 |
 National
Center for Infectious Diseases
National
Center for Infectious DiseasesSpecial Pathogens Branch
All About Hantaviruses
Technical Information | Contact Us
|
||||||||
 National
Center for Infectious Diseases National
Center for Infectious DiseasesSpecial Pathogens Branch All About Hantaviruses Technical Information | Contact Us |
||||||||
Technical Information
For health care providers, public health professionals, educators, and
others
Printer-Friendly Version
Back to Technical Information Index
Clinical Disease Manifestations of HPS
Discusses the symptoms and development of the HPS syndrome and how
clinicians may diagnose the disease.
Treatment
Discusses treatment of HPS and outlines the rationale for suggested treatment
options.
Histopathology
Explains the characteristic anatomical changes seen in HPS patients at the
microscopic level.
Pathogenesis
Discusses the characteristic mechanisms of disease development in HPS patients.
Diagnostics
Describes the various tests considered diagnostic for HPS.
Epidemiology
Describes demographic characteristics of HPS patients and discusses
risk factors for the disease.
Ecology
Describes the rodent carriers of HPS and their distribution; discusses
transmission of HPS from rodents to humans.
Virology
Discusses the nature and structure of hantaviruses, and the epidemiology
and analysis of the viruses causing HPS.
Prevention
Directs readers to guidelines for prevention of HPS both in the laboratory and
in a workplace or home setting.
Submitting Specimens to CDC
Explains protocol and provides forms for submitting suspected HPS specimens to
CDC.
Technical Questions and Answers
HPS Case Definition
The following case definition was published in:
"Case Definitions for Infectious Conditions Under Public Health Surveillance"
in
Morbidiy and Mortality Weekly Report, May 02, 1997/Vol 46, No RR10;1
Hantavirus Pulmonary Syndrome (Revised 9/96) Clinical description
Hantavirus pulmonary syndrome (HPS), commonly referred to as hantavirus disease,
is a febrile illness characterized by bilateral interstitial pulmonary infiltrates
and respiratory compromise usually requiring supplemental oxygen and clinically
resembling acute respiratory disease syndrome (ARDS). The typical prodrome consists
of fever, chills, myalgia, headache, and gastrointestinal symptoms. Typical
clinical laboratory findings include hemoconcentration, left shift in the white
blood cell
count, neutrophilic leukocytosis, thrombocytopenia, and circulating immunoblasts.
Clinical case definition
An illness characterized by one or more of the following clinical features:
A febrile illness (i.e., temperature greater than 101.0 F {greater than 38.3 C}) characterized by bilateral diffuse interstitial edema that may radiographically resemble ARDS, with respiratory compromise requiring supplemental oxygen, developing within 72 hours of hospitalization, and occurring in a previously healthy person.
An unexplained respiratory illness resulting in death, with an autopsy examination demonstrating noncardiogenic pulmonary edema without an identifiable cause
Laboratory criteria for diagnosis
Detection of hantavirus-specific immunoglobulin M or rising titers of hantavirus-specific immunoglobulin G,
or
Detection of hantavirus-specific ribonucleic acid sequence by polymerase chain reaction in clinical specimens,
or
Detection of hantavirus antigen by immunohistochemistry.
Case classification Confirmed: a clinically compatible case that is laboratory confirmed
Comment
Laboratory testing should be performed or confirmed at a reference laboratory. Because the clinical illness is nonspecific and ARDS is common, a screening case definition can be used to determine which patients to test. In general, a predisposing medical condition (e.g., chronic pulmonary disease, malignancy, trauma, burn, and surgery) is a more likely cause of ARDS than HPS, and patients who have these underlying conditions and ARDS need not be tested for hantavirus.
Clinical Disease Manifestations
Presentation and First Evaluation
Patients with HPS typically present in a very nonspecific way with a relatively short febrile prodrome lasting 3-5 days. In addition to fever and myalgias, early symptoms include headache, chills, dizziness, non-productive cough, nausea, vomiting, and other gastrointestinal symptoms. Malaise, diarrhea, and lightheadedness are reported by approximately half of all patients, with less frequent reports of arthralgias, back pain, and abdominal pain. Patients may report shortness of breath, (respiratory rate usually 26 - 30 times per minute). Typical findings on initial presentation include fever, tachypnea and tachycardia. The physical examination is usually otherwise normal.
HPS Clinical Presentation
| Most Frequent | Frequent | Other |
| fever | headaches | shortness of breath |
| chills | nausea, vomiting | dizziness |
| myalgias | abdominal pain | arthralgia |
| diarrhea | back or chest pain | |
| cough | sweats | |
| malaise |
The diagnosis is seldom made at this stage, as cough and tachypnea generally do not develop until approximately day seven. Once the cardiopulmonary phase begins, however, the disease progresses rapidly, necessitating hospitalization and often ventilation within 24 hours.
Signs that make a diagnosis of HPS unlikely include rashes, conjunctival or
other hemorrhages, throat or conjunctival erythema, petechiae, and peripheral or
periorbital edema.
Clinical Assessment
If a hantavirus infection is suspected, a CBC and blood chemistry should be repeated every 8 to 12 hours.
A fall in the serum albumin and a rise in the hematocrit may indicate a fluid shift from the patient's circulation into the lungs. The white blood cell count tends to be raised with a marked left shift. The percentage of white blood cells precursors may be as high as 50% and atypical lymphocytes are frequently present, usually at the time of onset of pulmonary edema.
In about 80% of individuals with HPS, the platelet count is below 150,000 units. A dramatic fall in the platelet count may herald a transition from the prodrome to the pulmonary edema phase of the illness.
The most severe cases of HPS develop disseminated intravenous coagulation (DIC), but, unlike the hantavirus-related hemorrhagic fevers (HFRS) seen in Asia, this is uncommon.
Coagulation Abnormalities in HPS
A Fatal Case
| Day 1 | Day 3 | |
| PT | 13.5 (NL) | 29.8 (up) |
| PTT | 32.8 (NL) | >240 (up) |
| Fibrinogen | 144 (down) | |
| Fibrin Split Products | >4000 (up) |
Proteinuria, and mild elevations of transaminases, CPK, amylase, and creatinine have also been reported.
When metabolic acidosis, prolongation of PT and PPT times and rising serum lactate levels develop, the prognosis is poor. Marked renal insufficiency has mainly been noted among cases from the southeastern United States although some degree of renal insufficiency, assessed by elevated serum creatinine levels, has been noted in 15% of all patients.
The combination of atypical lymphocytes, a significant bandemia, and thrombocytopenia
in the setting of pulmonary edema is strongly suggestive of a hantavirus infection.
Disease Development
Within 24 hours of initial evaluation, most patients develop some degree of hypotension and progressive evidence of pulmonary edema and hypoxia, usually requiring mechanical ventilation. The patients with fatal infections appear to have severe myocardial depression which can progress to sinus bradycardia with subsequent electromechanical dissociation, ventricular tachycardia or fibrillation.
Hemodynamic compromise occurs a median of 5 days after symptom onset--usually dramatically within the first day of hospitalization. In contrast to HFRS, overt hemorrhage occurs rarely in HPS, although hemorrhage is occasionally seen in association with disseminated intravascular coagulation. In contrast to septic shock, HPS patients have a low cardiac output with a raised systemic vascular resistance. Poor prognostic indicators include a plasma lactate of greater than 4.0 mmol/L or a cardiac index of less than 2.2 L/min/m2 Whilst pulmonary edema and pleural effusions are common, multiorgan dysfunction syndrome is rarely seen. However, HPS patients sometimes have mildly impaired renal function. Survivors frequently become polyuric during convalescence and improve almost as rapidly as they decompensated.
Differential Diagnosis
The prodromal phase of HPS is indistinguishable clinically from numerous other viral infections. Often the only guide to the etiology of the patient's illness is the blood picture, which may show circulating immunoblasts, which appear as large atypical lymphocytes, and thrombocytopenia. However, unlike other viral infections, HPS patients usually have concurrent left-shifted neutrophilia with circulating myelocytes.
In the cardiopulmonary stage of the disease, the patients have a diffuse pulmonary edema. The most frequent cause for such a picture is silent myocardial infarction so it is important to obtain an ECG and echocardiogram early to aid in the assessment. Intensivists at the University of New Mexico, where many of the patients have been managed, have found that a echocardiogram also helps to distinguish these patients from patients with ARDS as cardiac function is depressed to a much greater degree in the HPS patients and cardiac output does not respond to fluid challenge as it tends to with ARDS.
Infections in the immunocompetent which might present with a non-specific prodrome leading to acute cardiopulmonary deterioration as in HPS include leptospirosis, Legionnaire's disease, mycoplasma, Q fever, chlamydia, and in regions where the organisms are present, septicemic plague, tularemia, coccidioidomycosis and histoplasmosis. Non-infectious conditions such as Goodpasture's syndrome should also be considered. Lack of coryza aids the clinical distinction between HPS and Influenza A infection.
It must be remembered that HPS is relatively uncommon and in the immunocompromised PCP, CMV, cryptococcus, aspergillus and graft vs. host disease are far more likely to be the cause of diffuse pulmonary infiltrates than a hantaviral infection.
