CHEMICAL-SPECIFIC HEALTH CONSULTATION:
TREMOLITE ASBESTOS
AND OTHER RELATED TYPES OF ASBESTOS
September 2001
Prepared by
U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES
Agency for Toxic Substances and Disease Registry
Division of Toxicology
Atlanta, Georgia 30333
ACKNOWLEDGMENTS
The Agency for Toxic Substances and Disease Registry acknowledges Peter R. McClure, Ph.D., DABT, and Gloria W. Sage, Ph.D., from Syracuse Research Corporation for their assistance in developing this consultation.
The agency acknowledges Anne Olin as the editor of this document, and the contributions from the following Division of Toxicology scientific staff: Susan Kess, M.D., M.P.H; Carolyn A. Tylenda, D.M.D., Ph.D.; Rich Nickle; Yee-Wan Stevens, M.S.; Sharon Wilbur, M.A.; Malcolm Williams, D.V.M., Ph.D.; Cassandra Smith, M.S.; Doug Hanley, M.D.; John Wheeler, Ph.D., DABT; and Ed Murray, Ph.D.
CONTENTS
EXECUTIVE SUMMARY
Introduction
Definitions of Terms Used To Discuss Health Effects from Asbestiform Minerals
Chemistry of Amphibole Minerals
Occurrence of Tremolite Asbestos
Occurrence in Vermiculite
Occurrence in Chrysotile
Occurrence in Talc
Detection and Analysis of Asbestos in Air Samples
Potential for Human Exposure to Asbestos
Health Effects from Asbestos: Overview
Consensus Issues and Conclusions
Unresolved Issues and Discussions
Deposition and Clearance of Inhaled Asbestos Fibers: Overview
Mechanisms of Asbestos Fiber Toxicity: Overview
Health Effects from Tremolite Asbestos
Nonmalignant Respiratory Effects: Pulmonary Fibrosis and Pleural Changes
Lung Cancer
Mesothelioma
Overall Health Effects Weight of Evidence
Clinical Aspects of Diseases Associated with Exposure to Asbestos
Conclusions
Recommendations
References
Footnotes
The U.S. Department of Health and Human Services (DHHS) is addressing public health concerns regarding a fibrous amphibole that occurs in vermiculite ore in the Libby, Montana, area. Scientists agree that exposure to this mineral increased the risk of nonmalignant respiratory and pleural disorders, lung cancer, and mesothelioma in groups of people who worked in the now closed Libby vermiculite mine and mill. These health problems are similar to those experienced by workers exposed to other types of asbestos before modern workplace air regulations were established. The Agency for Toxic Substances and Disease Registry (ATSDR) has prepared this chemical-specific health consultation to provide support for public health decisions regarding individuals exposed to fibrous amphibole from Libby vermiculite or other related asbestos-containing materials. Key technical terms used in discussing asbestos-related health problems are defined after the Introduction.
Physical and Chemical Properties, Occurrence, and Detection: Tremolite Asbestos
Asbestos is the name of a group of highly fibrous minerals with separable, long, and thin fibers. Separated asbestos fibers are strong enough and flexible enough to be spun and woven, are heat resistant, and are chemically inert. Minerals with these asbestos characteristics are said to have an asbestiform habit.Regulatory agencies such as the U.S. Environmental Protection Agency (EPA) and Occupational Safety and Health Administration (OSHA) recognize six asbestos minerals: chrysotile, a serpentine mineral; and five amphibole minerals, actinolite asbestos, tremolite asbestos, anthophyllite asbestos, crocidolite asbestos, and amosite asbestos. Nonasbestiform amphibole minerals are not included in U.S. health regulations regarding asbestos because there is insufficient evidence that they will produce adverse health effects of the same type and severity produced by chronic exposure to asbestos.
Samples of the fibrous amphibole in the Libby vermiculite ore, popularly referred to as tremolite asbestos, were recently analyzed by U.S. Geological Survey scientists. On the basis of variable chemical composition, several different mineral names were assigned to the samples: winchite, richterite, tremolite, actinolite, ferro-edenite, and magnesio-arfvedsonite. All of these are classified as amphibole minerals. Most of the samples showed both asbestiform and nonasbestiform habits. Since it is known that this mix of fibrous amphibole increased the risk of typical asbestos-related diseases in groups of people who worked in the Libby, Montana, mine and mill, proposals have been made to consider changing U.S. asbestos regulations to include other asbestiform amphiboles in addition to the five mentioned previously.
Nonasbestiform tremolite is the predominant form of tremolite in the earth's crust, but there are many reports of tremolite asbestos occurring around the world in specific locations (including some locations in Maryland and California) and natural materials. Tremolite asbestos has only rarely been found in commercially mined deposits. It has never been a nationally important commercial source of asbestos in the United States. Two minerals of commercial importance that have been reported to contain tremolite asbestos or other amphibole asbestos are vermiculite and talc.
Before 1990, the now closed mine in Libby, Montana, was a significant source of vermiculite in the United States. In 1998, vermiculite was mined in the United States predominantly in South Carolina and Virginia and was also imported from South Africa and China. A 1984 study reported that the percentage of tremolite asbestos fiber by weight varied from 3.5% to 6.4% in raw vermiculite ore from Libby, Montana. In contrast, several studies of vermiculite mined elsewhere (South Carolina, Virginia, and South Africa) reported that levels of amphibole asbestos were either not detectable or only present at much lower levels than those found in the Libby vermiculite.
Talc ores can also contain a range of other minerals. In the United States, commercial talc is categorized into cosmetic grade, which is free of asbestos, and industrial grade, which may contain other asbestiform or nonasbestiform minerals, depending on intended use. For example, one important U.S. source of industrial-grade talc is a mixture referred to as tremolitic talc. Analysis by OSHA scientists shows that the tremolite in this talc is nonasbestiform.
The combined use of light microscopy, electron microscopy, and energy dispersive X-ray analysis offer the most accurate approach to identify asbestos and estimate concentrations in air samples or bulk samples that may become airborne upon disturbance. For the purposes of counting asbestos fibers in these samples, regulatory agencies commonly count as fibers those particles of asbestos minerals that have lengths ≥5 μm and length:width ratios ≥3:1. For other purposes, such as detecting fibers in bulk building materials, asbestos particles with length:width ratios ≥5:1 are counted. Typical air concentrations of asbestos fibers in ambient air are 0.00001 to 0.0001 fibers per milliliter (fiber/mL). Recent exposure limits for U.S. workplaces are 0.1 to 0.2 fiber/mL.
Exposure Potential: Tremolite Asbestos
Occupational exposure to tremolite asbestos may occur in workers involved in mining, milling, and handling of other ores and rocks that may contain tremolite asbestos (e.g., vermiculite or talc). Residents who live close to mining, milling, or manufacturing sites that involve tremolite asbestos-containing material may be potentially exposed to higher levels of airborne asbestos than levels in general ambient air. EPA, ATSDR, and other agencies currently are investigating past and current exposure to fibrous amphibole found in Libby, Montana, vermiculite. In addition, ATSDR is currently conducting medical testing of individuals who lived close to or worked in the Libby vermiculite mine and mill.
Asbestos can be found in a variety of building materials such as insulation, ceiling or floor tiles, and cement pipes. Amphibole asbestos has been found in some vermiculite sources that have been used as home and building insulation. Workers or homeowners involved in demolition work, maintenance, repair, or remodeling of buildings containing these products can be exposed to higher airborne fibrous amphibole levels than levels in general ambient air. Exposure can occur only when building materials containing asbestos are disturbed in some way to release particles and fibers into the air. When asbestos-containing materials are solidly embedded or contained, exposure will be minimal.
