Learning Objective
At the end of the case study, you should be able to:
Describe a strategy for testing and reporting carbapenemase-producing isolates of Enterobacteriaceae
Case:
A 56-year old woman who has been in the hospital for four days for treatment of metastatic breast cancer develops fever with shaking chills. Two sets of blood culture bottles become positive for gram-negative bacilli. The identification of the organism via an automated identification and antimicrobial susceptibility testing (ID/AST) system is Klebsiella pneumoniae.
The antimicrobial susceptibility profile of the organism is shown below: | Agent | MIC (mcg/mL) | Interpretation | | Amikacin | >32 | R | | Ampicillin | >32 | R | | Cefazolin | >32 | R | | Ceftazidime | >32 | R | | Ceftriaxone | >32 | R | | Cefepime | >32 | R | | Ciprofloxacin | >4 | R | | Gentamicin | 4 | S | | Imipenem | 4 | S | | Meropenem | 4 | S | | Piperacillin-Tazobactam | >128/4 | R | | Tobramycin | >16 | R | | Trimethoprim-Sulfamethoxazole | >4/76 | R | | Automated ESBL test: | | negative |
Question 1. This multi-drug resistant K. pneumoniae shows carbapenem MIC results (imipenem and meropenem) that are in the susceptible range but are unusually high. Do these results need to be confirmed? Is additional testing warranted? Answer: Yes to both questions. The MIC test should be repeated to confirm the unusual carbapenem results. In addition, according to the most recent Clinical and Laboratory Standards Institute (CLSI) document M100-S18, the imipenem and meropenem susceptible MIC results of 4 mcg/mL suggest that this K. pneumoniae may contain a carbapenem inactivating enzyme, such as a KPC beta-lactamase (Klebsiella Pneumoniae Carbapenemase). Consequently, additional testing is necessary to determine if a K. pneumoniae isolate, for which the imipenem or meropenem MICs are 4 mcg/mL, produces a carbapenemase. Carbapenemase producers may result in clinical failure when a patient is treated with a carbapenem even if the MIC or zone diameter results for carbapenems are in the susceptible range. Question 2: How should we confirm the original results? Answer: The original results from the ID/AST system showing increased imipenem and meropenem MICs can be confirmed using the same type of card or panel. Alternatively, you may choose a different method of testing (either MIC or disk diffusion) to confirm the unusual carbapenem phenotype (i.e., MICs in the upper end of the susceptible range, which are 2-3 doubling dilutions higher than the typical MICs for carbapenem-susceptible K. pneumoniae isolates – [e.g., see 2007 EUCAST data http://www.srga.org/eucastwt/WT_EUCAST.htm, see below]). By disk diffusion, zones of inhibition between 17 and 21 mm around meropenem disks are often seen with Enterobacteriaceae isolates for which the imipenem and meropenem MICS are 2-4 mcg/mL. 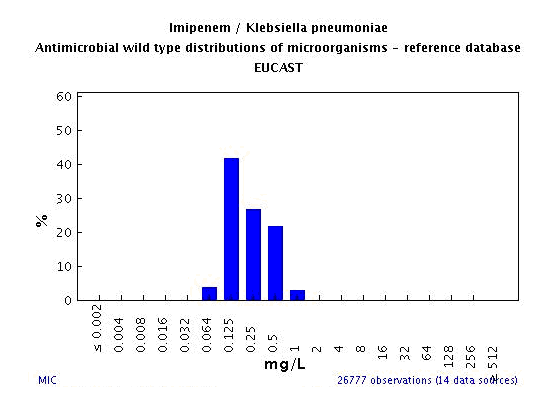 Question 3. Question 3. How would testing for a carbapenemase impact the susceptibility test report? Answer: Detecting a carbapenemase in the isolate of K. pneumoniae would indicate that the “susceptible” results for ertapenem, imipenem, meropenem, and the newly released doripenem, need to be interpreted with caution. These drugs may not be effective clinically, particularly for treating systemic infections like bacteremia. This is analogous to testing for ESBL-producing organisms that may yield MIC results for extended-spectrum cephalosporins in the susceptible range, even though those drugs will not be effective clinically. Thus, current methods for detecting carbapenem-resistant Enterobacteriaceae often lack sensitivity for detecting KPC and other carbapenemase producers and require the use of supplemental tests to improve detection. Question 4: Will ertapenem MICs also increase with carbapenemase producers? Answer: Yes. However, while ertapenem is a much more sensitive indicator of carbapenem resistance, it is less specific for carbapenemase production. In other words, the increase in the ertapenem MICs may be due to mechanisms of resistance other than carbapenemases. For example, ertapenem MICs often increase when an organism loses one or more of its porins (water channels in the cell wall that allow passage of antimicrobial agents and other molecules inside the cell). The presence of beta-lactamases other than carbapenemases (like AmpC or CTX-M beta-lactamases) together with porin changes, can result in ertapenem MICs in the intermediate or resistant range, while imipenem and meropenem remain susceptible (and in some instances clinically active). Question 5: Which isolates should be tested for carbapenamase activity? Answer: Isolates that meet the following two criteria should be tested for carbapenamase activity: 1.) one or more carbapenem results are susceptible, with MIC or disk diffusion zone diameters that are close to the standard CLSI susceptible breakpoints (see below), and 2.) one or more third-generation cephalosporin results are resistant.
