, it is vital to quickly and correctly identify infected individuals at ports of entry. Moreover, to maximize the utilization of our limited stockpile of antiviral medications (targeting treatment to individuals with confirmed pandemic influenza) and to minimize the disruption of community mitigation measures (limiting isolation and voluntary home quarantine to individuals and households with confirmed pandemic influenza), a field-based test to rapidly diagnose pandemic influenza is essential.
The Federal Government must intensify its efforts for improving biosurveillance to ensure that we are not "blind" during the next pandemic or any other public health crisis. Accelerating the development of rapid diagnostics is key for effectively implementing disease control measures.
Expanding Medical Capacity to Care for Large Numbers of Ill Patients
The projected demand for medical care during a severe pandemic would overwhelm the Nation's present healthcare system. Nationwide, many hospitals and emergency departments are already operating at or near capacity and may not have the capability to treat the surge of patients who will need care during a severe pandemic. Our Nation's current acute inpatient capacity is limited by the number of appropriately staffed and equipped hospital beds. Expanding acute care capacity, by increasing the number of hospital beds and training additional medical staff, would require substantial and recurring investments with long lag times. A severe pandemic will impact all communities nearly simultaneously and at the peak of the outbreak could result in many times the normal demand for acute medical care. There may be little opportunity to shift resources from one part of the country to another. To meet this demand and to ensure delivery of the best quality healthcare possible under such circumstances, individual communities need to plan for dramatic increases in patient care capabilities. Up to this point, contingency planning for pandemics and other disasters has generally attempted to improve medical "surge capacity." However, it is unrealistic for communities to be able to increase the numbers of staffed acute care hospital beds to a level necessary for pandemic preparedness. Clearly, alternative strategies are warranted.
Therefore, many hospitals plan to boost their current capacity to treat ill patients through the use of existing resources for alternative and auxiliary settings for healthcare and by expanding the number of available healthcare providers with retirees and volunteers. Healthcare workers and first responders will be on the front lines during an influenza pandemic. Ensuring the availability of protective measures for these critical workers will be essential to our efforts to protect the health and safety of the public. Community planning efforts will require the coordination of many providers and organizations and must address how healthcare facilities can best share assets in order to assure the greatest benefit for the largest number of people in the most ethical manner. The Federal Government has incorporated both funding and guidance to assist in planning for the strengthening of mass casualty care capacity. Community mitigation measures offer an additional strategy to help narrow the gap between existing acute care capacity in the United States and the projected demand for medical care. The combined efforts of maximizing available surge capacity and reducing the demand for acute care by community mitigation will help, but new strategies are required and much work remains to address our Nation's challenge of mass casualty care.
The stockpiling of critical medical materiel, including a reassessment of antiviral medication stockpile goals, is one area that the Federal Government needs to address in the coming year. Most healthcare facilities have "just-in-time" inventory for medical supplies, which means that hospitals normally have on hand only a few days of basic medical supplies including personal protective equipment and medications. Although the Federal Government has stockpiled medical supplies, the scope and scale of a pandemic in terms of the demand for medical materiel would be unprecedented. Limited global production capacity of antiviral medications was one factor that had constrained antiviral drug stockpile goals. Recent expansion of domestic antiviral drug production capacity -- up to 80 million regimens per year of oseltamivir -- has made increased stockpiling possible. This, in combination with several recent analyses suggesting substantial potential benefits of prophylactic antiviral drug use, has prompted a reassessment of pandemic antiviral drug use, strategies, and potential stockpiling targets. Accordingly, significant work remains at the Federal, State, local, and healthcare facility level to address the projected demand for antiviral medications, personal protective equipment (including surgical masks and respirators), antibiotics, ventilators, and other medical materiel required during a pandemic.
Accelerating Vaccine Development and Production
The Federal Government is investing in the expansion of vaccine manufacturing capacity, the advanced development of new cell-based vaccines, antigen-sparing technologies or adjuvants, and the establishment and maintenance of pre-pandemic vaccine stockpiles.
The challenge of producing enough pandemic vaccine for the U.S. population is significant, but an even greater challenge is producing and providing worldwide access to pandemic vaccine. The global capacity for influenza vaccine production is approximately 350 million doses per year. This could be scaled up to 500 million doses per year in an emergency such as a pandemic. Even with the current investments in expanded capacity, global vaccine production will remain well below projected demand during a pandemic. The U.S. Government is committed to working with the pharmaceutical industry, our international partners, and WHO to address this gap. But commitment alone is not sufficient. We need imaginative solutions to this problem, and we require a unified effort that engages our best scientists in addressing this challenge.