Recently, small amounts of amphibole asbestos have been found in some samples of vermiculite-containing consumer garden products by EPA and in some talc-containing crayons by the U.S. Consumer Product Safety Commission (CPSC). EPA recommended that consumers can reduce possible exposure by limiting the production of dusts when using the garden products. CPSC concluded that the risk is extremely low that children might be exposed to asbestos fibers through inhalation or ingestion of crayons containing asbestos and transitional fibers. The U.S. manufacturers of these crayons, however, have agreed to eliminate talc from their products in the near future.
Health Effects from Asbestos or Tremolite Asbestos
It is known that exposure to any asbestos type (i.e., serpentine or amphibole) will increase the likelihood of lung cancer, mesothelioma (a tumor of the pleura or peritoneum that is rare in the general population), and nonmalignant lung and pleural disorders including interstitial pulmonary fibrosis (asbestosis), pleural plaques, pleural thickening, and pleural effusions. This conclusion is based on observations of these diseases in groups of workers with cumulative exposures ranging from about 5 to 1,200 fiber-year/mL. Such exposures would result from 40 years of occupational exposure to air concentrations of 0.125 to 30 fiber/mL. The conclusion is supported by results from animal and mechanistic studies.
Based on an analysis of the epidemiologic data, EPA calculated that lifetime continuous exposure to asbestos air concentrations of 0.0001 fiber/mL could result in up to 2-4 cancer deaths (lung cancer or mesothelioma) per 100,000 people. This air concentration is within reported ranges of ambient air levels (0.00001 to 0.0001 fiber/mL). The EPA analysis has been extensively discussed and reviewed in the scientific literature. EPA is in the process of reviewing and possibly updating their cancer risk estimates for asbestos.
Important determinants of asbestos toxicity include exposure concentration, duration, and frequency, and fiber dimensions and durability. Long and thin fibers are expected to reach the lower airways and alveolar regions of the lung, to be retained in the lung longer, and to be more toxic than short and wide fibers or particles. Wide particles are expected to be deposited in the upper respiratory tract and not to reach the lung and pleura, the sites of asbestos-induced toxicity. Short, thin fibers, however, may also play a role in asbestos pathogenesis. Fibers of amphibole asbestos such as tremolite asbestos, actinolite asbestos, and crocidolite asbestos are retained longer in the lower respiratory tract than chrysotile fibers of similar dimension.
Diseases from asbestos exposure take a long time to develop. Most cases of lung cancer or asbestosis in asbestos workers occur 15 or more years after initial exposure to asbestos. Asbestos-exposed tobacco smokers have greater than additive risks for lung cancer than do asbestos-exposed nonsmokers (i.e., the risk is greater than the individual risks from asbestos and smoking added together). The time between diagnosis of mesothelioma and the time of initial occupational exposure to asbestos commonly has been 30 years or more. Cases of mesotheliomas have been reported after household exposure of family members of asbestos workers and in individuals without occupational exposure who live close to asbestos mines.
As with other forms of asbestos, chronic exposure to airborne tremolite asbestos is expected to increase risks of lung cancer, mesothelioma, and nonmalignant lung and pleural disorders. Evidence in humans comes from epidemiologic studies of tremolite asbestos-exposed groups of vermiculite miners and millers from Libby, Montana. This evidence is supported by reports of increased incidences of nonmalignant respiratory diseases, lung cancer, and mesothelioma in villages in various regions of the world that have traditionally used tremolite-asbestos whitewashes in homes or have high surface deposits of tremolite asbestos and by results from animal studies.
Clinical Diagnosis for Asbestos-Related Diseases
The chest X-ray is the most common and important tool to detect lung and pleural disease caused by chronic exposure to tremolite asbestos or other types of asbestos. Results from pulmonary function tests and high resolution computerized tomography can also be used in the diagnosis.
Biopsy to detect asbestos fibers in pieces of lung tissue, although not needed to make a clinical diagnosis, is the most reliable test to determine asbestos exposure. Less invasive tests can be conducted to detect asbestos fibers or asbestos bodies in bronchoalveolar lavage fluid or in sputum. These tests, however, do not reliably indicate how much asbestos a person may have been exposed to, or predict whether disease will develop.
Treatment Options for Asbestos-Related Diseases
Treatment options for patients diagnosed with nonmalignant lung or pleural disease from chronic exposure to asbestos are few. Preventing of further exposure and ceasing any tobacco smoking activities are the most important steps individuals can take to minimize development of health problems. Once established, these diseases may remain stable or progress in severity in the absence of further exposure. The diseases rarely regress. Treatment options for patients diagnosed with asbestos-related cancer of the lung or pleura are restricted to resection and/or chemotherapy.
Pleural effusions are early manifestations of inhalation exposure to high concentrations of asbestos; the fluid contains varying amounts of red blood cells, macrophages, lymphocytes, and mesothelial cells. Pleural effusions may be an early indication of mesothelioma and warrant further evaluation. Early identification of mesothelioma and intervention may increase chances of survival.
Additional research may help to develop therapeutic methods to interfere with the development of asbestos-induced lung and pleural disorders and to cause the disorders to regress once they are established.
Recommendations
Prevention of exposure and cessation of any tobacco smoking activities are the most important steps that individuals can take to prevent or minimize the development of asbestos-related health problems.
People who were exposed to asbestos and who smoke are expected to be unusually susceptible to asbestos-related lung cancer and asbestosis and are encouraged to cease smoking. Studies of asbestos workers indicate that asbestos-exposed smokers have greater than additive risks for lung cancer and asbestosis than asbestos-exposed nonsmokers.
Individuals residing or working in buildings with insulation or other building materials that may potentially contain asbestiform minerals (for example, vermiculite from the Libby, Montana, mine) are encouraged to ensure that the insulation material is solidly contained and not able to be disturbed and become airborne. If the material is to be removed, special procedures must be followed that minimize the generation of dust and specify appropriate locations for disposal. Individuals can obtain information about asbestos removal and disposal procedures from the 10 regional offices of the EPA.
Further evaluation of the progression of disease associated with exposure to Libby, Montana, vermiculite contaminated with asbestos is warranted. EPA, ATSDR, and other agencies currently are investigating exposure levels that Libby, Montana, residents (including children) who were not employed in the vermiculite mines and mills may have and are experiencing. In addition, ATSDR is currently conducting medical testing of individuals potentially exposed to fibrous amphibole associated with vermiculite in Libby, Montana.
The U.S. Department of Health and Human Services (DHHS) is addressing public health concerns regarding a fibrous amphibole that occurs in vermiculite ore in the Libby, Montana, area. Vermiculite was mined and milled in Libby from 1923 until 1990. In 1963 the mine was acquired from the Zonolite Company by W.R. Grace Company, which marketed the vermiculite as Zonolite®.
The Libby amphibole mineral, popularly known as tremolite asbestos, has been assigned a number of different names by scientists over the years (Meeker et al. 2001; Wylie and Verkouteren 2000); however, scientists agree that exposure to the mineral increased the risk of nonmalignant respiratory and pleural disorders, lung cancer, and mesothelioma in groups of people who worked in the now closed Libby mine and mill. (1) These health problems are similar to those experienced by workers exposed to other types of asbestos before modern workplace air regulations were established.