Atypical Presentations
Atypical clinical presentations with prominent renal insufficiency have also been reported; therefore, HPS and infection due to Seoul virus, one of the Old World hantaviruses that cause HFRS, should be considered for patients with unexplained febrile nephropathies and appropriate laboratory findings. Asymptomatic illness is rare. However, an increasing number of acutely infected patients who develop either no cardiopulmonary disease or extremely mild pulmonary disease with minimal hypotension have been identified; one such patient was managed successfully as an outpatient.
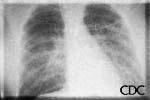
Radiologic Findings
HPS has a characteristic radiological evolution, beginning with minimal changes of
interstitial pulmonary edema, progressing to alveolar edema with severe bilateral
involvement. Pleural effusions are common and are often large enough to be evident
radiographically. Heart size is usually normal. Cardiac silhouette size on chest
radiographs is usually normal.
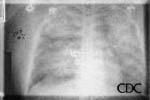
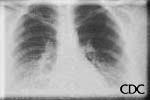
a) b)
 |
 |
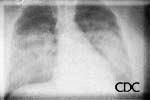 |
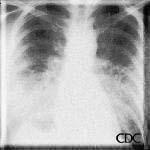
| Severe HPS. | Evolution of HPS, (1). | Evolution of HPS, (2). |
 |
 |
| Evolution of HPS, (3). | Large Effusion Associated With HPS. |
Images courtesy D. Loren Ketai, M.D.
Approximately one-third of patients show evidence of pulmonary edema in the initial
radiograph. Forty-eight hours after the initial radiograph, virtually all patients
demonstrate interstitial edema and two-thirds have developed extensive bibasilar or
perihilar airspace disease.
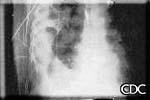
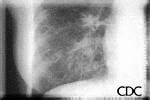
This radiograph shows the interstitial changes of early HPS. At the lung bases are Kerley B lines, short linear opacities which are perpendicular to the pleural surfaces. The longer linear opacities radiating from the lung hilum are known as Kerley A lines. Together these findings are classically seen in heart failure, but are also seen in HPS. Peribronchial cuffing is also seen well on this film. The bronchi viewed end on are surrounded by a "cuff" of edema. This makes the bronchi appear as prominent circular opacities, appearing as "ring-like" shapes next to pulmonary blood vessels.
 |
 |
| Marked interstitial edema with hilar indistinctness, Kerley B lines, in HPS. | Detail showing Kerley B lines. |
Images courtesy D. Loren Ketai, M.D.
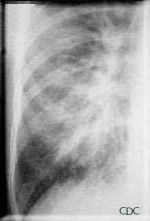
The lack of peripheral distribution of the initial airspace disease, the prominence of
interstitial edema and the presence of pleural effusions early in the disease process help
distinguish HPS from ARDS. There is, however, overlap in the radiographic appearance of
the two diseases. Atypical pneumonias such as that caused by mycoplasm pneumonia can
produce radiographic findings similar to early HPS, although the clinical illness tends
to be much less severe.
 |
 |
| Mycoplasm pneumonia can show prominent interstitial opacities, including Kerley B lines. | Detail of mycoplasm pneumonia. |
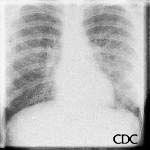
Hyperacute hypersensitivity reactions, mitral stenosis, acute myocardial infarctions, all can cause interstitial edema with a normal heart size, and are also in the radiologic differential diagnosis of early HPS.
 |
| Mitral stenosis can also cause interstitial edema with a normal-sized cardiac silhouette. |
Images courtesy D. Loren Ketai, M.D.
Treatment
There is no specific treatment or cure for hantavirus infection. Treatment of patients with HPS remains supportive in nature. Patients should receive appropriate, broad-spectrum antibiotic therapy while awaiting confirmation of a diagnosis of HPS. Care during the initial stages of the disease should include antipyretics and analgesia as needed.
If there is a high degree of suspicion of HPS, patients should be immediately
transferred to an emergency department or intensive care unit (ICU) for close
monitoring and care. Patients presenting with fulminant illness due to HPS have
a poor prognosis despite ICU care. ICU management should include careful assessment,
monitoring and adjustment of volume status and cardiac function, including inotropic
and vasopressor support if needed. Fluids should be administered carefully due
to the potential for capillary leakage. Supplemental oxygen should be administered
if patients become hypoxic. Equipment and materials for intubation and mechanical
ventilation should be readily available since onset of respiratory failure may
be precipitous.
Intravenous ribavirin, a guanosine analogue, has not been shown to be effective
for treatment of HPS despite its effects on a related disease, hemorrhagic fever
with renal syndrome (HFRS), which is caused by Old World hantaviruses. Controlled
trials showed a reduction in case-fatality for HFRS patients treated with ribavirin.
However, despite in vitro activity of ribavirin against SNV, neither an open-label
trial conducted during the 1993 outbreak nor an attempted placebo-controlled
trial demonstrated clinical benefit for HPS. Ribavirin is not recommended for
treatment of HPS and is not available for this use under any existing research
protocol.
Take-home Message for Care Providers
Rapid transfer to ICU
Careful monitoring
Fluid balance
Electrolyte balance
Blood pressure
Histopathology
No single pathognomonic lesion is found that would permit certain histopathologic diagnosis of HPS. In fact, the incipient stages of ARDS can create a picture of pulmonary edema similar to HPS. However, the total picture is rather distinctive. Pathology in HPS patients is characterized mainly by pulmonary findings, as well as findings in the spleen, liver, and lymph nodes.
Grossly, the lungs are dense, rubbery and heavy, usually weighing twice as much as the average lung. They are often found floating in a pool of yellow serous fluid within the pleural cavity.
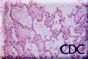 |
Image 1.: Low-power photomicrograph
showing interstitial pneumonitis and intraalveolar edema. |
A.The pathologic lesions are primarily vascular with variable degrees of generalized capillary dilation and edema. Morphologic changes of the endothelium are uncommon and, when present, consist of prominent and swollen endothelial cells. Histopathologic lesions are mainly seen in the lung and spleen. In most cases, the lungs reveal a mild to moderate interstitial pneumonitis with variable degrees of congestion, edema, and mononuclear cell infiltration. The cellular infiltrate is composed of small and enlarged mononuclear cells with the appearance of immunoblasts. Focal hyalin membranes are observed, as well as extensive intraalveolar edema and fibrin. Neutrophils are scanty, and the respiratory epithelium is intact in typical cases, with no evidence of cellular debris, nuclear fragmentation, or type II pneumocyte hyperplasia.
 B. |
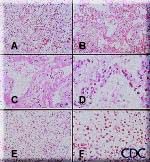
Image 2.: Photomicrographs showing histopathological
features less commonly seen in cases of HPS. A. Lung showing extensive interstitial and alveolar fibrosis. Note the increased interstitial cellularity with numerous fibroblasts. B. Patchy areas of alveolar septal thickening and prominent hyaline membranes. C. Higher magnification showing typical dense laminated hyaline membranes. D. Alveolar septum showing prominent type II pneumocyte proliferation. E. Abundant polymorphonuclear leukocytes fill alveolar spaces with focal destruction of alveolar septa. F. Higher power magnification showing the antraalveolar exudate composed mainly of polymorphonuclear leukocytes, red blood cells, and fibrin. Original magnifications: A & B, x 50; C, x 100; D, x 158; E, x 50; F, x 158. Images courtesy Sherif R. Zaki, MD, Ph.D. |
Among patients who die after a longer-than-average course of the disease, and in lung biopsy specimens from survivors, the histopathologic changes are more characteristic of exudative and proliferative stages of diffuse alveolar damage. In these cases, proliferation of reparative type II pneumocytes, severe edematous and fibroblastic thickening of the alveolar septa with severe airspace disorganization, and distortion of lung architecture can be seen.
 |
Images 3. and 4.: Immunoblasts in the periarteriolar sheath of the spleen. Note prominent nucleoli and high nuclear to cytoplasmic ratio. Images courtesy Sherif R. Zaki, MD, Ph.D. | 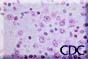 |
Other typical histopathologic findings are seen in lymphoid tissues of HPS patients. These include the presence of immunoblasts within the red pulp and periarteriolar sheaths of the spleen and paracortex, within sinuses of lymph nodes, and in the peripheral blood.
Pathology and Pathogenesis
Immunohistochemistry analysis has shown that viral antigens are distributed primarily within the endothelium of capillaries throughout various tissues from patients with HPS. Marked accumulations of hantaviral antigens are seen in the pulmonary microvasculature and in follicular dendritic cells within the lymphoid follicles of spleen and lymph nodes. Hantaviral nucleic acids can also be localized to endothelial and inflammatory cells in tissues from HPS cases by using in situ hybridization. Electron micrographic studies confirm the infection of endothelial cells and macrophages in the lungs of HPS patients. Typical hantaviral inclusions are seen frequently in pulmonary endothelial cells, and their identity can be confirmed by immunolabeling. In the heart, endothelial staining is mainly in the capillaries of the myocardium and varies from focal immunostaining in some cases to diffuse and extensive staining in others. Occasionally, staining of endothelial cells lining the endocardium is observed.