In that spirit, the United States is working in collaboration with WHO and international partners throughout the world to enhance surveillance and pandemic preparedness, including increasing vaccine development and vaccine access. Four important principles guiding our international effort include:(1) transparency; (2) rapid reporting; (3) sharing of data; and (4) scientific cooperation. The United States continues to call on countries everywhere to share influenza samples openly and rapidly, without preconditions. Withholding influenza viruses from the Global Influenza Surveillance Network greatly threatens global public health and is inconsistent with the spirit of the legal obligations member nations have all agreed to undertake through adherence to the International Health Regulations.
The World Health Assembly has recently adopted a resolution on pandemic influenza preparedness that makes clear that member states must continue to share influenza specimens and viruses with WHO's Global Influenza Surveillance Network. The resolution also emphasizes the need for increased vaccine access. The United States has invested more than $1 billion in the development of new vaccine technologies that will benefit the international community. The United States strongly supports and finances WHO's efforts to meet the long-term global need for an influenza vaccine through the Global Pandemic Influenza Action Plan to Increase Vaccine Supply. In addition, the United States is committed to working with member states and WHO to explore other avenues to meet the near-term need for greater access to influenza vaccines, including pre-pandemic vaccines.
Distributing and Tracking Countermeasures
Medical countermeasures have little utility if they cannot be delivered quickly to those in need, yet the logistical challenges of rapidly allocating, distributing, and administering countermeasures to 300 million Americans are substantial. Although we have made significant investments in distribution capacity since 2002 through the Strategic National Stockpile, State and local grant programs, and the Cities Readiness Initiative, much work remains. Guidance and resources have been provided to State, local, tribal, and territorial governments to facilitate completion of distribution plans for medical countermeasure stockpiles. Recipients of pandemic influenza supplemental funding are required to complete and exercise these plans.
Countermeasure allocation and distribution is important for preparing our Nation for pandemic influenza and other naturally occurring infectious diseases, as well as for chemical and nuclear attacks. In the future we may be faced with the need to prioritize scarce medical resources during a major disaster. The pandemic efforts could well serve as a template for allocating and distributing life-saving countermeasures against other threats. The ongoing guidance development process for prioritizing and deploying countermeasures during a pandemic represents our first steps in addressing this complex ethical and logistical challenge.
Implementing Community Mitigation and Building Community Resilience
Although the community mitigation strategy may significantly reduce illness and death, implementing this strategy will not be easy. It is critical that emergency planners better understand the challenges associated with the implementation of the Community Mitigation Guidance. It is also important that they are aware of any potential adverse consequences due to these interventions. Minimizing the negative impact will require communities to undertake appropriate planning and exercises to pre-identify vulnerable populations and persons at risk and to develop population-specific strategies. To enhance individual and community adherence to these community mitigation measures, the Federal Government must continue to work with the private sector, public health, education, and community-based and faith-based organizations to address feasibility concerns, develop clear and appropriate public messaging, and minimize any adverse consequences associated with implementation. Pandemic exercises provide an ongoing opportunity to continually test and refine these plans.
The community mitigation strategy developed by the United States offers hope, especially to countries with limited access to medical countermeasures. The United States is reaching out to partners in the international community by informing them of our approach and assisting them in the adaptation of our strategy.
Translating our Pandemic Preparedness Efforts to Seasonal Influenza
Each year, more than 200,000 Americans are hospitalized and 36,000 die due to seasonal influenza. Increased awareness of available antiviral therapies and advances in vaccine production and point-of-care diagnostics, as a result of pandemic preparedness efforts, could translate to more effective treatment for seasonal influenza. Promoting healthy behaviors, such as hand washing and cough etiquette, could help reduce the spread of seasonal influenza and other respiratory diseases. Seasonal influenza also provides an opportunity to reinforce these behaviors, promote vaccination of nontraditional high-risk groups and healthcare workers, and test workplace policies and practices, such as having ill individuals remain home, so that we are better prepared to respond should a pandemic occur. How our Nation invests today in pandemic preparedness will serve us well when a pandemic does occur and could also prevent illness and death associated with seasonal influenza.
A Year of Progress
Much has been accomplished since the release of the National Plan in May 2006. Over the past year, the United States and the international community have mobilized to confront the threat of an influenza pandemic at its source, bycontaining H5N1 poultry outbreaks and rapidly identifying associated cases of human disease. The United States has made pivotal contributions to control the international spread of H5N1 by working with affected countries and international partners to detect, contain, and prevent animal outbreaks, reduce human exposure to the virus, and enhance planning and preparedness for future outbreaks.