The Agency for Toxic Substances and Disease Registry (ATSDR) prepared this chemical-specific health consultation to provide support for public health decisions regarding Libby, Montana, and other locations where tremolite asbestos and related asbestos can be found. This document :
- defines terms used to discuss health effects from asbestiform minerals;
- discusses the chemistry of amphibole minerals;
- discusses the occurrence of tremolite asbestos in the earth's crust;
- discusses common methods to detect asbestos in air samples;
- discusses the potential for human exposure to asbestos;
- presents overviews of health effects from asbestos, deposition and clearance of asbestos in the lung, and mechanisms of asbestos toxicity;
- evaluates the weight of evidence that tremolite asbestos can cause mesothelioma, lung cancer, and nonmalignant disorders of the lung and pleura;
- discusses clinical diagnosis for asbestos-related diseases; and
- recommends actions to protect the public from possible health problems from tremolite asbestos and other forms of asbestos.
Evidence that nonasbestiform amphiboles may cause the same effects as amphibole asbestos is outside of the scope of this health consultation. The reader is referred to earlier reports (American Thoracic Society 1990; OSHA 1992) that discuss this issue and to epidemiological studies of workers exposed to mixtures of nonasbestiform amphibole minerals and other nonasbestos minerals including silica, taconite, and talc. For regulatory purposes, the Occupational Safety and Health Administration (OSHA 1992) concluded that there was insufficient evidence that nonasbestiform forms of tremolite, actinolite, and anthophyllite will produce adverse health effects of the same type and severity produced by chronic exposure to amphibole asbestos (OSHA 1992; Vu 1993). Nevertheless, the reader should be aware that repeated exposure to excessive amounts of insoluble dusts of any type can cause adverse health effects including interstitial pulmonary fibrosis (ACGIH 1996; OSHA 1992).
Definitions of Terms Used To Discuss Health Effects from Asbestiform Minerals
Definitions of key technical terms are provided because there has been variable use of some of them in the scientific literature and popular press.
Amphibole: A large group of silicate minerals with more than 40-50 members (Leake 1978; Leake et al. 1997). The molecular structure of all amphiboles consists of two chains of SiO4 molecules that are linked together at the oxygen atoms. In the earth's crust, amphibole minerals are mostly nonasbestiform; asbestiform amphiboles are relatively rare (Veblen and Wylie 1993; Zoltai 1979, 1981). See definitions of asbestiform, mineral, and mineral habit. Also see the Chemistry of Amphibole Minerals section.
Asbestiform: A habit of crystal aggregates displaying the characteristics of asbestos: groups of separable, long, thin, strong, and flexible fibers often arranged in parallel in a column or in matted masses (Veblen and Wylie 1993; Zoltai 1979, 1981). See definitions of mineral and mineral habit. Figure 1 shows a scanning electron micrograph of an asbestiform amphibole mineral showing a parallel arrangement of long fibers. Mineralogists call asbestiform amphibole minerals by their mineral name followed by "asbestos" (Leake 1978). Thus, asbestiform tremolite is called tremolite asbestos.
Asbestos: A group of highly fibrous minerals with separable, long, thin fibers often arranged in parallel in a column or in matted masses (Veblen and Wylie 1993; Zoltai 1979, 1981). Separated asbestos fibers

Scanning electron micrograph of asbestiform amphibole from a former vermiculite mining site near Libby, Montana. Source: U.S. Geological Survey and U.S. Environmental Protection Agency, Region 8, Denver, Colorado.
are generally strong enough and flexible enough to be spun and woven, are heat resistant, and are chemically inert (Veblen and Wylie 1993). See definitions of fibrous and mineral.
Currently, U.S. regulatory agencies, such as the Environmental Protection Agency (EPA) and OSHA, recognize six asbestos minerals: the serpentine mineral, chrysotile; and five asbestiform amphibole minerals, actinolite asbestos, tremolite asbestos, anthophyllite asbestos, amosite asbestos (also known as asbestiform cummingtonite-grunerite), and crocidolite asbestos(also known as asbestiform riebeckite) (ATSDR 2001a; OSHA 1992; Vu 1993). Proposals have been made to update asbestos regulations to include other asbestiform amphibole minerals such as winchite asbestos and richterite asbestos (Meeker et al. 2001; Wylie and Verkouteren 2000). See the Chemistry of Amphibole Minerals section.
Asbestosis: Interstitial fibrosis of the pulmonary parenchymal tissue in which asbestos bodies (fibers coated with protein and iron) or uncoated fibers can be detected (American Thoracic Society 1986). Pulmonary fibrosis refers to a scar-like tissue in the lung which does not expand and contract like normal tissue. This makes breathing difficult. Blood flow to the lung may also be decreased, and this causes the heart to enlarge. People with asbestosis have shortness of breath, often accompanied by a persistent cough. Asbestosis is a slow-developing disease that can eventually lead to disability or death in people who have been exposed to high amounts of asbestos over a long period. Asbestosis is not usually of concern to people exposed to low levels of asbestos. For more information, see the Health Effects from Asbestos: Overview section.
Cleavage fragment: Microscopic particles formed when large pieces of nonasbestiform amphiboles are crushed, as may occur in mining and milling of ores. Within a population of nonasbestiform amphibole cleavage fragments, a fraction of the particles may fit the definition of a fiber adopted for counting purposes. Populations of asbestos fibers can be readily distinguished from populations of nonasbestiform cleavage fragments, but sometimes it can be difficult to distinguish an isolated nonasbestiform cleavage fragment from an isolated asbestos fiber (Crane 2000; OSHA 1992). See definitions of asbestiform, fiber, fibrous, and mineral habit.
Fiber: Any slender, elongated mineral structure or particle. For the purposes of counting asbestos fibers in air samples, regulatory agencies commonly count particles that have lengths ≥5 μm and length:width ratios ≥3:1 as fibers. For detecting asbestos fibers in bulk building materials, particles with length:width ratios ≥5:1 are counted as fibers. See the Detection and Analysis of Asbestos in Air Samples section for more details.
Fiber-year/mL: Epidemiologic studies of groups of asbestos-exposed workers commonly express exposure in cumulative exposure units of fiber-year/mL. This exposure measure is calculated by multiplying a worker's duration of exposure (measured in years) by the average air concentration during the period of exposure (measured in number of fibers/mL of air).
Fibrous: A mineral habit with crystals that look like fibers (Zoltai 1981). A mineral with a fibrous habit is not asbestiform if the fibers are not separable and are not long, thin, strong, and flexible (Veblen and Wylie 1993; Zoltai 1979; 1981).
Interstitial: A term used as an adjective relating to spaces within a tissue or organ. Pulmonary interstitial fibrosis refers to fibrosis (scarring) occurring within lung tissue.
Mesothelioma: Cancer of the thin lining surrounding the lung (the pleura) or the abdominal cavity (the peritoneum). Mesotheliomas are rare cancers in general populations. Mesotheliomas annually accounted for an average of 1.75 deaths per million in the U.S. general population for the period 1987-1996 (NIOSH 1999). For U.S. white males (the U.S. group with the highest mortality rate), the rates were 3.61 per million in 1987 and 2.87 per million in 1996 (NIOSH 1999). See the Health Effects from Asbestos: Overview section for more information.
Mineral: Any naturally occurring, inorganic substance with a crystal structure. Naturally occurring, inorganic substances without a crystal structure (such as amorphous silica) are called mineraloids (Veblen and Wylie 1993).
Mineral Habit: The shape or morphology that single crystals or crystal aggregates take during crystal formation (Veblen and Wylie 1993). Mineral habit is influenced by the environment during crystal formation. Habits of single crystals include prismatic, acicular, platy, and fiber. Habits of crystal aggregates include asbestiform, fibrous, lamellar, and columnar.
Parenchyma: The functional cells or tissue of a gland or organ; for example, the lung parenchyma. The major lung parenchymal abnormality associated with exposure to asbestos is the development of scar-like tissue referred to as pulmonary interstitial fibrosis or asbestosis.