Functional impairment of vascular endothelium is central to the pathogenesis of HPS.
However, the pathogenesis of HPS is complex, and a myocardial depressant may contribute
significantly to the mortality of this disease. It is unclear how the shock syndrome
relates to factors such as viral distribution and immunologic and pharmacological
mediators of capillary permeability. There appears to be compartmentalization of a
selective immune response in the lungs of HPS patients in combination with extremely high
levels of viral antigens in the pulmonary vasculature. This feature suggests that the
mechanism of inflammatory cell recruitment in the lungs of HPS patients may result from
specific attraction and adherence of a selective population of inflammatory cells to an
activated pulmonary microvascular endothelium.
Diagnostics
A positive serological test result, evidence of viral antigen in tissue by
immunohistochemistry, or the presence of amplifiable viral RNA sequences in blood or
tissue, with compatible history of HPS, is considered diagnostic for HPS.
Serologic Assays
At the time of the 1993 outbreak in the Four Corners area, cross-reactive antibodies to the previously known hantaviruses (e.g., Hantaan, Seoul, Puumala, and Prospect Hill viruses) were found in the acute- and convalescent-phase sera of some of the initial HPS patients. Tests based on specific viral antigens from SNV have since been developed and are now widely used for the routine diagnosis of HPS. CDC uses an enzyme-linked immunosorbent assay (ELISA) to detect IgM antibodies to SNV and to diagnose acute infections with other hantaviruses. This assay is also available in some state health laboratories.
An IgG test is used in conjunction with the IgM-capture test. Acute- and convalescent-phase sera should reflect a four-fold rise in IgG antibody titer or the presence of IgM in acute-phase sera to be diagnostic for hantaviral disease. Note that acute-phase serum sent as an initial diagnostic specimen may not yet have IgG. IgG antibody is long lasting, and sera of patients retrospectively identified appear to have retained antibody for many years. The SNV IgG ELISA has therefore been used in serologic investigations of the epidemiology of the disease and appears to be appropriate for this purpose. Investigations of selected populations using this assay have confirmed that infections with the virus are not common and that mild or inapparent infections are rare.
A Western blot assay using recombinant antigens and isotype-specific conjugates for IgM-IgG differentiation has also been developed and its results are generally in agreement with those of the IgM-capture format.
Epitope mapping of SNV antibodies has been used to identify immunodominant epitopes of 43 and 31 amino acids in the nucleocapsid protein and G1 glycoprotein, respectively. The immunodominant epitope of G1 is conserved among SNV strains from a broad geographical area, despite extensive nucleotide sequence heterogeneity, and this feature constitutes the basis of a type-specific assay for SNV-like antibodies. Antibodies from HPS patients separated by more than 3000 km have been shown to react with the dominant G1 epitope.
Also in use is a rapid immunoblot strip assay (RIBA), an investigational prototype assay to identify serum antibody to recombinant proteins and peptides specific for SNV and other hantaviruses.
Serologic confirmation of hantaviral infections has traditionally been done with
neutralizing plaque assays, which have been recently described for SNV. However, these
specific assays are also not commercially available.
Isolation
Isolation of hantaviruses (see below) from human sources is difficult, and the viruses
causing HPS seem to be no exception to this rule. To date, no isolates of SNV-like viruses
have been recovered from humans, and therefore virus isolation is not a consideration for
diagnostic purposes.
Immunohistochemistry (IHC)
IHC testing of formalin-fixed tissues with specific monoclonal and polyclonal antibodies can be used to detect hantavirus antigens and has proven to be a sensitive method for laboratory confirmation of hantaviral infections. IHC has an important role in the diagnosis of HPS in patients from whom serum samples and frozen tissues are unavailable for diagnostic testing and in the retrospective assessment of disease prevalence in a defined geographic region.
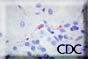 |
Caption: A. High power magnification showing immunostaining of renal interstitial capillaries using Peromyscus serum. Images courtesy Sherif R. Zaki, MD, Ph.D. |
Polymerase Chain Reaction (PCR)
Reverse transcriptase-PCR (RT-PCR) can be used to detect hantaviral RNA in fresh frozen lung tissue, blood clots, or nucleated blood cells. However, RT-PCR is very prone to cross-contamination and should be considered an experimental technique. Differences in viruses in the United States complicate the use and sensitivity of RT-PCR for the routine diagnosis of hantaviral infections.
Epidemiology
Case Characteristics
For the latest counts and descriptive statistics on confirmed cases of HPS in the
United States, please see the HPS Case Information page at
http://www.cdc.gov/ncidod/diseases/hanta/hps/noframes/caseinfo.htm. Information on this
page is updated as the case count changes.
HPS an Old Disease, Newly Recognized
Although the high-profile investigation of the HPS syndrome emphasized public health
authorities' warnings about new and emerging infectious diseases, HPS has turned out to be
a newly identified, but not a "new," disease (see Tracking a Mystery Disease, at
http://www.cdc.gov/ncidod/diseases/hanta/hps/noframes/history.htm). In fact, the earliest
case of a serologically confirmed SNV infection was in a person who developed an
HPS-compatible illness in July 1959 and was found to have IgG antibodies in September
1994. The earliest case of HPS to be confirmed by IHC with direct visualization of
hantaviral antigens in postmortem tissue involved a patient who died in 1978.
Risk Factors for Disease
Little is known about activities that lead to a greater risk of infection. However, an early case-control study suggests that increased numbers of rodents in the household is the strongest risk factor for infection. Entering rarely opened or seasonally closed buildings may also contribute to infection. Among the confirmed cases of HPS for which exposure information is available, 70% of the patients in the case control study had exposures closely associated with peridomestic activities, such as cleaning, in homes that showed signs of rodent infestation. Four clusters of HPS cases involving 2-4 persons have been documented; for each cluster, exposure probably occurred within a shared, enclosed structure. Taken together, these observations suggest that disturbing or inhabiting closed, actively rodent-infested structures may constitute an important risk factor for contracting HPS
Potentially occupationally acquired SNV infections have been recognized but are infrequent. Among documented U.S. cases of HPS, patients with potential occupational exposures have included grain farmers, an extension livestock specialist, field biologists, and agricultural, mill, construction, utility and feedlot workers. Many of these individuals had concurrent peridomestic exposures. Among U.S. mammalogists and rodent workers with varying degrees of rodent exposure, the seroprevalence of SNV antibodies was 1.14%. In contrast, a recent HPS seroprevalence study focused on selected occupational groups with frequent contact with rodents and their excreta (e.g., farm workers, laborers, professionals, home repairers, service industry and park service workers, heating and plumbing contractors, utility workers, and technicians) found no evidence of SNV infection.
Travel to and within all areas where hantavirus infection has been reported is not considered a risk factor for infection with HPS. The possibility of exposure to hantavirus for campers, hikers, and tourists is very small and is reduced further if steps are taken to reduce rodent contact.
Ecology
Reservoir and Reservoir Distribution: United States
All hantaviruses known to cause hantavirus pulmonary syndrome (HPS) are carried by New World rats and mice of the family Muridae, subfamily Sigmodontinae. The subfamily Sigmodontinae contains at least 430 species, which are widespread in North and South America. The rodent hosts of HPS are generally not associated with urban environments, although some species, including the deer mouse, Peromyscus maniculatus, and white-footed mouse, Peromyscus leucopus, will enter human habitation in rural and suburban areas.
Several hantaviruses that are pathogenic for humans have been identified in the United States. In general, each virus has a single primary rodent host. Other small mammals can be infected as well but are much less likely to transmit the virus to other animals or humans. The deer mouse is the host for Sin Nombre virus (SNV), the primary causative agent of HPS in the United States. The deer mouse is common and widespread in rural areas throughout much of the United States. Although prevalence varies temporally and geographically, on average about 10% of deer mice tested throughout the range of the species show evidence of infection with SNV.
Other hantaviruses associated with sigmodontine rodents and known to cause HPS include New York virus, which is hosted by the white-footed mouse; Black Creek Canal virus, which is hosted by the cotton rat, Sigmodon hispidus; and Bayou virus, which is hosted by the rice rat, Oryzomys palustris. Nearly the entire continental United States falls within the range of one or more of these host species. Several other sigmodontine rodent species in the United States are associated with additional hantaviruses that have yet to be implicated in human disease.
Recent studies have confirmed that infected deer mice are present in every habitat type--from desert to alpine tundra, although the prevalence of infection is higher in certain middle-altitude habitats. Surveys of rodents throughout the United States suggest that SNV is distributed in all locations where P. maniculatus is found. Related hantaviruses are also found throughout the geographic range of their rodent carriers. Given that P. maniculatus and P. leucopus are commonly found in the peridomestic setting and typically have higher population densities than other rodents, cases of HPS can be expected to occur throughout the range of these rodent species. Other implicated species, such as S. hispidus and O. palustris, generally do not live in such close proximity to human habitats, and this factor may decrease the probability of human exposure to viruses shed by these rodents.
