|
Introduction to ACCE
ACCE, which takes its name from the four components of evaluation—analytic validity, clinical validity, clinical utility and associated ethical, legal and social implications—is a model process for evaluating data on emerging genetic tests. The process includes collecting, evaluating, interpreting, and reporting data about DNA (and related) testing for disorders with a genetic component in a format that allows policy makers to have access to up-to-date and reliable information for decision making.
An important by-product of this process is the identification of gaps in knowledge. The ACCE approach builds on a methodology originally described by Wald and Cuckle (1) and on terminology introduced by the Secretary's Advisory Committee on Genetic Testing (2).
Additional information and ACCE reports are available here. |
|
Components of ACCE
The ACCE wheel (Figure 2) shows the relation among each of the four components of evaluation and the elements of each component. At the hub are the clinical disorder being evaluated and the setting in which testing is done (e.g., classic cystic fibrosis in the setting of prenatal screening). The evaluation process begins only after the clinical disorder and setting have been clearly established. Specific questions 1 through 7 in Table 1 help to define the disorder, the setting, and the type of testing.
|
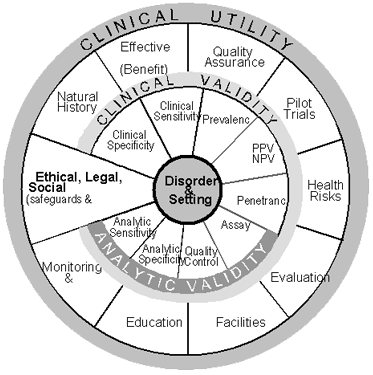 Figure 1
Figure 1. The ACCE evaluation process for genetic testing |
The analytic validity of a genetic test defines its ability to accurately and reliably measure the genotype of interest. This aspect of evaluation focuses on the laboratory component. The four specific elements of analytic validity include analytic sensitivity (or the analytic detection rate), analytic specificity, laboratory quality control, and assay robustness. Analytic sensitivity defines how effectively the test identifies specific mutations that are present in a sample. Analytic specificity defines how effectively the test correctly classifies samples that do not have specific mutations (although the term “mutation” is used here, the terms “polymorphism” or “variant” may be more appropriate for certain situations). Quality control assesses the procedures for ensuring that results fall within specified limits. Robustness measures how resistant the assay is to changes in pre-analytic and analytic variables. Specific questions 8 through 17 in Table 1 help organize the information available to document analytic validity.
The clinical validity of a genetic test defines its ability to detect or predict the associated disorder (phenotype). The four elements of analytic validity are all relevant to assessing clinical validity, along with six additional elements: clinical sensitivity (or the clinical detection rate), clinical specificity, prevalence of the specific disorder, positive and negative predictive values, penetrance, and modifiers (gene or environmental). Penetrance defines the relation between genotype and phenotype and allows the frequency of the clinical expression of a genotype (expressivity) to be determined. Clinical sensitivity measures the proportion of individuals who have a well-defined clinical disorder (or who will get the disorder in the future) and whose test values are positive. Clinical specificity measures the proportion of individuals who do not have the well-defined clinical disorder and whose test results are negative. Prevalence measures the proportion of individuals in the selected setting who have, or who will develop, the phenotype. The positive and negative predictive values more meaningfully define the genetic test performance by taking into account clinical sensitivity, clinical specificity and prevalence. Specific questions 18 through 25 in Table 1 help organize the information available to document clinical validity.
The clinical utility of a genetic test defines the elements that need to be considered when evaluating the risks and benefits associated with its introduction into routine practice. The natural history of the specific disorder needs to be understood so that such considerations as optimal age for testing might be taken into account. Another factor to be considered is the availability and effectiveness of interventions aimed at avoiding adverse clinical consequences (if no interventions are available, for example, testing may not be warranted). Quality assurance assesses procedures in place for controlling pre-analytic, analytic, and post-analytic factors that could influence the risks and benefits of testing. Pilot trials assess the performance of testing under real-world conditions. Health risks define adverse consequences of testing or interventions in individuals with either positive or negative test results. Economic evaluation helps define financial costs and benefits of testing. Facilities assess the capacity of existing resources to manage all aspects of the service. Education assesses the quality and availability of informational materials and expertise for all aspects of a screening service. Monitoring and evaluation assess a program's ability to maintain surveillance over its activities and make adjustments. Specific questions 26 through 41 in Table 1 help organize the information available to document clinical utility.
