 |
|
 |
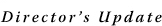

An Occasion to Commemorate
Welcome to the final issue of the NCI Cancer Bulletin for 2006. This issue is very special, not just because it's the conclusion of the Bulletin's third year, but because it's dedicated to an important event: the 35th anniversary of the enactment of the National Cancer Act of 1971 (NCA 1).
On such a momentous occasion, I felt it appropriate to highlight two former members of Congress who have been instrumental in making it possible for NCI to be the worldwide leader in cancer research, a position from which we have been able to accelerate the pace of scientific progress.
Read more 2 

35 Years of Progress
 December 23, 2006, marks the 35th anniversary of the passage of the NCA, a landmark statute that committed the United States' will and resources toward reducing the burden of cancer, and entrusted the leadership of that effort to NCI.
December 23, 2006, marks the 35th anniversary of the passage of the NCA, a landmark statute that committed the United States' will and resources toward reducing the burden of cancer, and entrusted the leadership of that effort to NCI.
The NCA was passed despite federal spending constraints, in a year when NCI's budget was less than $200 million. NCI Director Dr. Carl G. Baker had addressed a special Senate panel, chaired by Senator Ralph Yarborough (D-Tex.), earlier that year. The concerns he expressed, as well as the bold vision he laid out for the panel, were eventually encompassed in the NCA. This Act established a greater Executive Branch relationship with the National Cancer Program, converting the position of NCI director to a Presidential appointment and mandating that the NCI director administer the National Cancer Program with the advice of a President-appointed National Cancer Advisory Board (NCAB). In addition, the NCA authorized NCI to submit its annual budget request (the "bypass budget") directly to the President. Read more 3 
 |
The NCI Cancer Bulletin is produced by the National Cancer Institute (NCI). NCI, which was established in 1937, leads the national effort to eliminate the suffering and death due to cancer. Through basic, clinical, and population-based biomedical research and training, NCI conducts and supports research that will lead to a future in which we can identify the environmental and genetic causes of cancer, prevent cancer before it starts, identify cancers that do develop at the earliest stage, eliminate cancers through innovative treatment interventions, and biologically control those cancers that we cannot eliminate so they become manageable, chronic diseases.

For more information on cancer, call 1-800-4-CANCER or visit http://www.cancer.gov.

NCI Cancer Bulletin staff can be reached at ncicancerbulletin@mail.nih.gov.
|
|
 |
 |
An Occasion to Commemorate
Welcome to the final issue of the NCI Cancer Bulletin for 2006. This issue is very special, not just because it's the conclusion of the Bulletin's third year, but because it's dedicated to an important event: the 35th anniversary of the enactment of the National Cancer Act of 1971 (NCA 4).
On such a momentous occasion, I felt it appropriate to highlight two former members of Congress who have been instrumental in making it possible for NCI to be the worldwide leader in cancer research, a position from which we have been able to accelerate the pace of scientific progress.
One of those people is a man who, these days, works just blocks from the White House, former Representative Paul Rogers. Among the pictures, letters, plaques, and other mementos of his career as a public servant that adorn his office is a black-and-white photo that shows Rep. Rogers and fellow Congressional leaders gathered around President Nixon during the signing of the National Cancer Act, a bill that brought sweeping changes and unprecedented new authorities to NCI.
That landmark legislation, in fact, came to pass, in large part, because of Rep. Rogers' perseverance. In the summer of 1971, he was chair of the House Subcommittee on Health and the Environment, which was considering a bill passed by the Senate known as the "Conquest of Cancer Act." This bill would have, in effect, made NCI an independent agency inside the National Institutes of Health. Rep. Rogers, who was under pressure from Republicans and Democrats alike to move quickly and pass the bill, had doubts. He thought the bill, by granting NCI such a unique position within NIH, could lead to its breakup. That, he believed, would be tragic.
He convened weeks of hearings and marshalled support from the scientific community to help "educate the House and the public on the matter of cancer and how it should be handled." The bill that was enacted is an example of the best of American bipartisanship.