Lower population density, a lesser propensity for peridomestic encroachment and a narrower geographic and ecologic distribution (and perhaps differing virulence) may explain the lack of human disease associated with hantaviruses (or genetic sequences thought to represent additional hantaviruses) from meadow and California voles (Microtus pennsylvanicus and californicus, respectively), the western harvest mouse (Reithrodontomys megalotis), and the brush mouse (Peromyscus boylii).
Reservoir Distribution Outside the United States
HPS is more common in South America than in North America. Cases have been identified in Argentina, Chile, Uruguay, Paraguay, Brazil, and Bolivia. Andes virus causes HPS in Argentina and Chile and is the only hantavirus known to have been transmitted from person to person. Andes, Bermejo, Hu39694, Lechiguanas, Maciel, Oran, and Pergamino viruses have been linked to HPS cases in Argentina. Bermejo and Laguna Negra virus cause HPS in Bolivia, and Laguna Negra virus is also linked to HPS in Paraguay. Araraquara, Castelo dos Sonhos, and Juquitiba viruses have been associated with HPS in Brazil.
In 1999, an outbreak in Panama marked the first cases of HPS identified in Central America. This outbreak led to the identification of another hantavirus, Choclo virus, which is associated with the rodent host Oligoryzomys fulvescens. The broad geographic distribution of sigmodontine rodents suggests that human cases of HPS will eventually be identified from all countries in the Americas.
Numerous other New World hantaviruses associated with sigmodontine rodents have been identified by molecular methods, but so far, few of them have not been linked to human illness. It is likely that HPS does not occur in the Old World and is confined to the New World distribution of Sigmodontine rodents.
Infection in the Host
Hantaviruses do not cause overt illness in their reservoir hosts. Although infected rodents shed virus in saliva, urine, and feces for many weeks or months or for life, the quantity of virus shed can be at its greatest approximately 3--8 weeks after infection. Field data suggest that transmission in host populations occurs horizontally and that this occurs more frequently among male than female rodents. Transmission from rodent to rodent is believed to occur primarily after weaning and through physical contact, perhaps through aggressive behavior, such as fighting. Studies of the genomic sequences indicate that the virus has probably evolved concurrently with its rodent host over a long period of time.
Occasional evidence of infection (antibody) is found in numerous other species of rodents and their predators (e.g., dogs, cats, and coyotes), indicating that many (perhaps any) mammal species coming into contact with an infected host might become infected. No evidence supports the transmission of infection to other animals or to humans from these "dead-end" hosts. However, domestic cats and dogs may bring infected rodents into contact with humans.
Ticks, fleas, mosquitoes, and other biting insects have not been implicated in the transmission of HPS. Nevertheless, species of Peromyscus in the western United States are susceptible to infection with the plague bacterium (Yersinia pestis), and may act as hosts for plague-carrying fleas. Control of rodents without concurrent control of fleas might therefore increase the risk of human plague as the rodent fleas seek an alternative food source.
Transmission
Human infection occurs most commonly through the inhalation of infectious aerosolized saliva or excreta. Persons visiting laboratories where infected rodents were housed have been infected after only a few minutes of exposure to animal holding areas. Transmission can occur when dried materials contaminated by rodent excreta are disturbed and inhaled, directly introduced into broken skin or conjunctiva, or possibly, when ingested in contaminated food or water. Persons have also acquired HPS after being bitten by rodents. High risk of exposure has been associated with entering or cleaning rodent-infested structures.
Person-to-person transmission has not been associated with HPS cases in the United States. However, person-to-person transmission, including nosocomial transmission of Andes virus, was well documented for a single outbreak in southern Argentina, and it was suspected to have occurred much less extensively in another outbreak in Chile that was associated with the same virus. Therefore, universal precautions are recommended for healthcare workers treating HPS patients.
Virology
Hantaviruses
Hantaviruses belong to the bunyavirus family of viruses. There are 5 genera within the family: bunyavirus, phlebovirus, nairovirus, tospovirus, and hantavirus. Each is made up of negative-sensed, single-stranded RNA viruses. All these genera include arthropod-borne viruses, with the exception of hantavirus, which is rodent-borne.
Like other members of the bunyavirus family, hantaviruses are enveloped viruses with a genome that consists of three single-stranded RNA segments designated S (small), M (medium), and L (large). All hantaviral genes are encoded in the negative (genome complementary) sense. The S RNA encodes the nucleocapsid (N) protein. The M RNA encodes a polyprotein that is cotranslationally cleaved to yield the envelope glycoproteins G1 and G2. The L RNA encodes the L protein, which functions as the viral transcriptase/replicase. Within virions, the genomic RNAs of hantaviruses are thought to complex with the N protein to form helical nucleocapsids, which circularize due to sequence complementarity between the 5' and 3' terminal sequences of each genomic segment.
Hantaviruses replicate exclusively in the host cell cytoplasm. Entry into host cells is thought to occur by attachment of virions to cellular receptors and subsequent endocytosis. Nucleocapsids are introduced into the cytoplasm by pH-dependent fusion of the virion with the endosomal membrane. Transcription of viral genes is initiated by association of the L protein with the three nucleocapsid species. In addition to transcriptase and replicase functions, the viral L protein is also thought to have an endonuclease activity that cleaves cellular messenger RNAs (mRNAs) for the production of capped primers used to initiate transcription of viral mRNAs. As a result of this "cap snatching," the mRNAs of hantaviruses are believed to be capped and contain nontemplated 5' terminal extensions. The viral N and L mRNAs are thought to undergo translation at free ribosomes, whereas the M mRNA is translated in the endoplasmic reticulum. G1 and G2 glycoproteins form heterodimers and are then transported from the endoplasmic reticulum to the Golgi complex, where glycosylation is completed. The L protein produces nascent genomes by replication via a positive-sense RNA intermediate. Hantavirions are believed to form by association of nucleocapsids with glycoproteins embedded in the membranes of the Golgi, followed by budding into the Golgi cisternae. Nascent virions are then transported in secretory vesicles to the plasma membrane and released by exocytosis.
Hantaviruses Causing HPS
Sin Nombre virus (SNV) was first isolated from rodents collected on the premises of one of the initial HPS patients in the Four Corners region. Isolation was achieved through blind passage in Peromyscus maniculatus and subsequent adaptation to growth in Vero E6 cells. Additional viral strains have also been isolated from P. maniculatus associated with a fatal case in California and P. leucopus from the vicinity of probable infection of a New York case. Black Creek Canal virus was isolated from S. hispidus collected near the residence of a human case in Dade County, Florida.
Other Hantaviruses
Several members of the hantavirus genus cause different forms of hemorrhagic fever with renal syndrome (HFRS), an ancient disease first described in Russia in 1913. The four viruses that are associated with HFRS, each named for the region from where they were first isolated, have different primary rodent hosts: Apodemus agrarius (the striped field mouse) for Hantaan virus, Rattus norvegicus (the Norway rat) and Rattus rattus (the black rat) for Seoul virus, Clethrionomys glareolus (the bank vole) for Puumala virus, and Apodemus flavicollis (the yellow-necked field mouse) for Dobrava virus. Hantaan virus from Korea and Dobrava virus from Slovenia are associated with a severe form of HFRS characterized by renal failure that can precede pulmonary edema and disseminated intravascular coagulation (DIC), with estimated mortality rates of 5% to 15%. A moderate form of HFRS caused by Seoul virus (which, along with its host, is distributed worldwide) is responsible for thousands of Eurasian cases annually. Serologic evidence for infection with Seoul-like hantaviruses has been found in rodents in major cities of the United States, and this virus was recently implicated in human cases of HFRS in Baltimore. One report has also associated Seoul virus with chronic renal disease. A mild form of HFRS, caused by Puumala virus, is responsible for nephropathia epidemica in Scandinavia, with an estimated mortality rate of 1% to 3%.
Characteristics of Some Known Hantaviruses
| Hantaan | Seoul | Puumala | Prospect Hill | Sin Nombre | |
| Geographic Region | Asia | Worldwide | Northern Europe | U.S. | North America |
| Reservoir | Field Mouse | Domestic Rat | Bank Vole | Meadow Vole | Deer Mouse |
| Pathology | Renal | Renal | Renal | No known human disease | Pulmonary |
| Mortality | 5 - 15% | 1% | 1% | N/A | 50% |
Another hantavirus, Prospect Hill, has been previously isolated in the United States, but has not been implicated as a cause of human disease. Prospect Hill virus was originally isolated from Microtus pennsylvanicus (meadow vole) in Frederick, MD.