Pleura: A thin lining or membrane around the lungs or chest cavity. This lining can become thickened or calcified in asbestos-related disease.
Pleural: Having to do with or involving the pleura.
Pleural abnormalities: Abnormal or diseased changes occurring in the pleura. Pleural abnormalities associated with exposure to asbestos include pleural plaques, pleural thickening or calcifications, and pleural effusion.
Pleural calcification: As a result of chronic inflammation and scarring, pleura becomes thickened and can calcify. White calcified areas can be seen on the pleura by X-ray.
Pleural cavity: The cavity, defined by a thin membrane (the pleural membrane or pleura), which contains the lungs.
Pleural effusion: Cells (fluid) can ooze or weep from the lung tissue into the space between the lungs and the chest cavity (pleural space) causing a pleural effusion. The effusion fluid may be clear or bloody. Pleural effusions may be an early sign of asbestos exposure or mesothelioma and should be evaluated.
Pleural plaques: Localized or diffuse areas of thickening of the pleura (lining of the lungs or chest cavity. Pleural plaques are detected by chest X-ray, and appear as opaque, shiny, and rounded lesions.
Pleural thickening: Thickening or scarring of the pleura may be associated with asbestos exposure. In severe cases, the normally thin pleura can become thickened like an orange peel and restrict breathing.
Pulmonary interstitial fibrosis: Scar-like tissue that develops in the lung parenchymal tissue in response to inhalation of dusts of certain types of substances such as asbestos.
Serpentinite: Igneous or metamorphic rock chiefly composed of serpentine minerals such as chrysotile or lizardite (Jackson 1997). Chrysotile, when found, can occur in localities with serpentinite rock (Churchill et al. 2001).
Tremolite asbestos: A special form of the amphibole mineral, tremolite, that displays separable, long, thin fibers often arranged in parallel in a column or in matted masses. The fibers are generally strong enough and flexible enough to be spun and woven, are heat resistant, and are chemically inert.
Ultramafic rock: Igneous rock composed chiefly of dark-colored ferromagnesian silicate minerals (Jackson 1997). Asbestiform amphiboles, when found, can occur in localities with ultramafic rock (Churchill et al. 2001).
Vermiculite: A mineral belonging to the mica group of silicate minerals (Ross et al. 1993). Vermiculite has water molecules located between the silicate layers in the crystal structure. When heated, vermiculite expands to form a light-weight material that has been used for home and building insulation, as a soil amendment, and as a packing material. The process of heating and expanding vermiculite is called exfoliation or "popping". Raw vermiculite ore is processed to produce vermiculite concentrate, which is shipped to exfoliating plants to produce the finished vermiculite product.
The photograph in Figure 2 shows a sample of raw vermiculite ore from Libby, Montana, with asbestiform amphibole fibers mixed in with the vermiculite. Figure 3 shows processed vermiculite concentrate (before expansion) and exfoliated vermiculite (after expansion).
Chemistry
of Amphibole Minerals
The molecular structure of all amphiboles consists of two chains of SiO4 molecules that are linked together at the oxygen atoms (Jolicoeur et al. 1992; Skinner et al. 1988; Veblen and Wylie 1993). The chains are bonded together by cations (e.g. Ca, Mg, Fe) and hydroxyl molecules and stacked together to form crystals. The internal crystal structure of all amphiboles is the same, but there is a wide range of chemical variability within the amphibole group. Four subgroups of amphiboles are currently recognized: the magnesium-iron-manganese-lithium subgroup; the calcic subgroup; the sodic-calcic subgroup; and the sodic subgroup (Leake et al. 1997). Amphibole mineral names are based on ideal chemical compositions. The chemical composition of a specific mineral sample is likely to be close to, but not exactly the same as, the ideal chemical composition of its mineral name, because of natural chemical variability in minerals.
Tremolite (Ca2 Mg5Si8O22[OH]2) and ferro-actinolite (Ca2 Fe5Si8O22[OH]2) are mineral names currently applied to end members of a series (2) within the calcic amphibole subgroup in which the magnesium and iron content can vary widely (Leake et al. 1997;Verkouteren and Wylie 2000; Wylie and Verkouteren 2000). The ideal chemical composition of tremolite has no iron, ferro-actinolite contains no magnesium, and actinolite contains intermediate amounts of magnesium and iron (Leake et al. 1997). Figure 4 shows two other series within the amphibole group: 1) the tremolite-richterite series in which the calcium and sodium content can vary, and 2) the tremolite-winchite series in which the magnesium, calcium, and iron content vary. Some samples of the Libby amphibole show a chemical composition that is somewhere in the middle of the plane defined by the tremolite, richterite, and winchite corners of the cube in Figure 4.
From a chemical analysis of 30 amphibole samples from Libby mining and milling sites, the U.S. Geological Survey (USGS) assigned several different amphibole names to the samples: winchite, richterite, tremolite, actinolite, ferro-edenite, and magnesio-arfvedsonite (Meeker et al. 2001). These investigators noted that most of the amphibole samples displayed both asbestiform habits and nonasbestiform habits (from which cleavage fragments could be formed).
Occurrence of Tremolite
Asbestos
Nonasbestiform tremolite is the predominant form of tremolite that exists in the earth's crust (Veblen and Wylie 1993). There are many reports, however, of tremolite asbestos occurring in specific locations around the world.
Tremolite asbestos has only rarely been found in commercially mined deposits. Some tremolite asbestos has been mined in South Africa, India, Maryland, and South Korea, but it has never been a nationally important commercial source of asbestos in the United States. (Ross 1981). The extent of tremolite asbestos mining was small in Powhatan and Pylesville, Maryland, where it occurs with anthophyllite asbestos in ultramafic rocks (Ross 1981). In South Africa, tremolite asbestos was mined in the early twentieth century, but most amphibole asbestos recently mined in South Africa is amosite or crocidolite (Ross 1981). In contrast, as late as 1996, deposits of anthophyllite and tremolite asbestos were being commercially mined for use in asbestos cement in the South Rajasthan region of India (Mansinghka and Ranawat 1996).

Figure 2. Photograph of a sample of Libby, Montana, vermiculite ore. Fiber-like structures can be seen along the left edge of the piece of ore on the left. Source: U.S. Geological Survey and U.S. Environmental Protection Agency, Region 8, Denver, Colorado.

Figure 3. Photograph of vermiculite concentrate (on the right) and exfoliated vermiculite (on the left). Source: U.S. Geological Survey and U.S. Environmental Protection Agency, Region 8, Denver, Colorado.
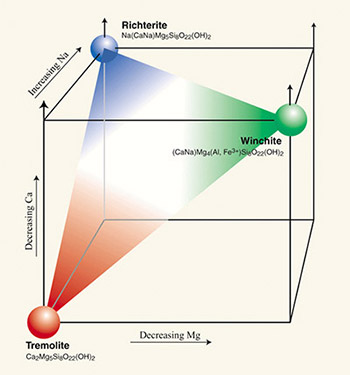
Figure 4. Relationships between magnesium, calcium, and sodium content and three amphibole mineral names: tremolite, winchite, and richterite. All three names have been assigned to various amphibole samples from former vermiculite mining and milling sites near Libby, Montana. Source: U.S. Geological Survey and U.S. Environmental Protection Agency, Region 8, Denver Colorado.