It was a similar spirit in the Senate - spurred by deep personal experience - that led former Senator Connie Mack, in the late 1990s, to champion the doubling of the NIH budget. Senator Mack's brother died from melanoma, he and his other brother had been treated for melanoma, and his wife and daughter battled cancer. So he has a personal understanding of the toll cancer can take.
"I have a picture on my desk at home of three little boys. I think we would have been 3, 4, and 5," he says. "It's a pretty dramatic reminder to me…about what we've experienced. It also gives me hope that we're going to be able to find a cure, but at the same time, a deep sense of frustration about how much more we have to do."
Sen. Mack's experience led him to the realization that it was time for a greater investment in research, particularly cancer, and he worked to bring more resources to the NIH and NCI.
As is often the case, it's individuals, and the policies or activities or movements they help start and sustain, that do the most good. And when the spirit to make a difference exhibited by people like Paul Rogers and Connie Mack takes hold, the true beneficiaries are not institutes and agencies, but healthier people.
I hope you enjoy this commemoration of the National Cancer Act and a look back at 35 years of progress. Have a joyful and safe holiday season.
Dr. John E. Niederhuber
Director, National Cancer Institute
|
 |
 |
 December 23, 2006, marks the 35th anniversary of the passage of the NCA, a landmark statute that committed the United States' will and resources toward reducing the burden of cancer, and entrusted the leadership of that effort to NCI.
December 23, 2006, marks the 35th anniversary of the passage of the NCA, a landmark statute that committed the United States' will and resources toward reducing the burden of cancer, and entrusted the leadership of that effort to NCI.
The NCA was passed despite federal spending constraints, in a year when NCI's budget was less than $200 million. NCI Director Dr. Carl G. Baker had addressed a special Senate panel, chaired by Senator Ralph Yarborough (D-Tex.), earlier that year. The concerns he expressed, as well as the bold vision he laid out for the panel, were eventually encompassed in the NCA. This Act established a greater Executive Branch relationship with the National Cancer Program, converting the position of NCI director to a Presidential appointment and mandating that the NCI director administer the National Cancer Program with the advice of a President-appointed National Cancer Advisory Board (NCAB). In addition, the NCA authorized NCI to submit its annual budget request (the "bypass budget") directly to the President.
The NCA also established a three-member President's Cancer Panel (PCP) to monitor and evaluate the National Cancer Program through periodic public hearings and an annual progress report.
Finally, NCA established 15 new centers for clinical research, training, and demonstration of advanced diagnostic and treatment methods, and it positioned NCI to facilitate regional cancer control programs, in cooperation with the states and other health agencies. Around the same time that he signed the NCA, President Nixon converted the U.S. Army's germ warfare facilities at Frederick, Md., into a cancer research facility, which now houses part of NCI's Center for Cancer Research 5 (CCR).
The NCA has been amended a number of times since 1971 to expand the authority of NCI, with a stronger emphasis on cancer control and research. NCI now has an annual budget of close to $4.8 billion, fueling programs that are among the most extensive and innovative in the world.
For example, NCA's original provisions for 15 Cancer Centers 6 has grown into today's network of 61 nationwide. At these centers, as well as other institutions and on the NIH campus in Maryland, NCI has trained tens of thousands of people from the United States and abroad.
NCI has synergized cancer research worldwide through its Office of International Affairs 7, coordinating research consortia and conferences, and supporting research activities in more than 60 countries. The results in the last 35 years: NCI-sponsored research activities domestic and abroad have produced 227,000 published journal articles (as indexed in PubMed) - more than 10,000 this year alone.
Beyond basic and clinical research, NCI's extensive communication efforts 8 are rooted in the NCA, which initially directed NCI to develop programs for rapid dissemination of cancer research to the international scientific community. This was later broadened to include cancer patients and their families, health professionals, and the public. Philanthropist and patient advocate Mary Lasker 9 is credited with a major role in enacting the NCA, and her legacy is carried on today by the growing legions of patient advocate representatives actively involved in the National Cancer Plan through NCI's Office of Liaison Activities 10.
