ISSN: 1080-6059
Volume 15, Number 1–January 2009
Dispatch
Enterovirus 71 Outbreak, Brunei
Sazaly AbuBakar, ![]() I-Ching Sam, Jaliha Yusof, Meng Keang Lim,
Suzana Misbah, NorAziyah MatRahim, and Poh-Sim Hooi
I-Ching Sam, Jaliha Yusof, Meng Keang Lim,
Suzana Misbah, NorAziyah MatRahim, and Poh-Sim Hooi
Author affiliations: University Malaya, Kuala Lumpur,
Malaysia (S. AbuBakar, I-C. Sam, S. Misbah, N. MatRahim, P.-S. Hooi); and Raja
Isteri Pengiran Anak Saleha Hospital, Bandar Seri Begawan, Brunei Darussalam
(J. Yusof, M.K. Lim)
Suggested citation for this article
Abstract
Enterovirus 71 (EV71) outbreaks occur periodically in the
Asia-Pacific region. In 2006, Brunei reported its first major outbreak of EV71
infections, associated with fatalities from neurologic complications. Isolated
EV71 strains formed a distinct lineage with low diversity within subgenogroup
B5, suggesting recent introduction and rapid spread within Brunei.
Enterovirus 71 (EV71), a member of the family Picornaviridae and the genus Enterovirus, is a common cause of hand, foot, and mouth disease in children. Infection with this virus is rarely complicated by severe neurologic disease, such as meningitis, brain stem encephalitis, neurogenic pulmonary edema, and acute flaccid paralysis. EV71 was first isolated in 1969 (1), and during the subsequent 30 years, outbreaks were reported in the United States, Europe, and Asia (2). Since 1997, several major outbreaks with deaths have occurred in the Asia-Pacific region, notably in Sarawak (East Malaysia), Peninsular Malaysia, Taiwan, Australia, Singapore, Japan, and Vietnam (3–10).
Brunei is situated on the island of Borneo (4°30´N, 114°E) and has a population of ≈370,000. From February through August 2006, Brunei experienced its first reported major outbreak of EV71. More than 1,681 children reportedly were affected, with 3 deaths resulting from severe neurologic disease. We report the virologic findings from this outbreak.
The Study
During March through October 2006, samples from at least 100 patients from Brunei diagnosed with hand, foot, and mouth disease or herpangina were received at the University Malaya Medical Center, Kuala Lumpur, Malaysia. Samples were inoculated into Vero and A549 cell cultures for virus isolation. EV71 was isolated from 34 patients (including 2 who died of severe neurologic complications), and an additional 7 isolates were obtained from Malaysian patients seen at the University Malaya Medical Center during the outbreak period in Brunei (Table 1). Adenovirus also was isolated from stool or rectal swabs of 4 patients, of whom 2 were coinfected with EV71; none had neurologic disease.
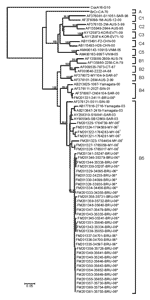 |
Figure. Phylogenetic relationships of enterovirus 71 partial viral protein (VP1) gene sequences. The prototype coxsackievirus A16 (CoxA16-G10) was used as the outgroup virus... |
Enteroviral RNA was extracted from cell cultures using QIAamp Viral RNA Mini Kit (QIAGEN, Hilden, Germany), and reverse transcription–PCR was performed to amplify the viral capsid protein (VP1) gene at nt positions 31–861. The primers used were VP1F 5´-CAGGCTAGCATGGGAGATAGGGTGGCAGATGTGATCGAGAGC-3´ and VP1R 5´-GGTGGATCCCAAAGGGTAGTAATGGCAGTACGACTAGTGCCGGT-3´. The 831-nt partial VP1 gene fragments were sequenced, and phylogenetic relations of the sequences were examined using selected enterovirus reference strains obtained from GenBank (Table 2). Sequences were aligned and phylogenetic trees were drawn using the neighbor-joining method (Figure), as described (12). Maximum-likelihood tree showed similar clustering and is not shown. The prototype coxsackievirus A16 (CoxA16-G10) was used as the outgroup virus for construction of the phylogenetic tree.