In certain Mediterranean regions, central and eastern Turkey, and New Caledonia in the South Pacific, soil containing tremolite asbestos has been used as stucco and for whitewashing of interior or exterior walls in certain villages (Baris et al. 1988a, 1988b; Bazas 1987; Bazas et al. 1985; Boutin et al. 1989; Constantopoulos et al. 1987a, 1992; Coplu et al. 1996; De Vuyst et al. 1994; Dumortier et al. 1998; Langer et al. 1987; Luce et al. 1994, 2001; McConnochie et al. 1987; Metintas et al. 1999; Sakellariou et al. 1996; Yazicioglu et al. 1980). This practice has declined as the health effects of inhalation exposure to tremolite asbestos have become better known.
Tremolite asbestos and chrysotile occur naturally in California, most commonly in areas of ultramafic rock and serpentinite (Churchill et al. 2001; Renner 2000). The Division of Mines and Geology of the California Department of Conservation has prepared a map identifying areas of ultramafic rock and serpentinite where tremolite asbestos and chrysotile may occur in El Dorado County, California (Churchill et al. 2001).
Before 1990, the now closed mine in Libby, Montana, was a significant source of vermiculite concentrate in the United States. According to a 1998 USGS report, vermiculite concentrate was produced in U.S. mines at Enoree and Woodruff, South Carolina, and in Louisa County, Virginia (USGS 1998b). U.S. imports of vermiculite in 1998 were supplied by South Africa and China (USGS 1998b). Twenty vermiculite exfoliating plants operated in 11 states in 1998.
In an early EPA-supported study, ~21% to 26% of the weight of raw ore samples and 0.3% to 7% of the weight of vermiculite concentrate samples from Libby were accounted for by asbestiform amphibole identified as tremolite-actinolite (Atkinson et al. 1982). In a 1984 study of samples from Libby, Montana, conducted by W.R. Grace, asbestiform amphibole percentage by weight varied from 3.5% to 6.4% in raw ore and from 0.4% to 1.0% in the concentrate (cited in Amandus et al. 1987a).
Amandus et al. (1987a) noted that among 599 fibers counted in eight airborne membrane filter samples from the Libby mine and mill, 96% and 16% had length:width ratios >10 and >50, respectively. Percentages of fibers with lengths >10, >20, and >40 μm were 73%, 36%, and 10%, respectively. McDonald et al. (1986b) reported that fibers in Libby air samples showed ranges for diameter, length, and length:width ratio of 0.1-2 m, 1-70 m, and 3-100, respectively. Greater than 60% of fibers were reported to be longer than 5 μm (McDonald et al. 1986b). These data are consistent with the asbestiform habit of the Libby amphibole.
When amphibole asbestos has been detected in vermiculite from other localities, the reported amounts have been lower than those in Libby vermiculite.
Moatamed et al. (1986) analyzed samples of vermiculite ores from Libby, Montana; Louisa County, Virginia; and South Africa for the presence of amphibole. Two samples of Montana unexpanded vermiculite ore were determined to have 0.08% and 2.0% amphibole by weight; two samples of expanded Montana vermiculite both showed 0.6% amphibole content. The South African unexpanded and expanded samples showed 0.4% and 0.0% amphibole content, respectively. The unexpanded and expanded Virginia samples were both determined to be 1.3% amphibole by weight.
The Virginia amphibole (identified as actinolite) and the South African amphibole (identified as anthophyllite) were predominately nonasbestiform, whereas the Montana amphibole (identified as actinolite) was predominately asbestiform (Moatamed et al. 1986). Numbers of fibrous amphibole particles in the Virginia samples were reported to be "extremely low" in comparison to the Montana samples. The infrequent, short fibrous structures were "most likely cleavage fragments." The South African vermiculite samples showed a "near absence of fibers" or "rare, short fibrous structures."
In another investigation, total asbestiform fibers (classified as tremolite-actinolite) represented less than 1% of the weight of samples of raw ore and vermiculite concentrate from Enoree and Patterson, South Carolina, compared with ~21% to 26% and 0.3% to 7% of the weight of raw ore and vermiculite concentrate samples, from Libby, Montana, respectively (Atkinson et al. 1982). Concentrations of particles with length > 5 μm in exfoliated vermiculite samples from South Carolina ranged from 0.7 to 11.7 x 106 fibers per g, whereas concentrations were higher in exfoliated Libby samples, ranging from 23 to 160 x 106 fibers per g (Atkinson et al. 1982). Transmission electron micrographs of nonasbestiform amphibole cleavage fragments from samples of Enoree vermiculite showed dramatic morphological differences from amphibole fibers from Libby vermiculite ore (Ross et al. 1993).
Amphibole (reported as tremolite) was detected in 26 of 57 samples of vermiculite with concentrations ranging from 0.01% to 6.4% in the samples with tremolite (Addison and Davies 1990). It was reported that "most of the amphibole in these samples was non-asbestiform." Further information was not provided in the report concerning where these samples came from and which ones may have contained asbestiform amphibole.
EPA (2000) investigated the occurrence of asbestos in vermiculite-containing garden products purchased in stores in several regions of the United States. These products ranged from products marketed as vermiculite to mixtures of vermiculite with other materials (e.g., soil or other minerals). In an initial investigation, asbestos was detected in 5 of 16 of the products tested, but only three products had sufficient levels that could be quantified. Reported weight concentrations of asbestos (identified as actinolite) were 0.30% and 0.33% for one product, 0.10% to 2.79% for another product, and 0.45% for the third (only one sample concentration was reported for this product). The second investigation detected asbestos in 17 of 36 garden products, but asbestos concentrations (identified predominantly as actinolite) were above 0.1% in only 5 of these products, ranging from 0.13 to 0.7% in an initial sampling. Further sampling showed that the concentrations in these "positive" products varied considerably, but no concentrations higher than the upper end of the initial ranges were reported.
To understand how much asbestos consumers may inhale when using vermiculite-containing garden products, EPA (2000) simulated exposure scenarios in enclosed conditions and in outside open air. From these simulations, EPA (2000) concluded that consumers "face only a minimal health risk from occasionally using vermiculite products at home or in their gardens." To further reduce the low health risk associated with occasional domestic use, EPA (2000) recommended 1) using vermiculite outdoors or in well-ventilated areas; 2) avoiding vermiculite dust by keeping vermiculite damp during use; and 3) avoiding bringing vermiculite dust into the home on clothing.
Amphibole asbestos, identified as tremolite asbestos or actinolite asbestos, has been reported to be a minor contaminant in some deposits of chrysotile in Quebec. Part of the evidence that tremolite asbestos exists in certain chrysotile deposits mined in Quebec comes from observations of higher concentrations of tremolite asbestos fibers than chrysotile fibers in autopsied lung tissues of certain miners and millers who were chronically exposed to chrysotile ores (see Case 1994 for review). Inhaled tremolite asbestos fibers are more persistent in lungs than inhaled chrysotile fibers.
The amount of tremolite asbestos or actinolite asbestos in chrysotile deposits, if present, is expected to vary from region to region and site to site. Tremolite was detected in 3 of 8 samples of commercial chrysotile using a method with detection limits of 0.01% to 0.05% that involved chrysotile digestion and energy-dispersive X-ray analysis (Addison and Davies 1990). Tremolite fibers in these samples were described as generally fine, straight, and needle-like with diameters around 0.2 m. Weight percentages accounted for by tremolite in the 3 "positive" samples were 0.02%, 0.08%, and 0.20%. The authors concluded, based on a combined analysis of results from this method, electron microscopy, and infrared spectrophotometry, that the tremolite in only one of the positive samples was asbestiform. In a wider survey of chrysotile samples using the same technique, tremolite was detected in 28 of 81 chrysotile samples; tremolite accounted for weight percentages in positive samples ranging from 0.01% to 0.6% (Addison and Davies 1990). The report did not indicate the extent to which the tremolite samples in the wider survey were asbestiform or nonasbestiform.