The fiscal challenges NCI is currently facing hearken back to the year that the NCA was signed. But NCI Director Dr. John E. Niederhuber has affirmed the institute's commitment to the legislation's original promise: "It is our responsibility to continually earn, and always merit, the public's trust," he said. "By investing in the three research spaces - the chemical, biological, and translational - and using powerful new technologies to integrate the discoveries from this investment, we will be able to generate better outcomes for all cancer patients and reduce the burden of cancer."
By Bill Robinson
|
 |
 |
 Paul Rogers was chairman of the House Subcommittee on Health and the Environment from 1971 to 1978, and was instrumental in passing the National Cancer Act of 1971 and the National Health Promotion and Disease Prevention Act of 1978. He spoke with Richard Folkers of NCI's Office of Communications about the National Cancer Act. Paul Rogers was chairman of the House Subcommittee on Health and the Environment from 1971 to 1978, and was instrumental in passing the National Cancer Act of 1971 and the National Health Promotion and Disease Prevention Act of 1978. He spoke with Richard Folkers of NCI's Office of Communications about the National Cancer Act.
The signing ceremony of the National Cancer Act on December 23, 1971, was an amazing moment of American bipartisanship.
It's the way we operated in those days.
What strikes you, as you look back on that day?
It went well, and everybody felt good about it. President Nixon said that he wanted to mount a drive and proposed $100 million. We finally worked out the differences between the two bodies, and I think everybody was pleased at that time with the actions taken and what should be done. We knew it would have to require good administration from the National Cancer Institute.
Why did the bill emphasize cancer centers?
We put in the bill provisions to set up geographically located cancer centers so that there would be comprehensive centers all over the nation. We wanted to do that so any information garnered here at NCI could immediately be transmitted to those cancer centers and they, in turn, could put that information to use, but also transmit it to local hospitals, so that people would begin getting upgraded care as soon as they got into the system. I think the care has been upgraded in the nation as a result of the designated Cancer Centers Program.
As a member of Congress, would a day ever go by when you didn't get a request for greater disease research funding?
Well, it's always a constant demand for more dollars. That still occurs, as you know. I think what finally resolved this was when they started saying, "Well, we will try to appropriate monies to go to institutes, and let the institute director and that facility out there decide where the priorities are."
In 1971, the word "survivor" was not used that often. How far have we come?
The knowledge has expanded tremendously from those days. And, of course, when we increased funding, that attracted so many other scientists. It was inevitable that the knowledge was going to come forth. I think NCI has handled things very well, in not being pressured just to go one particular route or the other, where they've taken a broader view of what is significant and what needs to be looked at and developed. I hope that will continue. I think it will.
|
 |
 |
In 1971, cancer epidemiology played an important, if underappreciated, role in NCI research. Studies rarely involved more than a few hundred participants, and were largely directed at how patterns of cancer risk in specific populations were affected by environmental and lifestyle factors.
Today, the molecular epidemiologist has a variety of tools, technologies, and opportunities. NCI has played a significant role in this story, designing important studies of familial cancer and providing ongoing support and direction for a national program in epidemiological research.
 11
"The advent of major advances in molecular science and technology offers the epidemiologist a chance to overcome some weaknesses of the classical approaches of the past," said Dr. Robert N. Hoover, director of the Biostatistics and Epidemiology Program in NCI's Division of Cancer Epidemiology and Genetics (DCEG) 12. This includes better measurement of exposures and outcomes, detection of susceptible subgroups, and insights into the biological mechanisms of disease. 11
"The advent of major advances in molecular science and technology offers the epidemiologist a chance to overcome some weaknesses of the classical approaches of the past," said Dr. Robert N. Hoover, director of the Biostatistics and Epidemiology Program in NCI's Division of Cancer Epidemiology and Genetics (DCEG) 12. This includes better measurement of exposures and outcomes, detection of susceptible subgroups, and insights into the biological mechanisms of disease.
A clear example of this process is the discovery that infection due to the human papillomavirus is responsible for most cases of cervical cancer. By learning about the etiology and epidemiology of this disease, basic researchers in NCI's CCR were able to develop a vaccine that was licensed for clinical use this year.
