ISSN: 1080-6059
Volume 15, Number 1–January 2009
Dispatch
Serotype G12 Rotaviruses, Lilongwe, Malawi
Nigel A. Cunliffe, ![]() Bagrey M. Ngwira, Winifred Dove, Osamu
Nakagomi, Toyoko Nakagomi, Arantza Perez, C. Anthony Hart,1 Peter N.
Kazembe, and Charles C.V. Mwansambo
Bagrey M. Ngwira, Winifred Dove, Osamu
Nakagomi, Toyoko Nakagomi, Arantza Perez, C. Anthony Hart,1 Peter N.
Kazembe, and Charles C.V. Mwansambo
Author affiliations: University of Liverpool, Liverpool, UK
(N.A. Cunliffe, W. Dove, O. Nakagomi, T. Nakagomi, C.A. Hart); College of
Medicine, Blantyre, Malawi (B.M. Ngwira, A. Perez); Baylor College of Medicine
Children's Foundation, Lilongwe, Malawi (P.N. Kazembe); Kamuzu Central Hospital,
Lilongwe (C.C.V. Mwansambo); and Nagasaki University, Nagasaki, Japan (O. Nakagomi, T. Nakagomi)
Suggested citation for this article
Abstract
To assess diversity of rotavirus strains in Lilongwe,
Malawi, we conducted a cross-sectional study of children with acute
gastroenteritis, July 2005–June 2007. Serotype G12 was identified in 30 (5%) of
546 rotavirus-positive fecal specimens. The G12 strain possessed multiple
electropherotypes and P-types, but their viral protein 7 sequences were closely
related, indicating that reassortment has occurred.
Rotavirus is the leading cause of severe, acute gastroenteritis, a disease that causes dehydration and death in infants and young children worldwide (1); an estimated 527,000 childhood deaths occur annually (www.who.int/immunization_monitoring/burden/rotavirus_estimates/en/index.htm). Because of the high death rates in children, vaccination to prevent severe disease outcomes after rotavirus infection is an essential public health strategy (2,3). Currently, 2 live attenuated oral rotavirus vaccines are becoming part of childhood immunization schedules in North America, Latin America, and Europe; Phase III clinical trials are underway in Africa and Asia (3).
Rotaviruses are segmented, double-stranded (ds) RNA viruses that possess a triple-layered protein capsid. The 11 dsRNA segments, upon separation by electrophoresis, exhibit profiles that can be broadly categorized into long and short RNA patterns termed electropherotypes. The rotavirus outer capsid comprises 2 neutralization antigens, VP7 and VP4, which respectively define the G (for glycoprotein) and P (for protease-sensitive) serotypes. The 5 globally most common rotavirus strain types comprise long electropherotype P[8] strains possessing G1, G3, G4, or G9 specificity and short electropherotype G2P[4] strains (4). Rotaviruses exhibit considerable diversity, including unusual combinations of electropherotypes and serotypes (which suggests viral reassortment); globally, rare G and P types predominate in some regions (5). For example, we have previously described serotype G8 to be a locally prevalent serotype in Blantyre, Malawi (6). More recently, rotavirus serotype G12 has emerged in multiple countries (7). Our study assesses diversity of rotavirus strains in Lilongwe, Malawi, in anticipation of introduction of a rotavirus vaccine in this country.
The Study
This 2-year, cross-sectional study was undertaken at Kamuzu Central Hospital in Lilongwe from July 2005 through June 2007. Children <5 years of age with acute gastroenteritis who received oral and/or intravenous rehydration therapy, were enrolled after parents or guardians gave written, informed consent. Study participants included outpatients and inpatients. A fecal sample was collected from each case-patient and stored at –80°C until rotavirus detection and characterization were undertaken.