Comparison of HFRS and HPS
| HFRS | HPS | |
| Major Target Organ | kidney | lung |
| First Phase | febrile | febrile "prodrome" |
| Second Phase | shock | shock, pulmonary edema |
| Evolution | oliguria, diureses, convalescence | diureses, convalescence |
| Mortality | 1 - 15% | 50% |
Epidemiology in the Virology Laboratory
During the outbreak in 1993, definitive proof that the agent causing HPS was a novel hantavirus was obtained using a genetic detection assay. Oligonucleotide primers were designed on the basis of regions of the M segment (G2 coding region) conserved among hantaviruses and were used in a nested RT-PCR assay to amplify hantavirus-specific DNA fragments from RNA extracted from the tissues of patients. The amplified DNA fragments were then sequenced. Comparative and phylogenetic analyses of derived sequence data demonstrated that the hantavirus associated with the HPS outbreak (SNV) was a novel virus most closely related to Prospect Hill virus (PHV). In addition, a direct genetic link was made between the human HPS cases and the virus harbored by peridomestic P. maniculatus rodents. Characterization of hantaviral genetic sequences recovered from human tissues demonstrated that these sequences were identical to those from rodents captured at the site of the patient's presumed infection. This characterization has continued to facilitate identification of the site of infection when more than one such site exists and therefore focus the public health response. These techniques also allow implication of a specific rodent host in areas of overlapping hosts.
Sin Nombre Virus Sequencing
The entire genomic sequence of SNV has subsequently been determined by using RNA extracted from autopsy material as well as RNA extracted from cell culture-adapted virus. The L RNA is 6562 nucleotides (nt) in length; the M RNA is 3696 nt long; and the S RNA is 2059 to 2060 nt long. Interestingly, when the prototype sequence (NMH10) of SNV detected in tissues from an HPS case was compared with the sequence of the SNV isolate (NMR11; isolated in Vero E6 cells from P. maniculatus trapped in the residence of the same case), only 16 nucleotide changes were found, and none of these changes resulted in alterations in amino acid sequences of viral proteins. It had been assumed that in the process of adaptation to cell culture, selection of SNV variants which grow optimally in cell culture would occur, and selected variants would differ genetically from the parental virus. Though NMH10 and NMR11 are identical in protein sequence, nucleotide substitutions in nontranslated regions of the genome could be responsible for altered viral phenotypes, as could changes in protein glycosylation or virus membrane components.
The nested RT-PCR assay developed during the initial HPS outbreak provided a rapid
method for the genetic characterization of novel hantaviruses that did not require a virus
isolate. Numerous new hantaviruses have been detected by RT-PCR in rodent tissues but have
yet to be associated with human disease. These include El Moro Canyon virus associated
with the western harvest mouse (Reithrodontomys megalotis), Tula virus with Microtus
arvalis and M. rossiaemeridionalis, Rio Segundo virus with the Mexican
harvest mouse (R. mexicanus), Isla Vista virus with the California vole (M.
californicus), and Prospect Hill-like viruses in Microtus species.
Phylogenetic Analysis
Phylogenetic analysis of Old World and American hantaviruses indicates that the relationship among hantaviruses corresponds with the phylogeny of their rodent hosts. Viruses of rodents belonging to the subfamily Murinae are monophyletic as are hantaviruses of arvicoline and sigmodontine rodents, suggesting that long-term virus-rodent coevolution is taking place. Hantavirus evolution is best understood as co-evolution within specific lineages in the rodent family Muridae. The apparent coupling between hantaviruses and their rodent hosts suggests that viruses of sigmodontine rodents share a common ancestor, as do viruses of the subfamily Arvicolinae and Murinae. This coupling also has a geographic and clinical correlate: viruses found in Old World murine rodents, including Hantaan virus (HTNV), Seoul virus (SEOV) and Dobrava virus, are associated with HFRS in Eurasia. By contrast, viruses carried by New World sigmodontine rodents, including SNV Black Creek Canal virus (BCCV) and Bayou virus (BAYV), are associated with HPS in the Americas. This distinction can narrow the search for a rodent host for newly discovered HPS-like diseases and suggest disease implications for the various new viruses being genetically amplified from rodents.
Sequence Divergence
Detection and characterization of Sin Nombre-like viruses in P. maniculatus and P. leucopus populations have shown that multiple phylogenetic lineages of SNV exist in North America, and in some cases similar viruses are detected in both Peromyscus species. Sequence divergence among SNV genes has been shown to be as high as 23% nucleotide dissimilarity and 7% amino acid dissimilarity. Comparison of SNV sequences with those of other hantaviruses provides no obvious explanation as to why SNV and related viruses cause HPS while other hantaviruses are associated with HFRS. The development of a reverse genetics system for manipulation of virus genomes and an animal model for studying pathogenesis will be necessary to define the molecular mechanism(s) of SNV pathogenicity.
Genomic reassortment by RNA viruses with segmented genomes is well documented and has the potential to produce viruses with altered biological activity, host range, and disease potential. For example, segment reassortment among influenza virus strains (antigenic shift) is thought to be responsible for influenza pandemics. Genomic reassortment among SNV variants is known to occur in nature, but the precise role of genomic reassortment in the epidemiology of HPS and HFRS is unknown.
Prevention Information
Special Pathogens Branch has several sources of information on HPS prevention issues.
Prevention of HPS in the Laboratory
Laboratory Management of Agents Associated with Hantavirus Pulmonary Syndrome:
Interim Biosafety Guidelines, at
http://www.cdc.gov/ncidod/diseases/hanta/labguide.htm#part2
This document provides interim biosafety guidelines for preventing laboratory-associated
infections with agents that cause hantavirus pulmonary syndrome. It is also part of the
supplement to "Hantavirus Infection-- Southwestern United States: Interim
Recommendations for Risk Reduction", published in the Morbidity and Mortality
Weekly Report, July 30, 1993, Volume 42, Number RR-11, Pages i-13.
Methods for Trapping and Sampling Small Mammals for Virologic Testing, at
http://www.cdc.gov/ncidod/dvrd/spb/mnpages/rodentmanual.htm
This manual is intended as a guide for those persons performing ecologic and
epidemiologic studies involving populations of rodents which are potentially infected with
hantavirus.
Controlling Rodent Infestations
How to handle small-scale infestations and how to prevent rodent infestations from occurring is covered in "How Do I Prevent HPS?", a series of pages designed for general readers in this web site, at http://www.cdc.gov/ncidod/diseases/hanta/hps/noframes/prevent.htm.
Guidelines for dealing with particularly heavy rodent infestations may be found at: "Special Precautions for Homes of Persons with Confirmed Hantavirus Infection or Buildings with Heavy Rodent Infestations", at http://www.cdc.gov/ncidod/diseases/hanta/hps/noframes/prevent4.htm.
"The Prevention of Hantavirus Disease" / "La Prevencion del Hantavirus" hand-out card. Downloadable direct from our web page, at http://www.cdc.gov/ncidod/diseases/hanta/hps/noframes/prevcard.htm. For use in HPS prevention efforts. This 3.3" x 11" card has simply written tips and pictures on rodent-proofing a home and cleaning up a rodent infestation. It's available in English and Spanish. The card can be downloaded in two versions: as two full-size web images, and as two 300 dpi .tif images that can be sent to a quick printer for production as two-sided handout cards.
See the Teaching Materials page at http://www.cdc.gov/ncidod/diseases/hanta/hps/noframes/teach.htm for other-prevention related materials available online and by mail from Special Pathogens Branch.
Handling Frequent Exposures to Rodents
Please see "Precautions for Workers in Affected Areas Who are Regularly Exposed to Rodents" at http://www.cdc.gov/ncidod/diseases/hanta/hps/noframes/prevent5.htm..
Updated Information on Respirator Use
Read the "Update On the Nomenclature and Use of Respirators as a Precaution for Hantavirus Infection, February, 1999" at http://www.cdc.gov/ncidod/diseases/hanta/hps/noframes/prevent7.htm..
Formal Risk Reduction Recommendations
See "Hantavirus Pulmonary Syndrome— United States: Updated Recommendations
for Risk Reduction" in the Morbidity and Mortality Weekly Report,
July 26, 2002, Vol. 51, No. RR-9.
Suspected HPS Specimen Submission Guidelines
Special Pathogens Branch (SPB), Division of Viral and Rickettsial Diseases performs a variety of diagnostic techniques for hantavirus pulmonary syndrome.
Who May Submit Specimens and How Should They Be Submitted?