To maximize the value of powerful new genomic tools, epidemiologists are taking a "more transdisciplinary team-based approach, which NCI is actively promoting through a number of strategic partnerships," said Dr. Joseph F. Fraumeni, Jr., DCEG director.
One such partnership is the Consortium of Cohorts 13, an international collaboration of intramural and extramural investigators managing 23 independently funded population cohorts that encompass 1.2 million individuals who have provided biospecimens including germline DNA. As cancers arise among these people, nested case-control studies should help evaluate molecular and biochemical biomarkers of susceptibility and early-stage disease.
In another initiative, Dr. Hoover, Dr. Gilles Thomas, and CCR's Dr. Stephen Chanock, who directs the NCI Core Genotyping Facility, are co-leading the Cancer Genetic Markers of Susceptibility (CGEMS) 14 project, along with Dr. David Hunter of the Harvard School of Public Health, to identify genetic alterations that make people susceptible to breast and prostate cancers. Here, too, nested case-control studies will be based on large, ongoing population cohort studies.
Since one of the limitations of genetic epidemiology in the past was testing for a specific, suspected risk factor, CGEMS represents an evolving model that will transform the approach and power of epidemiology. "Now we can look at nearly all regions of the genome for common genetic variants," said Dr. Chanock. "We're sure to uncover associations with cancer that will surprise us, and unraveling the science behind them is likely to have clinical payoffs because epidemiology is based on actual disease patterns in human populations."
|
 |
 |
 The unprecedented commitment to basic and clinical cancer research that came about through the NCA ushered in a revolutionary understanding of the molecular and cellular biology of cancer, which in turn has launched a new approach to treatment based on therapeutic targeting of molecular markers unique to cancer cells.
The unprecedented commitment to basic and clinical cancer research that came about through the NCA ushered in a revolutionary understanding of the molecular and cellular biology of cancer, which in turn has launched a new approach to treatment based on therapeutic targeting of molecular markers unique to cancer cells.
"If you go back 35 years, much of what is now commonplace was not known," said Dr. Thomas Waldmann, chief of the Metabolism Branch in NCI's CCR. With little support for basic science research available from industry prior to its signing, the NCA provided the funds necessary for investigators to commit their careers to elucidating the biological causes of cancer.
One early initiative funded under the NCA was the Cancer Virus Program, which resulted in the discovery of the first oncogenes. "What really emerged from the Cancer Virus Program was [an understanding of] molecular events that underlie the pathogenesis of cancer," explained Dr. Waldmann. "These, coupled with the results of the Human Genome Project, gave us new molecular targets for therapy, and we are just beginning to see the impact of these new targets, with drugs such as Gleevec and Herceptin."
Identifying these targets requires a large-scale, long-term commitment that was provided by the NCA. "It took us nearly 10 years to identify our first kidney cancer gene - the VHL gene," said Dr. Marston Linehan, chief of CCR's Urologic Oncology Branch, "but the subsequent elucidation of the function of that cancer gene's product has enabled the development and use of therapeutic agents to target the product and its pathway. Two such agents were approved by the FDA within the past year for the treatment of this disease."
Along with extensive support for laboratory science, the NCA has provided for a vast expansion in clinical trials to test new therapies, including the growth of large-scale cooperative groups that pool investigators and patients across the country to amass the data needed for confidence in the answers to clinical questions under study.
 |
 |
NCI Cancer Bulletin
Publication Break |
 |
| The NCI Cancer Bulletin will not be published on December 9 and 26. We will resume publication on our usual schedule with the January 2 issue. |
|
Over 20,000 patients a year now join clinical trials run by members of the Clinical Trials Cooperative Group Program. Funding provided by the NCA supports programs such as the Community Clinical Oncology Program and the Cancer Trials Support Unit, which allow community physicians and their patients to participate in clinical research projects. It also supports research at the NIH Clinical Center, which manages a broad selection of clinical trials that test new cancer agents.