Occurrence in TalcTalc occurs in mines along the Appalachian Mountains and in California and Texas; Germany; Florence, Italy; Tyrol, Austria; Transvaal, South Africa; and Shetland, Scotland (Amethyst Galleries 1999). In the United States in 1998, there were 15 talc-producing mines in 7 states. Companies in Montana, New York, Texas, and Vermont accounted for 98% of domestic production (USGS 1999). Industrial use of talc shows the following pattern: ceramics, 37%; paints, 19%; paper, 10%; roofing, 10%; plastics, 7%; cosmetics, 5%; rubber, 3%; and other uses, 9% (NTP 1993). The geological formation of talc may lead to the formation of other mineral phases including amphiboles and serpentines, including some in asbestiform habits. In the United States, commercial talc is categorized into cosmetic grade, which is free of asbestos, and industrial grade, which may contain other asbestiform or nonasbestiform minerals (NTP, 1993; Zazenski et al. 1995). Zazenski et al. (1995) noted that the Cosmetic, Toiletry, and Fragrance Association, the United States Pharmacopeia, and the Food Chemical Codex have established talc quality assurance specifications followed by U.S. cosmetic, pharmaceutical, and food companies that use talc to ensure the purity of their products.
Results of a survey of asbestos fibers in consumer cosmetic talc powders from Italian and international markets using electron microscopy, electron diffraction, and energy dispersive X-ray analysis showed that asbestos was detected in 6 of 14 talc samples from the European Pharmacopeia (Paoletti et al. 1984). Chrysotile was identified in 3 samples, 2 samples contained tremolite asbestos and anthophyllite asbestos, and 1 sample contained chrysotile and tremolite asbestos. The authors noted that, in all talc powders analyzed, fibrous talc particles frequently were present that were morphologically similar to amphibole asbestos fibers. Counting fibers as particles with aspect ratio >3:1 and width < 3 m, the percentages of particles that were asbestos fibers ranged from <0.03% to 0.13% for 4 samples, and were 18% to 22% for the other 2 samples. Paoletti et al. (1984) noted that the European Pharmacopeia, at that time, had not established analytical quality control of asbestos contamination.
Industrial talc currently mined in New York is called tremolitic talc because it contains significant quantities of nonasbestiform tremolite. Historical references in the scientific literature indicate that these talc deposits and their industrial products may contain asbestos (American Thoracic Society 1990; DOL 1980; NTP 1993; Wagner et al. 1982). In 1992, OSHA noted that the debate over the mineralogical content of the New York tremolitic talc ore was unresolved, but that the presence of asbestiform talc in the ore may have led to the identification of asbestiform tremolite and anthophyllite. More recently, a report from OSHA's Salt Lake Technical Center (Crane 2000) suggests that, in some cases, cleavage fragments of nonasbestiform tremolite and anthophyllite in the talc ore and products may have been inappropriately identified as asbestos. Crane (2000) described the New York talc ore as having nonasbestiform tremolite, mostly nonasbestiform anthophyllite, talc in both massive and asbestiform habits, and minor amounts of other minerals and mineraloids.
Talc has been used in the manufacture of crayons for many years. Recently, it was reported in the U.S. press that tremolite asbestos, anthophyllite asbestos, and chrysotile were detected in some crayons at concentrations ranging from 0.03% to 2.86% (CPSC 2000). In response, the Consumer Product Safety Commission (CPSC 2000) examined crayons from several U.S. manufacturers to determine whether asbestos was present. Trace amounts of anthophyllite asbestos were found in some of the crayons. The CPSC (2000) concluded that the risk that children would be exposed to fibers through inhalation or ingestion of talc-containing crayons is "extremely low," but recommended that, as a precaution, crayons should not contain these fibers. The manufacturers have agreed to reformulate their crayons using substitute materials (CPSC 2000).
Detection and Analysis of
Asbestos in Air Samples
The detection and analysis of
asbestos in air samples (and in bulk materials that may become
airborne) involve both fiber quantification and mineral
identification. The distribution of numbers of particles of
differing sizes in a sample is determined by microscopic
examination, performed using either light or electron microscopy.
For counting purposes, a fiber is defined as any particle with a
length ≥5 m and a length:width ratio ≥3:1. Concentrations in air
are reported as fiber/mL or fiber/cc. For the purposes of
determining asbestos content in bulk building material, EPA (2000)
uses an operational definition of fiber as any particle with a
length:width ratio ≥5:1. Electron diffraction and energy-dispersive X-ray
analysis give information on the chemical content and mineral
identity of the particles. The combined use of light microscopy,
electron microscopy (transmission and scanning), electron
diffraction, and energy-dispersive X-ray methods in analyzing air
and/or bulk material samples offers the most accurate approach to
estimating airborne asbestos concentrations.
Light Microscopic Methods The current standard method for determining airborne asbestos particles in the U.S. workplace is the National Institute for Occupational Safety and Health (NIOSH) Method 7400 which uses phase contrast light microscopy (PCM) (NIOSH 1994a, 1994b). Fibers are collected on a filter and counted with 400-450x magnification. The method does not accurately distinguish between asbestos and nonasbestos fibers, and cannot detect fibers thinner than about 0.25 m. Recent improvements in filter preparation allow for viewing at higher magnification (1250x) resulting in a several-fold improvement in sensitivity (Pang et al. 1989).
Phase contrast microscopy methods are widely used to assess occupational exposure to workers engaged in activities known to generate airborne asbestos fibers. However, in settings where large proportions of other particles or fibers (e.g., wool, cotton, glass) are present, the phase contrast microscopy will overestimate the asbestos fiber concentration without additional information.
Polarized light microscopy is frequently used for determining the asbestos content of bulk samples of insulation or other building materials (see, for example, NIOSH Method 9002 [NIOSH 1989] and OSHA method ID-191 [OSHA 1994]). This method also enables qualitative identification of asbestos types using morphology, color, and refractive index.
Electron Microscopic Methods Transmission electron microscopy (TEM) and scanning electron microscopy (SEM) methods can detect smaller fibers than PCM and can be used to determine mineral habit in bulk materials that may become airborne. NIOSH Method 7402, Asbestos by TEM, is used to determine asbestos fibers in the optically visible range and is intended to complement PCM (NIOSH Method 7400). Examination of a sample by either TEM or SEM allows the detection of much smaller fibers than light microscopy, and so more thorough data can be collected on fiber length and diameter distribution. Of these two methods, TEM has greater sensitivity for small fibers, and is the most common method for measuring asbestos in ambient air or inside schools or other buildings. SEM analysis usually images fibers that are more than 0.2 μm in diameter because of contrast limitations, while TEM can visualize fibers of all sizes.
Electron Diffraction and Energy-Dispersive X-ray Methods These methods determine crystal structure and elemental composition and are used to identify the mineral group to which a fiber or particle belongs. Modern transmission electron microscopes are equipped with instrumentation that examines individual particles by both of these methods, but scanning electron microscopy does not measure electron diffraction patterns. To distinguish between a nonasbestiform amphibole cleavage fragment and an asbestiform amphibole fiber of the same mineral type, information about mineral habit (which comes from light and electron microscopy) is needed.
Conversion Factors Conversion factors are used to compare results from epidemiologic studies that used different methods to measure airborne asbestos levels. Early studies often measured air concentrations in units of mass per volume of air or number of particles per volume of air, whereas more recent studies measure air concentrations in units of number of fibers (particles with lengths ≥5 μm and aspect ratio ≥3:1, determined by PCM or electron microscopy) per volume of air.
