|
|
||||||||||||||||
|
|
|
|
|
|||||||||||||
|
|
Control and Prevention Division of Cancer Prevention and Control 4770 Buford Hwy, NE MS K-64 Atlanta, GA 30341-3717 Call: 1 (800) CDC-INFO TTY: 1 (888) 232-6348 FAX: (770) 488-4760 E-mail: cdcinfo@cdc.gov Submit a Question Online |
|
|
|
NPCR–MERP E-Path Pilot Project WorkgroupThe E-Path Pilot Project Workgroup is a collaboration between CDC's National Program of Cancer Registries (NPCR), LabCorp, Inc., CDC's Public Health Information Network, and 18 state central cancer registries, including Alabama, Arizona, California, Colorado, Florida, Georgia, Michigan, Missouri, Nevada, New Hampshire, New Jersey, New York, North Carolina, Ohio, Oklahoma, Tennessee, Texas, and Virginia. States Participating in E-Path Pilot Project 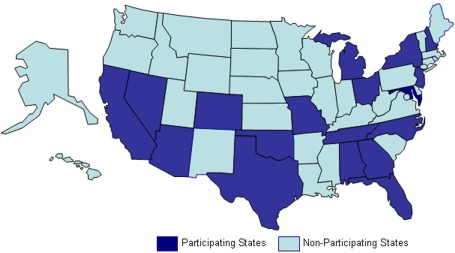 This workgroup will test electronic anatomical pathology reporting from a national laboratory to state central cancer registries. This workgroup will use the recently approved standard in the NAACCR Volume V Standard for Pathology Laboratory Electronic Reporting* and the business rules defined in the draft NAACCR ePath Reporting Process Guide. The workgroup will explore the feasibility of integrating electronic pathology reporting for cancer registries into the infrastructure for electronic laboratory reporting of communicable diseases and biosurveillance. This workgroup can help the cancer registry community use consistent standards for electronic pathology reporting that will improve the completeness, timeliness, and quality of cancer registry data. A Web conference is held every second Thursday of the month, and minutes are sent out the following Thursday. Recent Accomplishments
Phase II/III Project Activities
*Links to non-Federal organizations found at this site are provided solely as a service to our users. These links do not constitute an endorsement of these organizations or their programs by CDC or the Federal Government, and none should be inferred. CDC is not responsible for the content of the individual organization Web pages found at these links.
Page last reviewed: January 13, 2009
Page last updated: January 13, 2009 Content source: Division of Cancer Prevention and Control, National Center for Chronic Disease Prevention and Health Promotion |
|
|
|
|
||||||||||||
|