"I think translational research emerged through this act," said Dr. Waldmann. "Today, translational research is a buzzword, but it didn't really exist before then. Now, with the NIH Clinical Center so enormously powerful in allowing this kind of science, we can do basic science and translational research with something that's made here at the NIH. Then after it's approved by the FDA for use in a trial, we get to see the first results in our own patients."
|
 |
 |
 One of NCI's basic strategies in the campaign against cancer is to gather the best information about the disease and share it with researchers everywhere. This strategy was codified in the NCA, in which the NCI Director was charged with collecting, analyzing, and disseminating all data useful in the prevention, diagnosis, and treatment of cancer to researchers worldwide.
One of NCI's basic strategies in the campaign against cancer is to gather the best information about the disease and share it with researchers everywhere. This strategy was codified in the NCA, in which the NCI Director was charged with collecting, analyzing, and disseminating all data useful in the prevention, diagnosis, and treatment of cancer to researchers worldwide.
In the decades since the NCA was signed into law, additional legislation, such as the Public Health Service Act of 1996 15, broadened the scope of NCI's information sharing activities to include information for health professionals, patients and their families, and the public at large.
Today, NCI and its partners manage resources such as the Cancer Information Service 16, which offers confidential answers to specific questions about cancer. Information specialists respond to questions over the telephone, by e-mail, and via the Internet (LiveHelp at http://www.cancer.gov).
Another resource is the SEER (Surveillance, Epidemiology, and End Results) 17 database of cancer statistics, which contains the latest figures on cancer incidence and mortality in the United States. These statistics are the basis for many studies reported in the news, such as the Annual Report to the Nation on the Status of Cancer 18.
The public can view SEER data online as part of interactive maps and graphs 19 that show geographic patterns and time trends in cancer mortality in the United States.
NCI's oldest and main source of cancer information is the comprehensive Physician Data Query 20 (PDQ) database. Information in this database, which is accessible online, provides physicians and the public with information summaries 21 on cancer treatment, supportive care, screening, prevention, genetics, and complementary and alternative medicine, as well as an international registry 22 of cancer clinical trials and many other features. Available through Cancer.gov and the Web sites of several NCI licensing partners, PDQ information also has been translated into foreign languages, including Spanish and Japanese.
An early version of PDQ was the first online database of medical information for a particular disease. In 1982, health professionals accessed it through a dial-up connection to the National Library of Medicine 23. PDQ first became available on the World Wide Web in 1995.
As technology has advanced, so have the tools for information sharing. Some oncologists 24 are now using handheld computer devices to retrieve cancer information and identify open clinical trials during office visits with patients. And Cancer Control PLANET (http://cancercontrolplanet.cancer.gov) is a Web portal that provides easy access to data and research-based resources that can help state and local cancer control program planners and staff, and cancer prevention and control researchers to design, implement, and evaluate evidence-based cancer control programs.
The next frontier in cancer information sharing is an NCI initiative called caBIG 25™ (the cancer Biomedical Informatics Grid™). Launched in 2004, caBIG™ facilitates the sharing of data, tools, and knowledge by researchers and institutions. NCI is using this bioinformatics platform to build a network across the cancer community that supports the free exchange of information and ideas.
"caBIG™ can transform every aspect of cancer research," says NCI Deputy Director Dr. Anna Barker. "It is an information initiative for the 21st century with the potential to speed discoveries and improve the care of patients by linking researchers, physicians, and patients throughout the cancer community."
|
 |
 |
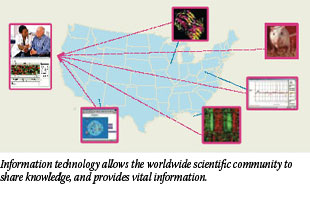
Cancer researchers have worked hard on ways to detect, treat, and prevent cancer during the last 35 years. But ironically, cancer incidence has gone up during this time, from approximately 400 per 100,000 persons in 1975 (according to data collected by NCI's SEER program) to a peak of nearly 510 per 100,000 in 1992; since then, it has effectively stabilized.