Health Departments:
Protocol For Submitting Hantavirus Specimens
Guidelines For Submitting
Specimens to the Special Pathogens Branch
Serology:
The following specimen types may be submitted:
Specimen packaging requirements:
Immunohistochemistry (IHC):
The following types of formalin-fixed or paraffin-embedded tissues may be submitted:
Specimen packaging requirements:
PCR/Virus Isolation:
The following types of samples may be submitted:
Specimen packaging requirements:
NOTES:
Hantavirus Pulmonary Syndrome Case Report Form
|
Case-patient Identification Number
|
||||||||||||||||||
_________________________ Case-patient's last name |
_________________________ First name |
_________________________ Middle name |
_______________________ Street Address |
_________________ City |
_______________ County |
__________ State |
__________ Zip |
(___)___________________ Home Telephone |
| Date of birth: ___/___/___ |
Age: ________ |
Sex:
|
|||||
| Race: | White | Black | Asian/ Pacific Islander |
American Indian/ Alaska Native |
Other: __________ |
||
| Ethnicity: | Hispanic | Non-Hispanic | Unk. | ||||
| Occupation: | ___________________________________ |
||||||
Onset date: ___/___/___ |
| Was patient Hospitalized? | Yes | No | Unknown |
Number of times hospitalized since onset of illness: _____ |
|||
| 1st Hospitalization | 2nd Hospitalization | ||||||
| Name of Hospital: | ________________________ | ________________________ | |||||
| Location of Hospital: | ________________________ | ________________________ | |||||
| Dates in Hospital: | ___/___/___ | to | ___/___/___ | ___/___/___ | to | ___/___/___ | |
| Record Number: | ________________________ |
________________________ |
|||||
| Did the patient have any of the following? | ||||
| Fever>101 F or>38.3 C: | Yes | No | Unk. | Highest Fever: __________ |
| Thrombocytopenia (platelets 150,000 mm3): | Yes | No | Unk. | Lowest platelet count: __________ |
| Elevated Hematocrit (Hct): | Yes | No | Unk. | Highest Hct: __________ |
| Elevated creatinine: | Yes | No | Unk. | Highest creatinine: __________ |
| WBC: __________ Total Neutrophils: __________(%) Banded Neutrophils:__________(%) Lymphocytes:__________(%) | ||||
| CXR with unexplained bilateral interstitial infiltrates or suggestive of ARDS? | Yes | No | Unk. | Date: ___/___/___ |
| Respiratory compromise requiring supplemental oxygen? | Yes | No | Unk. | |
| Oxygen saturation <90% at any time? | Yes | No | Unk. | |
| Was the patient intubated? | Yes | No | Unk. | Date: ___/___/___ |
| Has the patient received ribavirin? | Yes | No | Unk. | |
History of any relevant underlying medical conditions (i.e. COPD,
malignancy, immunosuppression, diabetes)?
Other possible explanations for acute illness (i.e. sepsis, burns, trauma)?
| Outcome of illness? | Alive | Dead | Unk. | If deceased, date of death: ___/___/___ |
| Was an autopsy performed? | Yes | No | Unk. | |
| If yes, was exam compatible with non-cardiogenic pulmonary edema? | Yes | No | Unk. | |
| Are tissue specimens (fresh-frozen or pariffin blocks) available for testing? | Yes | No | Unk. | |
| Is serum/blood specimen available for testing for hantavirus infection? | Yes | No | Unk. | |
| Has a specimen been tested for hantavirus infection at another laboratory? | Yes | No | Unk. | |
| If yes, where? ___________________________________ |
Type of specimen? __________ |
Results (i.e. titer, OD)? __________ |
||
| History of any rodent exposure in 6 weeks prior to onset of illness? | Yes | No | Unk. | |
If yes, date of contact: ___/___/___ |
||||
| Type of rodent: | Mouse | Rat | Other: __________ |
Unk. |
Place of Contact (town, county, state): _______________________________ |
||||
Comment:
State Health Dept. reporting case: ____________ |
State/local ID Number:_______________________ |
Date form completed: ___/___/___ |
|
Person completing report: __________________________________________________ |
|
Phone number: (___)_____-______ |
|
Name of patient's physician: _________________________________________________ |
|
Phone number: (___)_____-______ |
|
National Surveillance Laboratory Specimen Form
for Possible Cases of
Hantaviral Pulmonary Syndrome
Diagnostic Specimen Submission Form
| CASE-PATIENT IDENTIFICATION NUMBER: |
|
Case-Patient Name:
| ______________________________ | ______________________________ | _____ |
| Last | First | MI |
State Health Department Identifying Information:
Date Specimen(s) Received by State: ___/___/___ |
|
| State Health Department Lab Submitting Specimen(s): | ______________________________ |
| Name of State Lab Person Shipping Specimen(s): | ______________________________ |
| State Health Department Dept Laboratory Phone Number: | (_____)__________-__________ |
| Hospital Submitting Specimen(s): | ______________________________ |
Specimen(s) List: (circle specimen type)
| 1) Specimen ID Number: _______________ | Date Collected: ___/___/___ |
| 1 Serum | |||
| 2 Tissue | A Paraffin | B Formalin | C Fresh frozen |
| 3 Blood Clot |
| 2) Specimen ID Number: _______________ | Date Collected: ___/___/___ |
| 1 Serum | |||
| 2 Tissue | A Paraffin | B Formalin | C Fresh frozen |
| 3 Blood Clot |
| 3) Specimen ID Number: _______________ | Date Collected: ___/___/___ |
| 1 Serum | |||
| 2 Tissue | A Paraffin | B Formalin | C Fresh frozen |
| 3 Blood Clot |
| 4) Specimen ID Number: _______________ | Date Collected: ___/___/___ |
| 1 Serum | |||
| 2 Tissue | A Paraffin | B Formalin | C Fresh frozen |
| 3 Blood Clot |
| LABEL ALL SPECIMENS WITH: |
| 1) First and Last Name of Case-Patient |
| 2) Case-Patient ID Number |
| 3) State Laboratory Specimen ID Number |
| 4) The Date the Specimen was Collected |
| 5) Type of Specimen (e.g., lung, liver, heart, serum, etc. |
On Outside of the Box Label How Specimen Should be Stored (i.e. refrigerate, frozen, do not refrigerate)
| *Please send Case Report Form with this form and specimen(s). | CDC-Revised October, 1996 |
Centers for Disease Control and Prevention
Revised June 1998
|
HPS
Questions & Answers
Answers to Selected Questions by Participants of the Hantavirus Pulmonary Syndrome Clinical Update, 1999 Satellite Conference A CDC/Public Health Training Network Satellite Broadcast, May 27, 1999 |
Is there any information on re-infection with
hantaviruses, for example, in areas of South America where there
is high prevalence of antibodies indicating widespread exposure?
Have any correlates of protective immunity been identified in epidemiologic
studies?
Answer
Are the findings for HPS in
South America the same as those in the United States.? Is it the
same disease?
Answer
If HPS occurs mostly in rural areas,
why are the greatest number of cases affecting white males -- up
to 76%?
Answer
Why is HPS uncommon in children
in the United States?
Answer
What types of respirators or masks can
farmers and homeowners in rural areas use for protection against
hantavirus?
Answer
Is exposure to sunlight an effective means
of disinfecting materials contaminated with hantavirus? If so, how
long of an exposure is recommended?
Answer
If Sin Nombre is an enveloped virus, why
is it not destroyed by desiccation? Also, do you have data on how
long the virus is infective in dried feces, urine, and other excreta
of rodents?
Answer
You discussed the platelet count and the
blood smear in the diagnosis of HPS. What about the white blood
cell count (WBC) and hematocrit. Are they helpful?
Answer
Is there evidence to correlate immunologic
or immunogenetic characteristics (such as HLA type, immunosuppression)
with the severity of the disease?
Answer
Are there specific cytokine-blocking agents
or antibiotics that would be helpful in the treatment of acute HPS
cases?
Answer
What is known about virus attachment
and entry into host cells? Can this information be used to design
treatment strategies?
Answer
HPS patients seem to recover quite promptly
after their acute insult. Do they have residua, either from the
time on the ventilator or from their disease process?
Answer
What percent of the population in disease-endemic
areas have HPS antibody but have not presented with symptoms of
disease?
Answer
We recently had a Sin Nombre false-positive
result with a report from a commercial laboratory. The patient was
in ICU and ARDS was part of her clinical presentation: IgM was 1:80
and the IgG was 1:512 with > 1:80 being positive. When we tested
the patient's blood at our laboratory, it was negative, and another
diagnosis was clinically relevant to the patient. The commercial
laboratory is representing that they do Sin Nombre testing now --
not other related hantaviruses. Most of our hospitals know to send
all samples here, but the occasional mistake with a new employee
may occur. Do you know what test they have developed and its sensitivity
and specificity?
Answer
Is there any information on re-infection with hantaviruses, for example, in areas of South America where there is high prevalence of antibodies indicating widespread exposure? Have any correlates of protective immunity been identified in epidemiologic studies?
Answer:
There are no known re-infections with the homologous hantavirus;
virus neutralizing antibodies are formed. Closely related hantaviruses,
such as Seoul and Hantaan viruses, seem to cross-protect against
re-infection in experimental animals, and one might expect cross-protection
among the hantaviruses derived from sigmodontine rodents.
Dr. C. J. Peters, Special Pathogens Branch, CDC .
For discussion and
references, see:
Peters CJ. Hantavirus pulmonary syndrome in the Americas.
In: Scheld WM, Craig WA, Hughes JM, editors. Emerging Infections
II. Washington, D.C.: ASM Press; 1998. p. 17-64.
Note: It would be interesting to have observations from areas
of the Balkans where Dobrava virus and Puumala virus co-circulate;
these two viruses are more distantly related and thus cross-protection
might not be seen.
Are the findings for HPS in South America the same as those in the United States? Is it the same disease?
Answer:
Most disease seen in the United States is caused by Sin Nombre virus.