Older studies of health effects and occupational exposure measured dust exposure in units of million particles per cubic foot (mppcf). This method did not distinguish fibrous from nonfibrous particles and used relatively low magnification, so only the largest fibers were detected. The British Occupational Hygiene Society (BOHS 1968) suggested that an asbestos air concentration of 1 mppcf is roughly equal to 3 fiber/mL (detected by PCM).
To convert from PCM-measured to TEM-measured air concentrations, the National Research Council (NRC 1984) recommended that 1 PCM fiber/mL is roughly equal to 60 TEM fiber/mL, and that 1 PCM fiber/mL and 60 TEM fiber/mL are roughly equal to a mass concentration of 0.03 mg asbestos dust/m3 (i.e., 1 mg/m3 is roughly equal to 33 PCM fiber/mL or 2000 TEM fiber/mL). The NRC acknowledged that these conversion factors provide only rough estimates because converting from phase contrast microscopy counts to TEM counts can vary with different sizes of fibers, and converting from mass-per-volume units to fibers-per-volume units can vary with different mineral types and different sizes of fibers.
Epidemiologic studies of groups of asbestos-exposed workers commonly express exposure in cumulative exposure units (fiber-year/mL). This exposure measure is calculated by multiplying a worker's duration of exposure (measured in years) by the average air concentration during the period of exposure (measured in fiber/mL).
Potential for Human Exposure
to Asbestos
Occupational exposure to asbestos may occur and has occurred in workers involved in mining, milling, and handling of chrysotile (and other forms of asbestos) and vermiculite ores, in exfoliating vermiculite, and in mining, milling, and handling of other ores and rocks that may contain tremolite asbestos or other amphibole asbestos. Unless efforts are made to limit dust generation and release, and limit transport of dust on clothes to home environments, there is a probability of exposure to other workers, family members, and area residents.
Residents who live close to mining, milling, or manufacturing sites that involve asbestos-containing material may be potentially exposed to higher levels of airborne tremolite asbestos than levels in general ambient air. EPA, ATSDR, and other agencies currently are investigating levels of amphibole asbestos exposure that residents (including children) who were not employed in the vermiculite mines and mills may have and are experiencing. In addition, ATSDR is conducting medical testing of individuals potentially exposed to asbestiform minerals associated with vermiculite in Libby, Montana (ATSDR 2001b).
Asbestos fibers may be released to indoor or outdoor air by the disturbance of asbestos-containing building materials such as insulation, fire-proofing material, dry wall, and ceiling and floor tile, although the use of asbestos-containing building materials has declined sharply in recent years (HEI 1991). Amphibole asbestos has been found in some vermiculite sources that have been used as home and building insulation. Workers or homeowners involved in demolition work or asbestos removal, or in building or home maintenance, repair, and remodeling, potentially can be exposed to higher levels of airborne asbestos than levels in general ambient air. In general, exposure may occur only when the asbestos-containing material is disturbed in some way to release particles and fibers into the air. Exposure will be greatest when dry, friable (i.e., easily released) material is disturbed. When asbestos-containing materials are solidly embedded or contained, exposure will be negligible (USGS 1998b, 1999).
Typical concentrations of asbestos fibers (with lengths ≥5 μm) in urban and rural ambient air may be about 0.0001 or 0.00001 fiber/mL, respectively (ATSDR 2001a). In workplace air, recent U.S. regulations have limited asbestos air concentrations to 0.1 to 0.2 fiber/mL to protect against the development of pulmonary fibrosis and cancer (OSHA 1992, 1994). A study of indoor air of homes, schools, and other buildings that contain asbestos materials measured an average asbestos concentration of about 0.0001 fiber/mL (Lee et al. 1992). Most of the fibers in this study were identified as chrysotile; 2% of the fibers were identified as amphibole fibers. Indoor air concentrations are highly variable, however, and depend on the friability of the asbestos-containing material and on activities in which people are engaged.
As discussed in the Occurrence
of Tremolite Asbestos section, small amounts of amphibole
asbestos fibers have been identified in some samples of
vermiculite-containing consumer garden products from the United
States (EPA 2000). EPA (2000) concluded that consumers may face only
a minimal health risk from occasionally using vermiculite products
at home, and can reduce any risk by limiting the production of dusts
when using the products.
Health Effects from Asbestos: Overview
It is known that exposure to airborne asbestos fibers can increase the risk of lung cancer, malignant mesothelioma, and nonmalignant respiratory effects including pulmonary interstitial fibrosis (asbestosis), pleural plaques, pleural calcification, and pleural thickening. Epidemiologic studies have shown increasing risks for malignant or nonmalignant respiratory disease significantly associated with increasing measures of exposure intensity and duration among groups of occupationally exposed individuals. Results from studies of animals exposed by various routes of exposure and from mechanistic studies are consistent with these findings. Reviews of this evidence include those by the Agency for Toxic Substances and Disease Registry (ATSDR 2001a), the American Conference of Governmental Industrial Hygienists (ACGIH 1998), the American Thoracic Society (1990), Case (1991), Churg and Wright (1994), the Environmental Protection Agency (EPA 1986), the International Agency for Research on Cancer (IARC 1987a), Kamp and Weitzman (1997, 1999), Langer and Nolan (1998), Lippmann (1994), McDonald and McDonald (1997), Mossman and Churg (1998), Mossman et al. (1983, 1990), the National Toxicology Program (NTP 2001), the Occupational Safety and Health Administration (OSHA 1986, 1992), Stayner et al. (1996, 1997), Wylie et al. (1993), and the World Health Organization (WHO 1998).
Consensus Issues and Conclusions
There is general agreement among scientists and health agencies on the following issues and conclusions regarding health effects from asbestos.
(1) Exposure to any asbestos type (i.e., serpentine or amphibole) can increase the likelihood of lung cancer, mesothelioma, and nonmalignant lung and pleural disorders.
(2) Important determinants of toxicity include exposure concentration, exposure duration and frequency, and fiber dimensions and durability.
(3) Fibers of amphibole asbestos such as tremolite asbestos, actinolite asbestos, and crocidolite are retained longer in the lower respiratory tract than chrysotile fibers of similar dimension.
(4) Pulmonary interstitial fibrosis associated with deposition of collagen, progressive lung stiffening and impaired gas exchange, disability, and death occurred in many asbestos workers.
(5) Most cases of asbestosis or lung cancer in asbestos workers occurred 15 or more years after their initial exposure to asbestos.
(6) Asbestos-exposed tobacco smokers have greater than additive risks for lung cancer than do asbestos-exposed nonsmokers.
(7) The time between diagnosis of mesothelioma and the time of initial occupational exposure to asbestos commonly has been 30 years or more.
(8) Cases of mesotheliomas have been reported after household exposure of family members of asbestos workers and in individuals without occupational exposure who live close to asbestos mines.
Unresolved Issues and Discussions
(1) Does exposure to asbestos increase the risk for gastrointestinal cancer?
Results in support of a positive answer to this question include small increases in death rates from gastrointestinal cancer in some groups of asbestos-exposed workers and in some populations with high levels of asbestos fibers in drinking water, and a small but statistically significantly increased incidence of benign intestinal tumors in one National Toxicology Program (NTP) study of male rats exposed to chrysotile in their food for life (see ATSDR 2001a for citation of these studies). However, the increased gastrointestinal mortalities noted in workers and in populations exposed through drinking water were usually quite small, and consistent results were not found across studies. In addition, it is difficult to determine whether the increases were due to asbestos or to other factors (e.g., exposure to other chemicals, misdiagnosis, dietary factors, alcohol intake). The weight of the finding of intestinal tumors in chrysotile-exposed rats is counterbalanced by the facts that the tumors were both infrequent and benign, and that no significant increases in tumors occurred in five other NTP lifetime cancer bioassays of rats exposed to different forms of asbestos in their diet.