However, cancer incidence trends are not always easy to interpret as a measure of success - at least not in the short term. This is because changes in incidence are a function of many factors, including the increased use of screening, the introduction of new diagnostic technologies, and changes in the risk factor profile of the U.S. population, according to Dr. Eric J. (Rocky) Feuer, chief of NCI's Statistical Research and Applications Branch.
 26
"Due to screening and new diagnostic techniques, we're detecting cancer earlier than we used to," Dr. Feuer explains. "The introduction of new screening technologies causes the incidence to go up temporarily, not because there are necessarily more cases, but because we are finding cases earlier than if we waited for symptoms to develop. Although it is somewhat counterintuitive, a rise in incidence associated with screening can be a positive sign, since early detection coupled with effective treatment has the potential to extend life or even cure patients." 26
"Due to screening and new diagnostic techniques, we're detecting cancer earlier than we used to," Dr. Feuer explains. "The introduction of new screening technologies causes the incidence to go up temporarily, not because there are necessarily more cases, but because we are finding cases earlier than if we waited for symptoms to develop. Although it is somewhat counterintuitive, a rise in incidence associated with screening can be a positive sign, since early detection coupled with effective treatment has the potential to extend life or even cure patients."
"Changes of this type were particularly evident with prostate cancer," he continues, "when incidence went up significantly between 1988 and 1992 because changing biopsy techniques found more cancers and PSA screening was introduced. Incidence rates fell dramatically after screening rates stabilized and have since returned to a more moderate rising trend."
Dr. Feuer also notes that the risk profile of the U.S. population has changed in the past 35 years and is correlated with genuine changes in incidence rates of some types of cancer. "For example, tobacco use rates for white males started dropping in the late 1950s, leading to a flattening of lung and bronchus cancer incidence rates in the 1980s, followed by a decline starting in the late 1980s, 20 to 30 years after cessation trends changed."
Perhaps a clearer indication of the payoff for our nation's investment in cancer research is the decrease in mortality. U.S. mortality increased from 1975 to 1990, but has been falling since 1994. In addition, mortality rates of the four most common cancers - colorectal, breast, prostate, and lung - which together accounted for more than half of all U.S. cancer deaths in 2003, also showed falling rates.
These cancer mortality trends are linked to earlier screening and better detection methods, but they're also linked to changes in behavior, such as the decrease in smoking, declines in alcohol consumption in the early 1990s, and increases in sun protection in the late 1990s. Dr. Feuer notes, "It takes time to affect mortality rates. Some therapies that began in the 1970s and 1980s [such as adjuvant chemotherapy and hormone therapy for breast cancer] didn't start paying off in reduced mortality until the 1990s."
|
 |
 |
At the time the NCA was passed 35 years ago, the term chemoprevention - using drugs to prevent cancer in those at high risk - had yet to be coined. The first results from a chemoprevention clinical trial wouldn't be published until 1990 (an analog of vitamin A to prevent mouth and throat tumors), but by the end of that decade, the Breast Cancer Prevention Trial 27 would demonstrate that, compared with placebo, tamoxifen cuts the risk of breast cancer nearly in half in women at increased risk, a result that led it to be the first drug approved by the FDA for cancer risk reduction.
Since then, exciting results have also been seen in chemoprevention trials for prostate 28 and colon 29 cancer, while the STAR 30 trial, published earlier this year, showed that the osteoporosis drug raloxifene was as effective as tamoxifen at reducing breast cancer risk, but with fewer serious side effects.
"Medical approaches to cancer prevention have taken hold and are an exciting research frontier," says Dr. Peter Greenwald, director of NCI's Division of Cancer Prevention 31.
As the chemoprevention results demonstrate, the science of prevention has matured tremendously since the NCA was enacted. Improvements in statistical methods, for instance, have allowed researchers to determine with a good degree of certainty whether factors such as alcohol consumption, smoking, dietary and physical activity patterns, workplace exposures, and others increase - or decrease - the risk of certain cancers.
Improvements in technology that have allowed researchers to more closely scrutinize the molecular machinery of cancer have helped identify mutated genes that put a person at increased risk of specific cancers - the most well known and well studied of which may be BRCA1 32 and BRCA2 32 in breast and ovarian cancer.