Group A rotavirus antigen was detected by a commercial ELISA performed according to the manufacturer's instructions (Rotaclone; Meridian Diagnostics, Cincinnati, OH, USA). Among rotavirus antigen–positive specimens, specimens that exhibited color intensity at least equal to the positive control provided with the Rotaclone kit were selected for further strain characterization. Genotyping by multiplex, heminested reverse transcription-PCR (RT-PCR) was undertaken as previously described (6). Specimens that remained G nontypeable were analyzed by using a G12-typing primer (8), and those that could not be P-typed were further examined by a degenerate P[8] primer (9). Serotype G12 strains were further examined by polyacrylamide gel electrophoresis (PAGE) of rotavirus dsRNA followed by silver staining to determine the rotavirus electropherotype as previously described (10).
Full-length VP7 genes of G12 strains representing distinct electropherotypes were obtained by RT-PCR by using primers Beg 9 and End 9 (11). Amplification products were purified by using Minispin columns (Amersham, Buckinghamshire, UK) and sequenced by Cogenics Inc. (Hope End, Essex, UK). GenBank accession numbers representing the gene sequence encoding VP7 of each Malawi G12 strain examined are as follows (for each rotavirus strain, the prefix KCH is used to denote Kamuzu Central Hospital): KCH344 (EU573776); KCH1050 (EU573777); KCH1051 (EU573778); KCH1124 (EU573779); KCH569 (EU573780); KCH1074 (EU573781); KCH602 (EU573782).
Rotavirus was detected in 578 (38%) of 1,522 specimens, of which 419 (39%) of 1,070 were from inpatients and 159 (35%) of 452 were from outpatients. A total of 546 rotavirus- positive specimens were further characterized. The most commonly detected strain types included G1P[8] (47%), G8P[8] (12%), G1P[6] (10%), G8P[6] (7%), G8P[4] (6%), and G12 P[6] (4%) (Table). A total of 48 specimens (9%) could not be assigned a G and/or P type. Overall, G1 was the most common G-type (58%), followed by G8 (29%) and G12 (5%); P[8] was the most common P-type (64%) followed by P[6] (23%) and P[4] (7%).
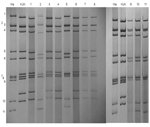 |
Figure 1. Polyacrylamide gel electrophoresis and silver staining of rotavirus double-stranded RNA of representative serotype G12 strains from Lilongwe, Malawi... |
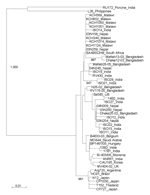 |
Figure 2. Strain designations are followed by country of origin. Malawi strain KCH1051 is genotype G12P[8] and has an indeterminate RNA profile... |
All but 2 G12 strains were detected in the second year of the study (July 2006 through June 2007). The G12 strains were associated with VP4 types P[6] (n = 22), P[8] (n = 5), P[4] (n = 1), and P[NT] (n = 2) and were investigated further by electropherotyping and nucleotide sequencing. Among the 30 G12 strains examined, 23 (77%) produced an identifiable electropherotype. Among G12P[6] strains, 11 displayed short electropherotypes (2 distinct patterns were recognized, with 1 predominant), and 9 strains had long electropherotypes (with 2 distinct patterns recognized). A single P[8] strain, KCH344, had a recognizable but distinct electropherotype (Figure 1). Two G12P[NT] strains possessed, respectively, short and long RNA profiles (data not shown). The single G12P[4] strain had no visible RNA after PAGE. Full-length VP7 sequences that could be successfully obtained by RT-PCR from G12 strains representing distinct electropherotypes were compared with each other and with published G12 sequences from elsewhere in the world (Figure 2). The VP7 genes of 7 Malawian G12 strains shared >99% nucleotide identity with each other, despite possessing a variety of electropherotypes and P-types, and were most closely related to recently identified G12 strains detected in Nepal, India, and South Africa.
Conclusions
Rotavirus was identified as a leading cause of gastroenteritis among infants and young children seeking hospital care in Lilongwe, Malawi; the virus was detected in 38% of all case-patients. The G1P[8] strain, globally the most common rotavirus strain type, was also the most commonly identified rotavirus strain in Lilongwe, comprising 47% of all characterized strains. Serotype G8, first identified in Blantyre, Malawi, in the late 1990s in association with the P[6] and P[4] VP4 types and considered to have arisen by viral reassortment from a bovine origin (6,12), was detected in 29% of strains in association with P-types P[8], P[6], and P[4]. The G8P[8] strain, comprising 41% of all G8 strains in this collection, is gaining increasing global recognition as an emerging strain type (13). In this study, we have also identified the globally common serotype G2, in association with VP4 types P[4] and P[6]. Notably, rotaviruses bearing the P[6] VP4 type comprised 23% of all characterized strains, thus providing further evidence of its prominence in Africa (4) (Table).
