Vaccines and Preventable Diseases:
Factsheet: Meningococcal Diseases and Meningococcal Vaccines
(April 25, 2006)
On This Page:
Content of this page derived from the 2005 ACIP statement.
Overview
With the introduction and widespread use of Hemophilus influenzae type b and Streptococcus pneumoniae conjugated vaccines, invasive disease due to these agents has been markedly reduced. The newly licensed tetravalent meningococcal conjugated vaccine (MCV4) should become a key addition to existing to control of Neisseria meningitidis. This document seeks to familiarize vaccination providers, partners, and the public with the epidemiology and clinical features of meningococcal disease, with the new conjugate meningococcal vaccine (MCV4), and the previously licensed polysaccharide meningococcal vaccine (MPSV4).
The Advisory Committee on Immunization Practices (ACIP) to the Centers for Disease Control and Prevention (CDC) has recently recommended routine vaccination of young adolescents with MCV4 at the pre-adolescent visit (11-12 years old). Introducing a recommendation for MCV4 vaccination in young adolescents (11-12 years old) may strengthen the role of the pre-adolescent visit and have a positive effect on vaccine coverage in adolescence. ACIP recommends that young adolescents see a health care provider at age 11-12 for a routine preventive visit, at which time appropriate immunizations and other preventive services should be provided. For those who have not previously received MCV4, ACIP recommends vaccination before high school entry (~15 years old) as an effective strategy to reduce meningococcal disease incidence in adolescents and young adults. Within 3 years, the goal is routine vaccination with MCV4 of all adolescents. ACIP recognizes that vaccine supply may be an issue in the first few years after licensure of MCV4. Other adolescents who wish to decrease their risk of meningococcal disease may elect to receive vaccine.
College freshmen who live in dormitories are at higher risk for meningococcal disease compared to other people of the same age. The ACIP recommends routine vaccination for college freshmen living in dormitories. Because of feasibility constraints in targeting freshmen in dormitories, colleges may elect to target their vaccination campaigns to all matriculating freshmen. The risk for meningococcal disease among non-freshmen college students is similar to that for the general population of similar age (18-24 years). Other college students who want to reduce their risk for meningococcal disease may elect to receive the vaccine.
Disease
- Invasive meningococcal disease occurs in three common clinical forms: meningitis (49% of cases), blood infection (33%) and pneumonia (9%); other forms account for the remainder (9%) of the cases.
- Onset can be abrupt and course of disease rapid.
- Case fatality rate is 10%-14%; 11%-19% of survivors suffer serious sequelae including deafness, neurologic deficit, or limb loss.
Epidemiology
- Rates highest in infancy with second peak in adolescence (see graph below) with the peak around 18 years of age (see figure)
- Annually, 1,400–2,800 cases of invasive meningococcal disease occur in the US.
- 20% of cases occurs among adolescents and young adults ages 14–24
- 16% of cases occurs among infants under 1 year of age
- College freshmen living in dormitories are at higher risk than general population of similar age
- Most cases are sporadic (97%); a minority is associated with outbreaks (3%)
- Disease is seasonal, with cases peaking in December and January.
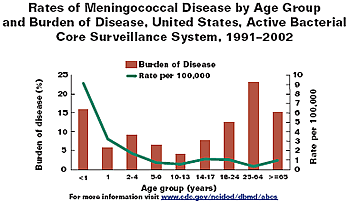
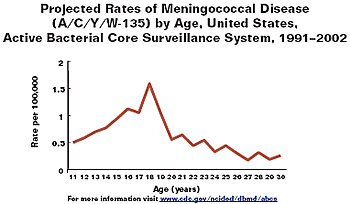
screen-reader version of both graphs
Causative Bacteria
- Meningococci are carried only by humans in the nasopharynx—their only reservoir
- Overall 5%-10% of the population carries the bacteria
- Adolescents and young adults have the highest carriage rates
- Few carriers develop disease
- Transmission occurs when close, face-to-face contact permits the exchange of salivary secretions from people who are ill or are carriers
- Worldwide, the vast majority of disease is caused by 5 serogroups (A, B, C, Y, W-135) of the bacterium
- In the United States, almost all cases are caused by serogroups B, C and Y; there is currently no licensed vaccine that protects against Serogroup B in the U.S.
The Vaccines
- Licensed in the United States in January 2005 for persons 2–55 years of age
(updated March 2008)
- Covers Serogroups A, C, Y and W-135
- Conjugate Group C vaccine is currently licensed and routinely used in many European countries
- It is likely that this or a similar vaccine will be licensed for younger age groups in the future
- Included in the Vaccines for Children (VFC) Program
- Cost to private sector per dose: $82.00
- Given intramuscularly as a single dose
- Need for revaccination not yet known
- Longer duration of protection and similar efficacy compared to MPSV4 expected in adolescents and adults
- Adverse reactions similar to Meningococcal Polysaccharide Vaccine (see below)
- Recommendations for use: in February 2005, ACIP voted to recommend vaccination with MCV4 in following groups:
- Adolescents
- Young adolescents at the pre-adolescent visit (11–12 years old)
- Adolescents (if not previously vaccinated) at high school entry (~15 years old)
- Adolescents who wish to decrease their risk may elect to receive
- Groups that have elevated risk of meningococcal disease
- College freshmen living in dormitories
- Microbiologists who are routinely exposed to isolates of N. meningitidis
- Military recruits
- Persons who travel to, or reside in countries in which N. meningitidis is hyperendemic or epidemic, particularly if contact with the local population will be prolonged
- Persons who have anatomic or functional asplenia or terminal complement component deficiencies (an immune system disorder)
- Licensed in 1981
- Included in the Vaccines for Children (VFC) program (no CDC contract)
- Cost to private sector per dose: $86.10
- Given subcutaneously as a single dose
- Generally not protective in children less than 2 years of age
- Good short-term (3–5 years) protection (85%) in older children and adults
- Antibody levels decrease markedly after 2–3 years, especially in children
- People at high risk need revaccination every 3–5 years
- Adverse reactions:
- Mostly mild injection site pain and redness
- Brief fever in 5 percent
- Severe allergic and neurological reactions: each <0.1/100,000
- Recommendations for use: MPSV4 is recommended for individuals who are at elevated risk aged 2–10 years and over 55 years (see MCV4 recommendations for list of groups at elevated risk)
- If MCV4 is unavailable, MPSV4 is an acceptable alternative for persons at elevated risk ages 11–54 years
- MPSV4 is not recommended and should not be administered routinely for adolescents ages 11–12 or for adolescents entering high school. Adolescents in these age groups are recommended only to receive MCV4
Additional Sources of Information
National Foundation for Infectious Diseases (NFID), The Changing Epidemiology of Meningococcal Disease Among United States Children, Adolescents, and Young Adults, November, 2004 ![]()
Raghunathan P, Bernhardt S, Rosenstein R. Opportunities for control of meningococcal disease in the United States. Annu Rev Med 2004;55:333–53
CDC. Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2005; In press.
CDC. Meningococcal disease and college students: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2000;49 (No. RR-7):11–21
Return to main Meningococcal Vaccination page
.pdf files: To view and print the .pdf files on this site, you will need Adobe Acrobat Reader. Use this link to obtain a free copy of Adobe Acrobat Reader (exit). We highly recommend that you upgrade to the latest version if haven't already.
Content last reviewed on June 7, 2005
Content Source: National Center for Immunization and Respiratory Diseases