The other SNV-related viruses in the United States (New York and
Monongahela) seem to cause a very similar disease. Two other viruses
in North America, Bayou and Black Creek Canal, cause HPS that fits
the surveillance case definition, and the cases were recognized
by clinicians as HPS. The few cases that have been evaluated
seem to have more renal failure and higher elevations of serum creatine
phosphokinase than the typical SNV infection. In South America,
all the recognized cases have been basically HPS, but there are
some clusters that seem to have more renal failure, petechiae and
bleeding manifestations, and/or involvement of children. In addition,
some have had facial flushing, which is not seen with HPS but is
seen with hemorrhagic fever with renal syndrome. It must be borne
in mind that cases are usually recognized by HPS surveillance or
by the dramatic manifestations of HPS, so there is a strong ascertainment
bias. Until there is at least a common protocol for evaluating cases
in South America, the different manifestations reported by various
groups need to be interpreted cautiously.
Dr. C. J. Peters, Special Pathogens Branch, CDC
For a summary and
references to South American publications, see:
Peters CJ. Hantavirus pulmonary syndrome in the Americas.
In: Scheld WM, Craig WA, Hughes JM, editors. Emerging Infections
II. Washington, D.C.: ASM Press; 1998. p. 17-64.
For a discussion
of milder SNV infections, see:
Kitsutani PT. Acute Non-HPS Sin Nombre Hantavirus Infection
in the U.S. Emerg Infect Dis 1999; (in press).
If HPS occurs mostly in rural areas, why are the greatest number of cases affecting white males -- up to 76%?
Answer: That is an interesting question and can best be answered by looking at the way we keep HPS records at CDC. As you noted, most (70%) of U.S. cases occur in rural areas. The most recent statistics show that 61% of cases are male and 75% are white, but only 45% of the cases are white males. While these data seem to show an increased risk for white males, this distribution of cases requires further clarification.
In addition to gender, CDC classifies confirmed cases of HPS by race. The U.S. population west of the Mississippi River, where the majority of HPS cases has occurred, is predominantly white, with the following breakdown: white (77%), Asian (7%), black (5%), American Indian (2%), and other (10%).
We also record Hispanic ethnicity. Individuals of Hispanic origin make up a large portion of the population of the Southwest, where Sin Nombre virus is endemic. To date, 10% of cases are Hispanic, 9% are white Hispanic, and 5.5% are white Hispanic males.
Clearly, there is a statistically significant increased risk associated
with being male. Males have a 1.5-fold higher risk for HPS than
females. This small increased risk is possibly due to occupational
exposure.
Dr. James Olson, Special Pathogens Branch, CDC.
Why is HPS uncommon in children in the United States?
Answer: As of June 1, 1999, 217 cases of HPS have been reported in the United States. Thirteen (6%) were 16 years of age or younger, although this age group represents 24.2% of the US population. The youngest reported patient was 10 years of age.
Two or more factors may explain the relative scarcity of HPS cases among children. The first is that children may be less likely to get infected because they do not perform the activities that would put them at risk for infection, such as cleaning in enclosed spaces. Even if they perform these types of activities or have their noses closer to the ground, their total lung exposure to virus may be less than an adult's; despite the fact that children breathe faster than adults, their minute volume is less. Alternatively, they may just be less likely to get infected due to nonspecific immune mechanisms. The second possibility is that children are as likely to get infected as adults, but less likely to develop HPS, the severest manifestation of infection. We would not generally know of these mild infections, but are aware of a 4-year-old boy who had a very mild illness and did not develop the severe cardiac and pulmonary syndrome. We know that HPS is a disease that reflects your immune response to the virus, so it is possible that children respond differently than adults.
The total picture of HPS infection among children is further complicated
by studies in South America. There appear to be proportionately
more children infected, more children with asymptomatic or mild
infections, and children with hemorrhagic manifestations after infections.
Some data suggest hantavirus transmission via breast-milk. Further
research will help answer this question and provide us a clue to
the immunology that underlines infection and disease development.
Dr. Ali Khan, Special Pathogens Branch, CDC.
For further information,
see:
Pini NC, Resa A, Laime GDJ, Lecot G, Ksiazek TG, Levis S,
et al. Hantavirus Infection in Children in Argentina. Emerg Infect
Dis 1998;4(1):85-7.
What types of respirators or masks can farmers and homeowners in rural areas use for protection against hantavirus?
Answer: For those who frequently handle or are frequently exposed to rodents in rural areas (such as mammalogists and pest control workers), CDC recommends wearing either a half-mask air-purifying (or negative-pressure) respirator or a powered air-purifying respirator (PAPR) with N-100 filters.
CDC does not recommend routine use of respirators by farmers and
homeowners in rural areas. CDC guidelines (MMWR 1993; 42,
RR-11) address specific risk-reduction measures for rural residents
(rodentproofing, environmental management, and trapping) and precautions
to be taken during activities that may pose increased risk of hantavirus
infection (cleanup of rodent infested areas). Cleanup of very heavy
rodent infestations or of homes associated with known cases of HPS
are special instances for which we do recommend respiratory protection,
and these tasks are best left to pest control or public health professionals.
There is no evidence that farmers operating farm machinery in open
fields (even though rodents may be crushed in the machinery) are
at increased risk. Under these conditions, the natural circulation
of air and virucidal properties of natural UV light make inhalation
of infectious aerosols less likely. The possibility of human exposure
is greater in indoor closed spaces, such as barns and sheds, that
may be infested with rodents. It is important that outbuildings
be rodent-proofed to the greatest extent possible. When effective
rodentproofing is not possible, snap traps (and, if necessary, rodenticides)
should be used continuously, and recommended precautions (concerning
airing out and cleanup of infestations) should be followed when
entering such buildings after periods of non-use.
Dr. James Mills, Special Pathogens Branch, CDC.
Is exposure to sunlight an effective means of disinfecting materials contaminated with hantavirus? If so, how long of an exposure is recommended?
Answer: Ultraviolet (UV) light is a very effective way to kill viruses under certain circumstances. Sunlight produces high intensities of UV and finely dispersed aerosols of the kind that infect humans are readily penetrated by the light. Virus inactivation has never been measured under those circumstances, but it must be very rapid.
However, the UV light must penetrate to the virus particle. One reason why the interior of structures may be dangerous is that the reflected white light from outside will not contain sufficient UV. Similarly, solids or liquids provide a challenge to UV penetration.
We also don't recommend UV lights for disinfection because of the
considerations above, the difficulties in assuring continued strength
of the radiation at the site for disinfection, and possible health
effects.
Dr. C. J. Peters, Special Pathogens Branch, CDC.
If Sin Nombre is an enveloped virus, why is it not destroyed by desiccation? Also, do you have data on how long the virus is infective in dried feces, urine, and other excreta of rodents?
Answer:
Studies on Hantaan virus
have shown that the virus infectivity cannot be recovered after
2 days upon desiccation. Studies with Sin Nombre virus are pending.
Dr. C. J. Peters, Special Pathogens Branch, CDC.
Answer:
Thrombocytopenia, left shift of the myeloid series, and appearance
of immunoblasts are consistent findings in almost all HPS patients.
The total white cell count is quite variable, ranging from as low
as 2,800 during prodrome, to over 100,000 /mm-3 in some severe HPS
cases. The elevation of the WBC is not an accurate indicator of
severity. The hematocrit is significantly elevated (>50 in men,
>48 in women) in only approximately 50% of cases, and is a function
of preceding existence of anemia, as well as severity of the capillary
leak syndrome.
Dr. Frederick Koster, University of New Mexico, School of Medicine.
Is there evidence to correlate immunologic or immunogenetic characteristics (such as HLA type, immunosuppression) with the severity of the disease?
Answer:
A search for correlates to severity of HPS has not revealed
any relationships to gender or ethnicity. Individuals under the
age of 15 years appear to have milder disease. Preliminary data
using HLA typing have suggested that individuals bearing one particular
B-locus allele, B*35, appear to be at higher risk for severe disease
than all other B alleles. No associations have yet been found for
alleles in other HLA genes. Other than supporting the notion that
T cells are critically involved in mediating the lung and/or cardiac
injury in HPS, the significance of these immunogenetic data is not
yet known.
Dr. Frederick Koster, University of New Mexico, School of Medicine.
Are there specific cytokine-blocking agents or antibiotics that would be helpful in the treatment of acute HPS cases?
Answer: The simple answer to this question is that there have been no studies of any cytokine blocking agents in HPS, so they should not be tried empirically. Such agents should only be used on an experimental protocol. However, the simple answer begs the question of whether an anti-cytokine protocol should be implemented.
There is evidence that certain cytokines are involved in the pathogenesis of HPS. Tumor necrosis factor (TNF) alpha or beta and interleukin 2 (IL-2) seem especially important. When direct measurements of these cytokines were made in acutely ill HPS patients, the serum levels were found to be not statistically different from those of normal volunteers or from critically ill control patients. However, evaluation of soluble receptors for TNF and IL-2 demonstrated that they were strikingly elevated in HPS patients, as compared with controls. Additionally, the levels of these soluble receptors were highest in severely ill patients, as compared with patients who had mild HPS. In patients who survived the acute illness, the levels of soluble cytokine receptors for TNF and IL-2 decreased during the convalescent period.