We have identified in Malawi the globally emerging rotavirus serotype G12, which was detected in 5% of all specimens. The single previous description of serotype G12 from the African continent was from Johannesburg, South Africa (14). G12 rotaviruses, first identified in the Philippines in 1987, have emerged over the past few years in numerous countries worldwide (7).
In our study, G12 was associated predominantly with the P[6] VP4 type and less commonly with P[8] and P[4]. Multiple electropherotypes were demonstrated within P[6] strains, including long and short profiles. In contrast, the VP7 sequence of strains representing each electropherotype and P-type were closely related, sharing >99% nucleotide identity. These findings suggest the introduction of a single G12 VP7 gene into local rotavirus strains, which have subsequently undergone reassortment to generate G12 strains harboring a constellation of electropherotypes and P-types. Similar diversity among G12 strains has recently been described in Nepal (8,15). The critical role that viral reassortment has played in generating extensive genetic diversity among G12 rotaviruses in Lilongwe mirrors the evolutionary mechanisms that have led to the global emergence of this serotype (7). The close relationship of the VP7 genes of Lilongwe G12 strains with G12 strains from Nepal and India suggests an origin in Asia, a hypothesis proposed by Rahman et al. (7).
Two current rotavirus vaccines, Rotarix (GSK Biologicals, Rixensart, Belgium) and RotaTeq (Merck & Co., Whitehouse Station, NJ, USA) offer the potential to greatly reduce childhood deaths from rotavirus gastroenteritis (2,3). The monovalent vaccine Rotarix comprises a human G1P[8] rotavirus strain, whereas the pentavalent vaccine RotaTeq is a human-bovine reassortant vaccine comprising human serotypes G1, G2, G3, G4, and P[8] on a bovine strain background. Although both vaccines are highly effective in preventing severe rotavirus gastroenteritis in North America, Latin America, and Europe, their efficacy in countries harboring a wider diversity of strain types is yet to be fully established (2,3). Other rotavirus proteins (e.g., VP6 and NSP4) may play a role in the protective immunity against rotavirus infection; G12P[6] strains detected in the current study share neither G- nor P-type with either of the 2 current vaccines and could theoretically challenge vaccine efficacy. Continued surveillance for serotype G12 in Malawi and elsewhere in Africa is needed, given the propensity of this emerging serotype to rapidly spread and establish itself within populations (7,8).
This study was funded by the World Health Organization.
Dr Cunliffe is Senior Clinical Lecturer in Medical Microbiology, University of Liverpool, and Honorary Consultant Microbiologist, Royal Liverpool Children's National Health Service Trust. His research focuses on the epidemiology and prevention of rotavirus gastroenteritis.
References
- Parashar UD, Gibson CJ, Bresee JS, Glass RI. Rotavirus and severe childhood diarrhea. Emerg Infect Dis. 2006;12:304–6.
- Glass RI, Parashar UD, Bresee JS, Turcios R, Fischer TK, Widdowson MA, et al. Rotavirus vaccines: current prospects and future challenges. Lancet. 2006;368:323–32. PubMed DOI
- Cunliffe N, Nakagomi O. Introduction of rotavirus vaccines in developing countries: remaining challenges. Ann Trop Paediatr. 2007;27:157–67. PubMed DOI
- Gentsch JR, Laird AR, Bielfelt B, Griffin DD, Banyai K, Ramachandran M, et al. Serotype diversity and reassortment between human and animal rotavirus strains: implications for rotavirus vaccine programs. J Infect Dis. 2005;192:S146–59. PubMed DOI
- Cunliffe NA, Bresee JS, Gentsch JR, Glass RI, Hart CA. The expanding diversity of rotaviruses. Lancet. 2002;359:640–2. PubMed DOI
- Cunliffe NA, Gondwe JS, Graham SM, Thindwa BD, Dove W, Broadhead RL, et al. Rotavirus strain diversity in Blantyre, Malawi, from 1997 to 1999. J Clin Microbiol. 2001;39:836–43. PubMed DOI
- Rahman M, Matthijnssens J, Yang X, Delbeke T, Arijs I, Taniguchi K, et al. Evolutionary history and global spread of the emerging G12 human rotaviruses. J Virol. 2007;81:2382–90. PubMed DOI
- Pun SB, Nakagomi T, Sherchand JB, Pandey DB, Cuevas LE, Cunliffe NA, et al. Detection of G12 human rotaviruses in Nepal. Emerg Infect Dis. 2007;13:482–4.