| | Standard CLSI susceptible breakpoints | Values suggesting carbapenemase activity* | | MIC (mcg/mL) | Disk (mm) | MIC (mcg/mL) | Disk (mm) | | Ertapenem | ≤2 | ≥19 | 2 | 19-21 | | Imipenem | ≤4 | ≥16 | 2-4 | N/A† | | Meropenem | ≤4 | ≥16 | 2-4 | 16-21 | * Isolates with intermediate or resistant carbapenem results need not be tested for carbapenemase activity. † N/A, not applicable (poor test performance)
Question 6: How can I test this K. pneumoniae isolate (or other isolates which meet the criteria listed above) for carbapenemase activity? Answer: The disk-based phenotypic confirmatory test for carbapenem inactivation (also called the modified Hodge test) detects carbapenemase production among the Enterobacteriaceae. In this assay, a carbapenem-susceptible organism, such as Escherichia coli ATCC 25923 is inoculated onto a Mueller-Hinton agar plate. Carbapenem disks are placed on the plate and the test organism (in this case, the K. pneumoniae isolate) is streaked from the disk (using an inoculating loop) towards the periphery of the plate or towards the center of the plate as shown in the figure below. A carbapenemase-negative organism, such as K. pneumoniae ATCC BAA-1705, should be inoculated on the other side of as disk as a control. A positive control (i.e., ATCC BAA-1706) should be tested at the same time (may be on a separate plate). If a carbapenemase is present in the test organism, it will diffuse in the agar away from the streak and inactivate the carbapenem in the zone of inhibition, allowing the E. coli to grow along the streak of the test organism towards the carbapenem disk. This carbapenem inactivation test is not specific for KPC beta-lactamases but will be positive for most carbapenem inactivating enzymes (such as VIM, IMP, or IMI beta-lactamases) (See figure below). 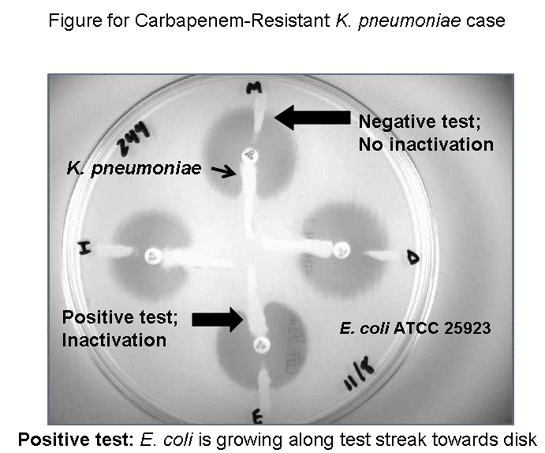 Question 7: How do I report the carbapenem results if the phenotypic confirmatory test for carbapenemase activity is positive? Answer: There are two options for reporting the results if the test is positive. Option one (for MIC results) is to report the carbapenem MIC results without interpretations and to add a comment to the report indicating that the clinical efficacy of carbapenems for treating infections caused by Enterobacteriaceae with carbapenem MIC results in the standard CLSI susceptible range, but with confirmed carbapenemase production, has not been established. For example, "Although this isolate tests as susceptible to carbapenems, such as impenem and meropenem, it contains an enzyme that inactivates these drugs. Carbapenem drugs should be used with caution." Option two (for disk diffusion results or MIC results) is to change the susceptible results for all carbapenems to either intermediate or resistant. A comment regarding the presence of a carbapenemase similar to the one above may also be added to the report. Check with your Laboratory Director, and the Pharmacy and Infectious Disease services, about the most appropriate way to report results for the phenotypic carbapenemase confirmation test in your institution. Question 8: The KPC beta-lactamase is a Class A enzyme and is an ESBL (i.e., it inactivates extended-spectrum cephalosporins). Why is the ESBL screen-test (with clavulanic acid) negative for this organism? Answer: K. pneumoniae isolates that produce a KPC beta-lactamase typically harbor other beta-lactamases as well. These often overcome the clavulanic acid used in the ESBL confirmation test producing a negative result. Some KPC-producing strains are ESBL screen test positive. Question 9: Should the preliminary culture and susceptibility report advise the physician that additional testing is being performed to detect carbapenemase production? Answer: Yes. The laboratory may issue a preliminary report that either suppresses the carbapenem results, or notes that the carbapenem susceptible results are unusual and require confirmation. A comment may be included that further testing to detect carbapenemase production by the isolate is in progress. The laboratory may also choose to alert the infectious disease service and infection control department that a multi-drug resistant K. pneumoniae isolate that is potentially carbapenem-resistant has been isolated. Question 10: Is there a PCR test for the genes encoding the KPC beta-lactamases? Answer: Yes, however, a negative PCR test for the genes encoding KPC beta-lactamases does not rule out the presence of other carbapenemase genes. Question 11: What other antimicrobial agents should be considered for testing against this isolate of K. pneumoniae? Answer: Several antimicrobial agents, including tigecycline and colistin, may show activity against carbapenem-resistant strains of K. pneumoniae; however, standardized methods of testing, and MIC and disk diffusion breakpoints have yet to be established by CLSI. The FDA breakpoints for tigecycline for Enterobacteriaceae are