Should a pandemic emerge, the United States is better positioned today to detect an outbreak earlier, to support an international effort to contain the pandemic in its earliest stages, to limit the spread of the pandemic, and to save lives. The Federal Government has developed mitigation strategies and guidelines for controlling influenza outbreaks in communities and has made significant investments in vaccines, antiviral medications, and research, which will safeguard our Nation and benefit the world. The unprecedented efforts to prepare for and respond to the threat of a pandemic underscore the Federal Government's resolve to protect human life.
Resiliency defines us as a Nation. Each American generation has faced and has risen to the challenges of its time. But no prior generation has ever anticipated and prepared for a pandemic. We have the opportunity to be the first generation to use our collective wisdom, determination, and resources to take on this task. The stakes are high, and our greatest enemy is complacency. We remain committed to this effort, not only for generations of Americans alive today, but also for the sake of generations to come.
Acknowledgements
This One Year Summary provides an overview of the steps that the Federal Government has taken to prepare the Nation for an influenza pandemic. While these accomplishments are credited to the U.S. Government, they result from the coordinated efforts of many Federal departments and agencies. It is not possible to capture the full depth and breadth of those activities in a report of this nature, but the following provides a very brief summary of some of them.
The Department of Agriculture (USDA) is conducting surveillance for influenza in domestic animals and animal products, monitoring wildlife in coordination with the Department of the Interior (DOI), and working to ensure an effective veterinary response to a domestic animal outbreak of highly pathogenic avian influenza. As part of an international effort, USDA has provided education, research, monitoring, and eradication assistance to affected countries to slow the spread of highly pathogenic avian influenza H5N1 overseas. USDA, in coordination with other key Federal and State departments and agencies as well as industry partners, has developed a detailed response plan to contain and eliminate a domestic outbreak of highly pathogenic avian influenza.
The Department of Commerce (DOC) is promoting pandemic preparedness across the private sector, both domestically and internationally, by creating guidance and executing outreach. DOC, in coordination with the Department of Health and Human Services (HHS), is playing a key role in pandemic preparedness in the Pacific Rim through leadership in the Asia Pacific Economic Cooperation (APEC) forum. Over the past year, DOC, in coordination with the Department of Homeland Security (DHS), has worked with private sector, academic, and government organizations to promote critical infrastructure preparedness. In addition, DOC has been actively involved, in coordination with the Department of the Treasury and other economic agencies, in evaluating the economic effects of proposed government policies meant to minimize the spread of pandemic influenza.
The Department of Defense (DOD) is working to protect American interests at home and abroad in the event of a pandemic. DOD supports the Department of State (DOS) in international engagement to promote global capacity to address an influenza pandemic and provides up-to-date information and pandemic risk-level assessments as an integral part of the global surveillance network for pandemic influenza. Domestically, DOD supports DHS and HHS in developing, planning, and exercising national and regional response efforts. In order to maintain readiness and to promote international efforts, geographic combatant commands continue to exercise, refine, and review their existing plans with Federal departments and agencies and international partners.
The Department of Education (ED) is working with schools to ensure that they have the proper tools to assist in mitigating the spread of a pandemic by preparing students, families, and staff. ED has convened expert roundtables, created guidance, provided resources, conducted outreach, and produced information campaigns for education stakeholders.
The Department of Health and Human Services is responsible for the overall coordination of the public health and medical emergency response during a pandemic. HHS, in coordination with other Federal departments and agencies, has provided guidance, conducted training and outreach, and developed tools critical to pandemic preparedness and response, at home and abroad. International efforts include establishment of regionally deployable response teams and provision of technical assistance with investigation of human H5N1 outbreaks. HHS has led initiatives to strengthen disease detection and surveillance, provide guidance on community mitigation measures to reduce the spread and impact of a pandemic, enhance medical capacity to care for large numbers of infected people, and acquire and accelerate distribution of countermeasures from the Strategic National Stockpile. HHS continues to support the establishment of a robust domestic capacity to manufacture influenza vaccine, foster the incorporation of modern vaccine production methods, support advanced development of dose-sparing technologies, and acquire a stockpile of pre-pandemic vaccine.
The Department of Homeland Security is responsible for the coordination of the overall Federal response during an influenza pandemic. DHS has pre-designated the Pandemic Influenza Principal Federal Official (PFO) and Regional PFOs for coordinating this response. DHS is ensuring continuity of government operations, the integrity of the Nation's critical infrastructure, and domestic security and is working with HHS to implement scalable border measures during a pandemic. Initiatives include outreach to governmental organizations, the private sector, and critical infrastructure entities, through education and guidance development as well as continued development of an integrated biosurveillance system. In coordination with other Federal departments and agencies, DHS is developing a coordinated government-wide concept of operations plan for response to a severe pandemic.