The phylogenetic tree, drawn on the basis of the alignment of the VP1 gene sequences, showed 3 independent genogroups (A, B, and C) with the prototype BrCr strain as the only member of genogroup A (11). Within each of genogroups B and C, 5 additional subgenogroups were identified, designated B1–B5 and C1–C5 (8,10). Although no definitions have been established, generally there is nucleotide variation of ≈16%–20% between genogroups and differences of ≈6%–12% between subgenogroups within each genogroup (5,11).
All Brunei and Malaysia isolates from 2006 clustered into subgenogroup B5, except for 1 Brunei isolate, which grouped to subgenogroup B4. Nucleotide sequences of the VP1 gene were highly similar (96%–100%) among all strains in subgenogroup B5. All Brunei B5 isolates were clustered in an independent lineage within subgenogroup B5 (99.9% bootstrap support), separate from the established Sarawak and Yamagata isolates from 2003 (8). Amino acid sequences were highly conserved among the Brunei B5 isolates, with 99%–100% similarity. No amino acid sequence changes were observed in the 2 isolates from patients who died.
Conclusions
The different genogroups of EV71 are widely distributed around the world (2). The continuing appearance of new EV71 subgenogroups in recent years in the Asia-Pacific region suggests that the virus is continuously evolving (5,8,9). The annual rate of evolution is estimated at 1.35 ×10–2 substitutions per nucleotide, similar to poliovirus (11). In some countries, outbreaks occur in a cyclical pattern every 3 years, predominantly caused by strains that are distinct from previous outbreaks (3,9). These strains often have been detected in other countries in the region in years preceding the outbreak. In some EV71 outbreaks, other enteroviruses cocirculate, particularly coxsackievirus A16 or EV71 from a different subgenogroup (3,8,10). On the basis of the samples received in the study, the Brunei 2006 EV71 outbreak was caused by subgenogroup B5 virus. Apart from the single isolate from subgenogroup B4, no other enteroviruses were isolated, although 2 patients also had adenovirus. Occasional EV71 and adenovirus co-infection has been reported (13), also without association with severe disease. The low sequence diversity and predominance of the Brunei B5 isolates in this outbreak suggest recent introduction and subsequent rapid spread, without the concurrent spread of other genogroups, subgenogroups, or enteroviruses.
Other than its northern coastline, Brunei is surrounded entirely by the East Malaysian state of Sarawak. In 2006, an outbreak of EV71 affected approximately 14,400 children in Sarawak (14). Thus, temporally and geographically, the Brunei and Sarawak outbreaks were related, raising the possibility that the same strains were involved. Sarawak had experienced EV71 outbreaks every 3 years (1997, 2000, and 2003), caused by subgenogroups B3, B4, and B5, respectively (3). However, no sequence results from the Sarawak 2006 outbreak are available for comparison. All subgenogroup B5 isolates reported seem to have diverged from an ancestral strain related to strain 5511/SIN/00 (GenBank accession no. AF376121), isolated in Singapore as early as 2000 (3). Subsequently, subgenogroup B5 emerged in Japan (8) and Sarawak (3) in 2003, before appearing in Peninsular Malaysia and Brunei in 2006. The source of the Brunei outbreak remains unclear, and it may not be one of these countries where subgenogroup B5 has already been reported. However, EV71 subgenogroup B5 clearly continues to diverge, and further subgenogroups are likely to arise.
In summary, the first reported major outbreak of EV71 in Brunei was caused by strains from subgenogroup B5 that were distinct from other reported B5 isolates, suggesting a recent introduction from an as-yet-unidentified source. Hence, continued molecular surveillance of EV71 in Asia is required to further our understanding of factors influencing the evolution of the virus and its association with emergence of outbreaks in the region.
Acknowledgments
We thank the Virology Laboratory, Department of Laboratory Services, RIPAS Hospital, and the Disease Control Division, Public Health Department, Ministry of Health of Brunei Darussalam, for their work during the outbreak.
The study was funded in part by Top Down grant 36-02-03-6002 from the Ministry of Science, Technology and Innovation, Malaysia, and by grant FQ016-2007A from University Malaya, Kuala Lumpur, Malaysia.
Dr AbuBakar is a professor and head of the Department of Medical Microbiology, Faculty of Medicine, University of Malaya, Malaysia. His research interests include pathogenesis and emerging virus infections.
References
- Schmidt NJ, Lennette EH, Ho HH. An apparently new enterovirus isolated from patients with disease of the central nervous system. J Infect Dis. 1974;129:304–9.