Meanwhile, for several cancer types, improvements in screening have allowed clinicians to detect cancers, or precancerous conditions, at very early stages, resulting in vastly improved outcomes for many thousands of patients.
Of the advances made over the last 35 years in cancer prevention, the substantial reduction in smoking prevalence has been among the most important.
Dr. Robert Croyle, director of NCI's Division of Cancer Control and Population Sciences 33, cites numerous factors that helped to bring about this reduction, including higher taxes on tobacco products, restrictions on smoking in workplaces and public places, comprehensive state-based tobacco control programs, local and national antismoking campaigns, and effective treatments for nicotine dependence.
"Research funded by NCI has helped us understand that, to be effective, we need to impact the individual and change the environment to support a nonsmoking norm," Dr. Croyle said.
This year marked a truly remarkable prevention milestone: the FDA's approval of an HPV vaccine 34, which protects against the two HPV types that are responsible for 70 percent of all cases of cervical cancer and is the first-ever vaccine designed specifically to prevent cancer.
As Dr. Greenwald stresses, though, the last three decades of cancer prevention research have made one conclusion abundantly clear: "More and more evidence has established that the way you live your life affects your chances of getting cancer.
"The key things most people should do are eat smaller portions of food, establish a balanced diet, limit high-calorie drinks, exercise throughout life, and, of course, don't start smoking and, if you do smoke, stop."
|
 |
|
Table of Links
| 1 | http://www3.cancer.gov/legis/1971canc.html |
| 2 | http://www.cancer.gov/ncicancerbulletin/NCI_Cancer_Bulletin_121206/page2 |
| 3 | http://www.cancer.gov/ncicancerbulletin/NCI_Cancer_Bulletin_121206/page3 |
| 4 | http://legislative.cancer.gov/history/phsa/1971 |
| 5 | http://ccr.nci.nih.gov |
| 6 | http://cancercenters.cancer.gov |
| 7 | http://www.cancer.gov/oia |
| 8 | http://www.cancer.gov/NCICancerBulletin/NCI_Cancer_Bulletin_071205 |
| 9 | http://www.laskerfoundation.org/about/albertmary.html |
| 10 | http://ola.cancer.gov |
| 11 | http://ghr.nlm.nih.gov/ghr/conditionsByCategory/
show/cancers |
| 12 | http://dceg.cancer.gov |
| 13 | http://epi.grants.cancer.gov/Consortia/cohort_mission.html |
| 14 | http://cgems.cancer.gov/index.asp |
| 15 | http://www3.cancer.gov/legis/phsa.html |
| 16 | http://cis.nci.nih.gov |
| 17 | http://seer.cancer.gov |
| 18 | http://www.cancer.gov/ncicancerbulletin/NCI_Cancer_Bulletin_091206/page5 |
| 19 | http://www3.cancer.gov/atlasplus |
| 20 | http://www.cancer.gov/cancertopics/pdq/cancerdatabase |
| 21 | http://www.cancer.gov/cancertopics/pdq/cancerdatabase#summaries |
| 22 | http://www.cancer.gov/cancertopics/pdq/cancerdatabase#clinical_trial |
| 23 | http://www.nlm.nih.gov |
| 24 | http://www.cancer.gov/ncicancerbulletin/NCI_Cancer_Bulletin_031505/page2 |
| 25 | http://cabig.cancer.gov/index.asp |
| 26 | http://progressreport.cancer.gov |
| 27 | http://www.cancer.gov/clinicaltrials/digestpage/BCPT |
| 28 | http://www.cancer.gov/pcpt |
| 29 | http://www.cancer.gov/newscenter/pressreleases/aspirin |
| 30 | http://www.cancer.gov/clinicaltrials/digestpage/STAR |
| 31 | http://www.cancer.gov/prevention |
| 32 | http://www.cancer.gov/cancertopics/factsheet/Risk/BRCA |
| 33 | http://cancercontrol.cancer.gov |
| 34 | http://www.cancer.gov/cancertopics/hpv-vaccines |
|
 |