The Department of the Interior is enhancing surveillance systems for identifying wildlife that may be carrying highly pathogenic avian influenza H5N1. In coordination with USDA, DOI has trained hundreds of biologists over the past year in sample and data collection and entry techniques for wild birds and effective monitoring of wild bird mortality. In addition, DOI continues to work with other Federal departments and agencies, academic institutions, non-governmental organizations, private citizens, and the media to raise awareness and enhance our understanding of and response to avian influenza.
The Department of Justice (DOJ) is enhancing pandemic preparedness across the justice system to ensure continuity of public safety and the justice system during a pandemic. Over the past year, DOJ has developed best-practices guidance, held a number of pandemic preparedness seminars, and worked in coordination with DHS and the Department of Transportation (DOT) to ensure the safety and functioning of the first responder community in the event of a pandemic.
The Department of Labor (DOL) is promoting employer preparedness to protect the health, safety, and welfare of the Nation's workforce as an essential component of maintaining critical infrastructure and economic security during a pandemic. Over the past year, DOL has issued guidance, developed in consultation with HHS, to help America's employers meet their responsibility to protect their employees during a pandemic. DOL has also issued guidance targeted to employers and employees in the healthcare industry, a workforce that is indispensable to the Nation's pandemic response.
The Department of State is leading and coordinating international efforts including U.S. engagement in a broad range of bilateral and multilateral initiatives that build cooperation and capacity to fight the spread of avian influenza and to prepare for a possible pandemic. These initiatives include the International Partnership on Avian and Pandemic Influenza, which has elevated pandemic influenza on national agendas and mobilized resources for the global effort, the North American Plan for Avian and Pandemic Influenza, and information on pandemic preparedness to American citizens overseas. DOS has also carried out public diplomacy activities through training foreign journalists, and sending experts abroad to speak to medical and veterinary health professionals, government officials, and the media.
The Department of Transportation is working to ensure that coordinated actions are taken by the transportation sector to limit spread of infection while preserving the movement of essential goods and services and limiting the impact of the pandemic on the economy. Over the last year, DOT has worked with the Emergency Medical Services (EMS) and 9-1-1 community to develop protocols and guidelines, conducted outreach and education campaigns for transportation stakeholders, and worked closely with DHS and other border stakeholders to plan for transportation and border measures.
The Department of the Treasury (Treasury) is working to ensure that the financial sector is prepared for a pandemic by communicating guidance, conducting outreach and education campaigns, and executing exercises with financial sector stakeholders. In addition, Treasury is preparing policy responses to pandemic-related international economic developments; for example, the Department is leading the Federal Government's engagement with the multilateral development banks (MDB) and international financial institutions (IFI), including encouraging MDB and IFI efforts to assist countries to address the threat of pandemic influenza.
The Department of Veterans Affairs (VA) operates the largest Federal integrated healthcare system and has increased pandemic preparedness by enhancing surveillance, stockpiling scarce resources, contributing to Federal Government efforts to develop critical guidance for healthcare facilities and healthcare workers, and participating in State and community-level preparedness activities. VA organized and exercised a leadership and incident command structure and created an advisory panel of experts in pandemic influenza and VA operations.
The Environmental Protection Agency (EPA) is assisting DHS and HHS in preparing the Nation's water sector (drinking water and wastewater) utilities by working to increase preparedness and maintain essential services in the event of an avian influenza or human pandemic influenza outbreak. EPA has engaged Federal, State, and local partners in order to develop and promote the establishment of mutual aid and assistance agreements so that water sector utilities can appropriately assist each other in time of need.
The Office of Personnel Management (OPM) is working to ensure that Federal departments and agencies have the workforce needed to complete critical missions during a pandemic. OPM has conducted outreach to Federal human capital stakeholders, created guidance for and provided technical assistance to human resources offices within Federal agencies, and designed pandemic-related educational materials and programs, including a new telework guide, specifically for Federal employees.
The United States Agency for International Development (USAID), in partnership with HHS and USDA, is assisting countries at risk, including those experiencing outbreaks of highly pathogenic avian influenza H5N1, in increasing public awareness and developing surveillance and diagnostic capacity at national, regional, and local levels toward timely notification of suspected cases of avian influenza. USAID has developed a stockpile of essential commodities for use by surveillance and response workers for effective disease containment during outbreaks and has shipped these commodities to over 70 countries to date.