Ethical, legal, and social implications surrounding a genetic test are represented in Figure 2 by a penetrating pie slice, implying that the safeguards and impediments should be considered in the context of the other components. Specific questions 42 through 44 in Table 1 help organize the information available to document ELSI issues. |
Table 1. The ACCE Model's List of Targeted Questions Aimed at a Comprehensive Review of Genetic Testing (3)
| |
| Disorder/Setting |
|
|
|
| |
|
1.
2.
3.
4.
5.
6.
7. |
What is the specific clinical disorder to be studied?
What are the clinical findings defining this disorder?
What is the clinical setting in which the test is to be performed?
What DNA test(s) are associated with this disorder?
Are preliminary screening questions employed?
Is it a stand-alone test or is it one of a series of tests?
If it is part of a series of screening tests, are all tests performed in all instances (parallel) or are only some tests performed on the basis of other results (series)? |
| Analytic Validity |
|
|
|
|
Sensitivity
Specificity
|
8.
9.
10
11.
12.
13.
14.
15.
16.
17.
|
Is the test qualitative or quantitative?
How often is the test positive when a mutation is present?
How often is the test negative when a mutation is not present?
Is an internal QC program defined and externally monitored?
Have repeated measurements been made on specimens?
What is the within- and between-laboratory precision?
If appropriate, how is confirmatory testing performed to resolve false positive results in a timely manner?
What range of patient specimens have been tested?
How often does the test fail to give a useable result?
How similar are results obtained in multiple laboratories using the same, or different technology? |
| Clinical Validity |
|
|
|
| |
Sensitivity
Specificity |
18.
19.
20. |
How often is the test positive when the disorder is present?
How often is the test negative when a disorder is not present?
Are there methods to resolve clinical false positive results in a timely manner? |
| |
Prevalence
|
21.
22.
23.
24.
25. |
What is the prevalence of the disorder in this setting?
Has the test been adequately validated on all populations to which it may be offered?
What are the positive and negative predictive values?
What are the genotype/phenotype relationships?
What are the genetic, environmental or other modifiers? |
| Clinical Utility |
|
|
|
| |
Intervention
Intervention
Intervention
Intervention
Intervention
|
26.
27.
28.
29.
30.
31. |
What is the natural history of the disorder?
What is the impact of a positive (or negative) test on patient care?
If applicable, are diagnostic tests available?
Is there an effective remedy, acceptable action, or other measurable benefit?
Is there general access to that remedy or action?
Is the test being offered to a socially vulnerable population? |
| |
Quality Assurance |
32. |
What quality assurance measures are in place? |
| |
Pilot Trials
Health Risks
Economic
|
33.
34.
35.
36.
|
What are the results of pilot trials?
What health risks can be identified for follow-up testing and/or intervention?
What are the financial costs associated with testing?
What are the economic benefits associated with actions resulting from testing? |
| |
Facilities
Education
|
37.
38.
39.
|
What facilities/personnel are available or easily put in place?
What educational materials have been developed and validated and which of these are available?
Are there informed consent requirements?
|
| |
Monitoring |
40.
41. |
What methods exist for long term monitoring?
What guidelines have been developed for evaluating program performance? |
| ELSI |
|
|
|
| |
Impediments
Safeguards |
42.
43.
44. |
What is known about stigmatization, discrimination, privacy/confidentiality and personal/family social issues?
Are there legal issues regarding consent, ownership of data and/or samples, patents, licensing, proprietary testing, obligation to disclose, or reporting requirements?
What safeguards have been described and are these safeguards in place and effective? |
|
|
References
- Wald N, Cuckle H. Reporting the assessment of screening and diagnostic tests. Br J Obstet Gynaecol 1989 Apr;96(4):389-96.
- Department of Health and Human Services, Secretary’s Advisory Committee on Genetic Testing. Request for public comment on a proposed classification methodology for determining level of review for genetic tests. Federal Register 2000;65(236):76643-76645.
- Haddow JE, Palomaki GE. ACCE: A Model Process for Evaluating Data on Emerging Genetic Tests. In: Human Genome Epidemiology: A Scientific Foundation for Using Genetic Information to Improve Health and Prevent Disease. Khoury M, Little J, Burke W (eds.), Oxford University Press, pp. 217-233, 2003.
|