Administration of TNF or IL-2 to animals causes syndromes that are quite similar to HPS. Both cytokines can cause myocardial depression, as is seen in HPS, via activation of intracellular nitric oxide in cardiac myocytes. IL-2 causes pulmonary edema and hypotension when it is given to humans as cancer chemotherapy. A plausible explanation for the cytokine findings in HPS is that TNF alpha or beta and IL-2 levels may have been strikingly increased in the period leading up to the acute illness, or that a surge of one or another or all of these cytokines led to rapid onset of pulmonary edema and shock. By the time of hospital admission and blood collection, the cytokine levels had either decreased to normal or were masked by binding to high levels of circulating soluble receptors. However, alternative explanations for the cytokine findings should not be ignored. For example, both forms of soluble TNF receptor (55 kd and 75 kd) and the soluble IL-2 receptor can be released from activated mononuclear cells, even in the absence of binding of the respective cytokine. Presumably, this release of soluble receptors represents a mechanism of slowing or modulating inflammation.
So, a more circumspect answer to the question may be that there is as yet insufficient evidence to attempt a therapeutic trial that hinges on blocking the actions of one of these cytokines. There are currently no agents available for blocking the action of IL-2. However, several anti-TNF agents have been developed, including a humanized murine monoclonal antibody to TNF alpha and a fusion protein of recombinant 75 kd receptor and the Fc portion of human IgG. These agents have failed to prove useful in sepsis resulting from microorganisms other than hantaviruses. Other agents that are potentially useful include the anti-inflammatory cytokines IL-10 and TGF-beta. Before trials with anti-cytokine agents are designed, a more definite association must be made between increased cytokine levels and the development of shock and pulmonary edema in patients with HPS. This will probably require earlier evaluation of cytokine profiles in patients who have not yet developed pulmonary edema.
As to the use of antibiotics in HPS, it is clear that no antibacterial
is likely to be an effective therapy. Nevertheless, patients should
be placed on broad spectrum antibiotics until the diagnosis of HPS
is well established, since bacterial shock is far more common than
hantaviral shock. The antiviral agent ribavirin is effective in
improving survival and shortening the length of illness in another
hantaviral illness, hemorrhagic fever with renal syndrome caused
by Hantaan virus. An open label trial of ribavirin in 1993-94 did
not demonstrate the drug to be effective in HPS. However, most patients
who received the drug were critically ill at the time, and it is
difficult to postulate that inhibition of the virus reverses shock.
An NIH-sponsored, double-blinded, placebo-controlled trial of ribavirin
administered earlier in the syndrome is currently under way, but
ribavirin should not be regarded as the standard of care.
Steven Q. Simpson, University of Kansas Medical Center, School of
Medicine.
What is known about virus attachment and entry into host cells? Can this information be used to design treatment strategies?
Answer:
The cellular receptor for pathogenic hantaviruses has been
recently identified as the b3 integrins. The b3 integrins have been
characterized as the receptors for many other viruses, such as adenovirus,
foot-and-mouth disease virus, coxsackievirus, and papillomavirus.
Antibodies to the b3 integrins can partially inhibit hantavirus
entry into cells in tissue culture experiments. Based on these results,
a therapeutic potential to anti-b3 antibodies has been suggested,
but it is too early in the hantavirus studies to know their real
application. More information is needed on the cause of the vascular
leakage and on hantavirus pathogenesis in general. In addition,
the above studies are hampered by the lack of any animal model for
diseases caused by the hantaviruses
Dr. Christina Spiropoulou, Special Pathogens Branch, CDC .
For more information on receptor studies, see:
Gavrilovskaya IN, Brown EJ, Ginsberg MH, and Mackow ER. Cellular entry of hantaviruses which cause hemorrhagic fever with renal syndrome is mediated by b3 integrins. J Virol 1999; 73:3951-59.
Gavrilovskaya IN, Shepley M, Shaw R, Ginsberg MH, and Mackow ER. B3 integrins mediate the cellular entry of hantaviruses that cause respiratory failure. Proc Natl Acad Sci USA 1998;95:7074-79.
Additional information on these outbreaks can be found in:
Chapparo J, Vega J, Terry W, Barra B, Meyer R, Peters CJ, et al. Assessment of person-to-person transmission of hantavirus pulmonary syndrome in a Chilean hospital setting. J Hosp Inf 1998;40:281-5.
Padula PJ, Edelstein A, Miguel SD, Lopez NM Rossi CM, Rabinovich RD. Hantavirus pulmonary syndrome outbreak in Argentina: molecular evidence for person-to-person transmission of Andes virus. Virology 1998; 241(2):323-30.
Parisi MDN, Enria DA, Pini NC, sabattini MS. Retrospective detection of clinical infections caused by hantavirus in Argentina. Medicina (Buenos Aires) 1996; 56(1):113.
Toro J, Vega JD, Khan AS, Mills JN, Padula P, Terry W, et al. An outbreak of hantavirus pulmonary syndrome, Chile, 1997. Emerg Infect Dis, 1998;4:687-94.
Wells RM, Sosa Estani S, Yadon ZE, et al. An unusual hantavirus outbreak in southern Argentina: Person-to-person transmission? Emerg Infect Dis 1997;3:171-4.
Wells RM, Sosa Estani S, Yadon ZE, Enria D, Padula P, Pini NC, et al. Seroprevalence of antibodies to hantavirus in health care workers and other residents of southern Argentina. Clin Inf Dis 1998;27:895-6.
Wells RM, Young J, Williams RJ, Armstrong LR, Busico K, Khan AS,
et al. Hantavirus transmission in the United States. Emerg Infect
Dis 1997;3:361-5.
HPS patients seem to recover quite promptly after their acute insult. Do they have residua, either from the time on the ventilator or from their disease process?
Answer:
Following recovery from HPS, patients often experience fatigue
and exercise intolerance for several months. Almost all patients,
including those not requiring mechanical ventilation, have evidence
for a modest degree of small airways obstruction, which persists
for more than one year after recovery. Evidence for decreased diffusion
capacity in the lung resolves after 3 to 6 months. A few patients
have mild proteinuria and mild pulmonary hypertension, but the number
of patients studied is too small to draw conclusions on the significance
of these findings.
Dr. Frederick Koster, University of New Mexico, School of Medicine.
What percent of the population in disease-endemic areas have HPS antibody but have not presented with symptoms of disease?
Answer:
Studies in the United States suggest that most people who
are infected with Sin Nombre virus (SNV) develop HPS. The prevalence
of antibody to SNV among healthy people residing in the disease-endemic
area is extremely low (0.3%). On the other hand, the prevalence
of antibodies to South American hantaviruses among health populations
in disease-endemic areas of South America may be considerably higher,
suggesting that there are inapparent infections or that the disease
is mild and unrecognized following infection with some hantaviruses.
Dr. James Olson, Special Pathogens Branch, CDC.
For further information, see:
Williams RJ, Bryan RT, Mills JN, Palma E, Vera I, de Velasquez F, et al. An outbreak of hantavirus pulmonary syndrome in western Paraguay. Am J Trop Med Hyg 1997;57:274-82.
We recently had a Sin Nombre false-positive result with a laboratory report from a commercial laboratory. The patient was in ICU and ARDS was part of her clinical presentation: IgM was 1:80 and the IgG was 1:512 with > 1:80 being positive. When we tested the patient's blood at our laboratory, it was negative, and another diagnosis was clinically relevant to the patient. The commercial laboratory is representing that they do Sin Nombre testing now -- not other related hantaviruses. Most of our hospitals know to send all samples here, but the occasional mistake with a new employee may occur. Do you know what test they have developed and its sensitivity and specificity?
Answer:
There are at least three tests we are aware of that are being
done for diagnosis. One, developed at the University of New Mexico,
is a strip that is treated with serum and then the resulting bands
are analyzed. This method has been compared to the ELISA and seems
to give very similar results in testing human diagnostic sera. The
other two have not been compared. One of these is an ELISA similar
to the one in use at CDC, but does not use the same controls and
we would not be surprised if it gave false-positives from time to
time. None of the tests for SNV are licensed by the FDA for diagnostic
use with human sera.
Dr. James Olson, Special Pathogens Branch, CDC.
For further information, see:
Ksiazek TG, Peters CJ, Rollin PE, Zaki S, Nichol ST Spiropoulou CF, et al. Identification of a new North American hantavirus that causes acute pulmonary insufficiency. Am J Trop Med Hyg 1995; 52(2):1017-23.
Back to Technical Information Index
![]() All
About Hantaviruses Home | General
Information |
Technical
Information | Contact
Us
All
About Hantaviruses Home | General
Information |
Technical
Information | Contact
Us
CDC Home | Search | Health Topics A-Z
This page last reviewed Thursday, April 28, 2005
Infectious Disease Pathology
Activity
Division of Viral and Rickettsial Diseases
National Center for Infectious Diseases
Centers
for Disease Control and Prevention
U.S. Department of Health and Human Services
![]()
![]()