- Bible JM, Pantelidis P, Chan PK, Tong CY. Genetic evolution of enterovirus 71: epidemiological and pathological implications. Rev Med Virol. 2007;17:371–9. PubMed DOI
- Ooi MH, Wong SC, Podin Y, Akin W, del Sel S, Mohan A, et al. Human enterovirus 71 disease in Sarawak, Malaysia: a prospective clinical, virological, and molecular epidemiological study. Clin Infect Dis. 2007;44:646–56. PubMed DOI
- AbuBakar S, Chee HY, Al-Kobaisi MF, Xiaoshan J, Chua KB, Lam SK. Identification of enterovirus 71 isolates from an outbreak of hand, foot and mouth disease (HFMD) with fatal cases of encephalomyelitis in Malaysia. Virus Res. 1999;61:1–9. PubMed DOI
- Kung SH, Wang SF, Huang CW, Hsu CC, Liu HF, Yang JY. Genetic and antigenic analyses of enterovirus 71 isolates in Taiwan during 1998–2005. Clin Microbiol Infect. 2007;13:782–7. PubMed DOI
- McMinn P, Lindsay K, Perera D, Chan HM, Chan KP, Cardosa MJ. Phylogenetic analysis of enterovirus 71 strains isolated during linked epidemics in Malaysia, Singapore, and Western Australia. J Virol. 2001;75:7732–8.
- Singh S, Chow VTK, Chan KP, Ling AE, Poh CL. RT-PCR. Nucleotide, amino acid, and phylogenetic analyses of enterovirus type 71 strains from Asia. J Virol Methods. 2000;88:193–204. PubMed DOI
- Mizuta K, Abiko C, Murata T, Matsuzaki Y, Itagaki T, Sanjoh K, et al. Frequent importation of enterovirus 71 from surrounding countries into the local community of Yamagata, Japan, between 1998 and 2003. J Clin Microbiol. 2005;43:6171–5. PubMed DOI
- Hosoya M, Kawasaki Y, Sato M, Honzumi K, Kato A, Hiroshima T, et al. Genetic diversity of enterovirus 71 associated with hand, foot and mouth disease epidemics in Japan from 1983 to 2003. Pediatr Infect Dis J. 2006;25:691–4. PubMed DOI
- Tu PV, Thao NT, Perera D, Huu TK, Tien NT, Thuong TC, et al. Epidemiologic and virologic investigation of hand, foot, and mouth disease, Southern Vietnam, 2005. Emerg Infect Dis. 2007;13:1733–41.
- Brown BA, Oberste MS, Alexander JP Jr, Kennett ML, Pallansch MA. Molecular epidemiology and evolution of enterovirus 71 strains isolated from 1970 to 1998. J Virol. 1999;73:9969–75.
- AbuBakar S, Wong PF, Chan YF. Emergence of dengue virus type 4 genotype IIA in Malaysia. J Gen Virol. 2002;83:2437–42.
- AbuBakar S, Shafee N, Chee HY. Adenovirus in EV71-associated hand, foot, and mouth disease. Lancet. 2000;355:146. PubMed DOI
- Sarawak Health Department/Ministry of Health Malaysia. Hand, foot and mouth disease [cited 2008 Sep 1]. Available from http://www.sarawak.health.gov.my/hfmd.htm#INFO9
Figure
Tables
Table 1. Enterovirus 71 from Brunei and Malaysia isolated in 2006
Table 2. Reference enterovirus 71 sequences used for phylogenetic analysis
Suggested Citation for this Article
AbuBakar S, Sam I-C, Yusof J, Lim MK, Misbah S, MatRahim N, et al. Enterovirus 71 outbreak, Brunei. Emerg Infect Dis [serial on the Internet]. 2009 Jan [date cited]. Available from http://www.cdc.gov/EID/content/15/1/79.htm
DOI: 10.3201/eid1501.080264
Please use the form below to submit correspondence to the authors or contact them at the following address:
Sazaly AbuBakar, Department of Medical Microbiology, Faculty of Medicine, University Malaya, 50603 Kuala Lumpur, Malaysia; email: sazaly@um.edu.my
Please contact the EID Editors at eideditor@cdc.gov
The opinions expressed by authors contributing to this journal do not necessarily reflect the opinions of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.
This page posted December 23, 2008
Centers for Disease Control and Prevention, 1600 Clifton Rd, Atlanta, GA 30333, U.S.A
Tel: (404) 639-3311 / Public Inquiries: (404) 639-3534 / (800) 311-3435