
|

Notice to Readers: Pneumococcal Conjugate Vaccine Shortage Resolved
Since February 2004, CDC has recommended that
7-valent pneumococcal conjugate vaccine (PCV7), marketed
as Prevnar® and manufactured by Wyeth Vaccines (Collegeville, Pennsylvania), be administered to healthy children on an abbreviated schedule to conserve the limited supply
(1--3). Production capacity has been increased, and supply is
now sufficient to meet the national demand for vaccine on the routine, 4-dose schedule. Effective immediately, CDC, in consultation with the Advisory Committee on Immunization Practices, the American Academy of Family Physicians, and the American Academy of Pediatrics, recommends that providers resume administration of PCV7 according to the
routine schedule (4--6).
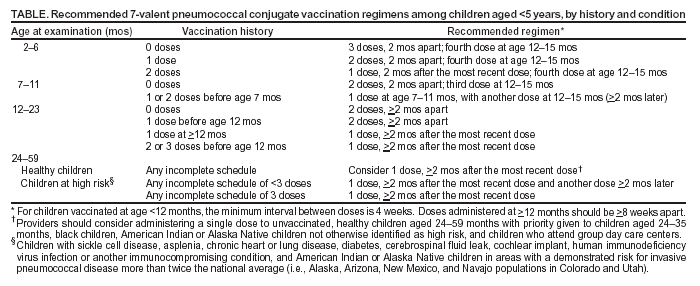
A vaccination schedule is provided for children who are
incompletely vaccinated (Table). The highest priority for
catch-up vaccination is to ensure that children aged <5 years at high risk for invasive pneumococcal disease because of certain
immunocompromising or chronic conditions (e.g., sickle cell disease, asplenia, chronic heart or lung disease,
diabetes, cerebrospinal fluid leak, cochlear implant, or human immunodeficiency virus infection) are fully vaccinated. Second priorities include vaccination of healthy children aged <24 months who have not received any doses of PCV7 and vaccination of healthy children aged <12 months who have not yet received 3 doses.
Because of the frequency of health-care provider visits by children during their first 18 months, catch-up vaccination
might occur at regularly scheduled visits for most children who receive vaccines from their primary-care providers. Programs that provide vaccinations but do not see children routinely for other reasons should consider a notification process to contact undervaccinated children.
Providers with questions about obtaining
Prevnar® should contact Wyeth's customer service department, telephone
800-666-7248. For public-purchased vaccine, including vaccines used in the Vaccines for Children Program, providers
should contact their state/grantee immunization projects to obtain vaccine. These projects should contact their project officers at the National Immunization Program at CDC for information regarding vaccine supply.
References
- CDC. Limited supply of pneumococcal conjugate vaccine: suspension of recommendation for fourth dose. MMWR 2004;53:108--9.
- CDC. Updated recommendations on the use of pneumococcal conjugate vaccine: suspension of recommendation for third and fourth dose.
MMWR 2004;53:177--8.
- CDC. Updated recommendations for use of pneumococcal conjugate vaccine: reinstatement of the third dose. MMWR 2004;53:589--90.
- CDC. Prevention of pneumococcal disease among infants and young children: recommendations of the Advisory Committee on
Immunization Practices. MMWR 2000;49(No. RR-9).
- CDC. Pneumococcal vaccination for cochlear implant candidates and recipients: updated recommendations of the Advisory Committee
on Immunization Practices. MMWR 2003;52:739--40.
- CDC. Recommended childhood and adolescent immunization schedule---United States, July--December 2004. MMWR 2004;53:Q1--3.
Table
Return to top.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication. |
Disclaimer
All MMWR HTML versions of articles are electronic conversions from ASCII text
into HTML. This conversion may have resulted in character translation or format errors in the HTML version.
Users should not rely on this HTML document, but are referred to the electronic PDF version and/or
the original MMWR paper copy for the official text, figures, and tables.
An original paper copy of this issue can be obtained from the Superintendent of Documents,
U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800.
Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to
mmwrq@cdc.gov.
Page converted: 9/16/2004
|

|