| MIC (mcg/ml): | | Susceptible | Intermediate | Resistant | ≤2
| 4
| ≥8
|
| Disk Diffusion (mm): | | Resistant | Intermediate | Susceptible | | ≤14 | 15-18 | ≥19 |
There are no CLSI or FDA breakpoints for colistin for Enterobacteriaceae, but the European Committee for Antimicrobial Susceptibility Testing (EUCAST) breakpoints for colistin and Enterobacteriaceae are:
| MIC (mcg/ml): |
Susceptible | Resistant | | ≤2 | ≥4 |
Colistin should not be tested by disk diffusion. Check with your Laboratory Director, and the Pharmacy and Infectious Disease services, about the most appropriate way to report results for colistin in your institution. Question 12: Have KPC beta-lactamases been detected in other Enterobacteriaceae besides K. pneumoniae? Answer: Yes. Isolates of E. coli, Salmonella, Citrobacter, Enterobacter, Serratia, and even non- Enterobacteriaceae, such as Pseudomonas aeruginosa have been shown to produce KPC beta-lactamases. The blaKPC genes can be located on plasmids, transposons, and integrons facilitating their wide distribution among many bacterial species. Question 13: Should carbapenemase-producing isolates of Enterobacteriaceae be reported to local or state health departments? Answer: In general, carbapenemase-producing Enterobacteriaceae have not been designated as reportable by most local or state health departments. However, there is considerable concern among public health officials in many states about the emergence of these organisms, or their spread in areas where such organisms have already been recognized. Check with your local or state health department for current policies on reporting. References:
- Anderson, K. F., D. R. Lonsway, J. K. Rasheed, J. Biddle, B. Jensen, L. K. McDougal, R. B. Carey, A. Thompson, S. Stocker, B. Limbago, and J. B. Patel. 2007. Evaluation of methods to identify the Klebsiella pneumoniae carbapenemase in Enterobacteriaceae. J Clin Microbiol 45:2723-5.
- Bratu, S., M. Mooty, S. Nichani, D. Landman, C. Gullans, B. Pettinato, U. Karumudi, P. Tolaney, and J. Quale. 2005. Emergence of KPC-possessing Klebsiella pneumoniae in Brooklyn, New York: epidemiology and recommendations for detection. Antimicrob Agents Chemother 49:3018-20.
- Chiang, T., N. Mariano, C. Urban, R. Colon-Urban, L. Grenner, R. H. Eng, D. Huang, H. Dholakia, and J. J. Rahal. 2007. Identification of carbapenem-resistant Klebsiella pneumoniae harboring KPC enzymes in New Jersey. Microb Drug Resist 13:235-39.
- Deshpande, L. M., P. R. Rhomberg, H. S. Sader, and R. N. Jones. 2006. Emergence of serine carbapenemases (KPC and SME) among clinical strains of Enterobacteriaceae isolated in the United States Medical Centers: report from the MYSTIC Program (1999-2005). Diagn Microbiol Infect Dis 56:367-72.
- Elliott, E., A. J. Brink, J. van Greune, Z. Els, N. Woodford, J. Turton, M. Warner, and D. M. Livermore. 2006. In vivo development of ertapenem resistance in a patient with pneumonia caused by Klebsiella pneumoniae with an extended-spectrum beta-lactamase. Clin Infect Dis 42:e95-8.
- Tenover, F. C., R. K. Kalsi, P. P. Williams, R. B. Carey, S. Stocker, D. Lonsway, J. K. Rasheed, J. W. Biddle, J. E. McGowan, Jr., and B. Hanna. 2006. Carbapenem resistance in Klebsiella pneumoniae not detected by automated susceptibility testing. Emerg Infect Dis 12:1209-13.
- Woodford, N., R. L. Hill, and D. M. Livermore. 2007. In vitro activity of tigecycline against carbapenem-susceptible and -resistant isolates of Klebsiella spp. and Enterobacter spp. J Antimicrob Chemother 59:582-3.
- Yigit, H., G. J. Anderson, J. W. Biddle, C. D. Steward, J. K. Rasheed, L. L. Valera, J. E. McGowan, Jr., and F. C. Tenover. 2002. Carbapenem resistance in a clinical isolate of Enterobacter aerogenes is associated with decreased expression of OmpF and OmpC porin analogs. Antimicrob Agents Chemother 46:3817-22.
- Yigit, H., A. M. Queenan, G. J. Anderson, A. Domenech-Sanchez, J. W. Biddle, C. D. Steward, S. Alberti, K. Bush, and F. C. Tenover. 2001. Novel carbapenem-hydrolyzing beta-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob Agents Chemother 45:1151-61.
|