- Iturriza-Gomara M, Green J, Brown DW, Desselberger U, Gray JJ. Diversity within the VP4 gene of rotavirus P[8] strains: implications for reverse transcription-PCR genotyping. J Clin Microbiol. 2000;38:898–901.
- Koshimura Y, Nakagomi T, Nakagomi O. The relative frequencies of G serotypes of rotaviruses recovered from hospitalized children with diarrhea: a 10-year survey (1987–1996) in Japan with a review of globally collected data. Microbiol Immunol. 2000;44:499–510.
- Gouvea V, Glass RI, Woods P, Taniguchi K, Clark HF, Forrester B, et al. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J Clin Microbiol. 1990;28:276–82.
- Cunliffe NA, Gentsch JR, Kirkwood CD, Gondwe J, Dove W, Nakagomi O, et al. Molecular and serologic characterization of novel serotype G8 human rotavirus strains detected in Blantyre, Malawi. Virology. 2000;274:309–20. PubMed DOI
- Steyer A, Poljsak-Prijatelj M, Bufon TL, Marcun-Varda N, Marin J. Rotavirus genotypes in Slovenia: unexpected detection of G8P[8] and G12P[8] genotypes. J Med Virol. 2007;79:626–32. PubMed DOI
- De Beer M, Page N, Dewar J, Steele D. Molecular analysis of rotavirus strains from the Johannesburg area in 2004: emergence of serotype G12 rotavirus strains. 9th International Symposium on Double Stranded RNA viruses, October 2006, Cape Town, South Africa (PW6.7).
- Uchida R, Pandey BD, Sherchand JB, Ahmed K, Yokoo M, Nakagomi T, et al. Molecular epidemiology of rotavirus diarrhea among children and adults in Nepal: detection of G12 strains with P[6] or P[8] and a G11P[25] strain. J Clin Microbiol. 2006;44:3499–505. PubMed DOI
Figures
Figure 1. Polyacrylamide gel electrophoresis and silver
staining of rotavirus double-stranded RNA of representative serotype G12
strains from Lilongwe, Malawi...
Figure 2. Strain designations are followed by country of origin. Malawi
strain KCH1051 is genotype G12P[8] and has an indeterminate RNA profile...
Table
Table. Distribution of rotavirus G and P types in Lilongwe, Malawi, July 2005–June 2007
Suggested Citation for this Article
Cunliffe NA, Ngwira BM, Dove W, Nakagomi O, Nakagomi T, Perez A, et al. Serotype G12 rotaviruses, Lilongwe, Malawi. Emerg Infect Dis [serial on the Internet]. 2009 Jan [date cited]. Available from http://www.cdc.gov/EID/content/15/1/87.htm
DOI: 10.3201/eid1501.080427
Please use the form below to submit correspondence to the authors or contact them at the following address:
Nigel A. Cunliffe, Department of Medical Microbiology, University of Liverpool, Duncan Bldg, Daulby St, Liverpool L69 3GA, UK; email: n.a.cunliffe@liv.ac.uk
Please contact the EID Editors at eideditor@cdc.gov
The opinions expressed by authors contributing to this journal do not necessarily reflect the opinions of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.
This page posted December 23, 2008
Centers for Disease Control and Prevention, 1600 Clifton Rd, Atlanta, GA 30333, U.S.A
Tel: (404) 639-3311 / Public Inquiries: (404) 639-3534 / (800) 311-3435