The available data do not support a definitive conclusion about whether the increased risk for gastrointestinal cancer observed in some of the epidemiologic studies is real or not. Some scientists believe the available evidence is substantial, others believe the evidence is inadequate to reach a firm conclusion, and still others believe the increased risks are probably due to other factors. ATSDR (2001a) and NTP (2001) concur, however, that it seems only prudent to consider increased risk of gastrointestinal cancer an effect of concern from exposure to asbestos.
(2) Are chrysotile fibers (or amphibole asbestos fibers) primarily responsible for mesotheliomas in certain groups of workers predominantly exposed to chrysotile?
Some investigators have proposed that chrysotile fibers may not be the primary cause of mesothelioma in humans exposed predominantly to chrysotile, whereas others have proposed that amphibole fibers are more potent than chrysotile in this regard (see Berman et al. 1995; Case 1991; Churg 1988; Churg and Wright 1994; Frank et al. 1998; Langer and Nolan 1998; Lippmann 1994; McDonald and McDonald 1997; Stayner et al. 1996). Tremolite asbestos fibers have often been detected at higher concentrations than chrysotile fibers in autopsied lung tissues of certain miners and millers who were chronically exposed to chrysotile ores that contained only very small amounts of tremolite asbestos (see Case 1994 for review). Part of the difficulty in ascribing primary responsibility in these mesothelioma cases is that chrysotile fibers are removed from the lung much more quickly than amphibole asbestos fibers, and data on fiber content in pleural or peritoneal tissue in human cases are few.
(3) Are amphibole asbestos types more potent than chrysotile in inducing asbestosis and lung cancer?
Some investigators have proposed that amphibole asbestos fibers, such as tremolite asbestos, are more potent than chrysotile fibers in inducing fibrotic lung disease and lung cancer (McDonald 1998; McDonald and McDonald 1997; McDonald et al. 1999; Mossman et al. 1990). Others propose that differences in the potency of chrysotile and amphibole-asbestos fibers in inducing lung cancer cannot be reliably discerned from available data (Berman et al. 1995; Stayner et al. 1996).
Despite the dispute in the scientific literature concerning issues (2) and (3), U.S. and international agencies concur that exposure to any type of asbestos (including chrysotile) can increase the risk for asbestosis, mesothelioma, and lung cancer in humans (e.g., ATSDR 2001a; EPA 1986; IARC 1987a; NTP 2001; WHO 1998).
(4) Should the U.S. regulatory definition of an asbestos fiber (length ≥5 μm with aspect ratio ≥3:1), established for purposes of quantifying exposure levels, be changed?
This issue has received continued debate since the establishment of the definition (see American Thoracic Society 1990; OSHA 1992, 1994; Wylie et al. 1993, 1997). At least part of the debate has involved uncertainties associated with the relative importance of long and short inhaled fibers in asbestos pathogenicity.
In support of the importance of longer fibers, animal carcinogenic responses to asbestos have been variously reported to be best correlated with the concentration of fibers with lengths ≥8 μm and diameters ≤0.25 μm (Stanton et al. 1981) and with the concentration of fibers with lengths ≥20 μm (Berman et al. 1995). Case-control analyses of fiber concentrations in autopsied lungs of mesothelioma subjects and subjects who died of other causes showed that increased risks for mesothelioma were significantly related to longer fibers. Fibers longer than 5 μm (Rodelsperger et al. 1999), 8 m (McDonald et al. 1989), or 10 m (Rogers et al. 1991) were implicated in different studies.
In contrast, analyses of autopsied human lung tissue of asbestos-exposed and nonexposed patients often show greater numbers of short (< 5 μm) than long (> 5 μm) retained fibers (Dodson et al. 1997, 1999), and short chrysotile fibers have been reported to be the most prevalent type of fibers found in parietal pleura tissue from asbestos-exposed autopsy cases (Sebastien et al. 1980). Also, significant inverse relationships have been observed between degree of fibrosis and retained amphibole fiber length in autopsy studies of chrysotile miners and millers (Churg and Wright 1989) and amosite-exposed shipyard and insulation workers (Churg et al. 1990). Significant correlations have also been observed in animal studies between carcinogenic response and concentrations of fibers with lengths shorter than 8 μm (Berman et al. 1995; Stanton et al. 1981). In addition, exceptions to the principle that long and thin structures are required for a carcinogenic response to asbestos or other fibers have been reported in animal studies (Davis et al. 1991; Stanton et al. 1981). For example, carcinogenic responses in rats to two tremolite asbestos samples were markedly higher than the predicted response from Stanton's regression curve relating probability of tumor to the number of particles with lengths ≥8 μm and diameters ≤ 0.25 μm (Stanton et al. 1981). In addition, one of seven talcs tested had high numbers of particles with lengths ≥8 μm and diameters ≤ 0.25 μm, but did not produce tumors (Stanton et al. 1981).
(5) What are the molecular events involved in the development of asbestos-induced respiratory and pleural effects and how are they influenced by fiber dimensions and mineral type?
Identification of the molecular and cellular events of asbestos-induced disease has been the subject of extensive research within the past two decades (see Mechanisms of Asbestos Toxicity: Overview section). However, much remains unknown, and the precise steps in pathogenic pathways are not fully established.
(6) What are the actual risks for malignant or nonmalignant respiratory disease that may exist at exposure levels below air concentrations (0.1-0.2 fiber/mL) established as recent occupational exposure limits?
Asbestosis: Based on its review of available data, a task group convened by the World Health Organization (WHO 1998) concluded that "asbestotic changes are common following prolonged exposure of 5 to 20 fiber/mL" and that "the risk at lower exposure levels is not known."
Alternatively, based on an analysis that extrapolated from data for asbestosis mortalities in a group of asbestos textile workers, Stayner et al. (1997) concluded that there was an excess risk of 2/1,000 for asbestosis mortality for men exposed for 45 years to an airborne asbestos concentration of 0.1 fiber/mL. Other scientists have criticized the applicability of the Stayner analysis to general population environmental exposures, noting that this group of asbestos textile workers displayed higher mortality rates than other groups of asbestos workers (Case et al. 2000; Hodgson and Darnton 2000).
Lung Cancer and Mesothelioma: Based on an analysis of data from epidemiologic studies of workers who were exposed to asbestos before modern occupational exposure limits were established, EPA (1986) calculated by extrapolation that lifetime exposure to asbestos air concentrations of 0.0001 fiber/mL could result in up to 2 to 4 excess cancer deaths (lung cancer or mesothelioma) per 100,000 people. This air concentration is within reported ranges of ambient air levels (0.00001 to 0.0001 fiber/mL). The EPA analysis has been extensively discussed and reviewed in the scientific literature (Camus et al. 1998; Hodgson and Darnton 2000; Hughes 1994; Landrigan 1998; Lash et al. 1997). EPA is in the process of reviewing and possibly updating their cancer risk estimates for asbestos.
(7) Can lung cancer be attributed to asbestos exposure (regardless of fiber type) in the absence of pulmonary fibrosis?
Some scientists have supported the hypothesis that asbestosis is a necessary prerequisite for asbestos-induced lung cancer, but there is also evidence that an increased risk for lung cancer occurs in asbestos workers without obvious asbestosis (see Henderson et al. 1997; Hillerdal and Henderson 1997; Hughes and Weill 1991; Jones et al. 1996; Wilkinson et al. 1995). Hillerdal and Henderson (1997) concluded from their review of the data that "there was an increasing body of evidence that, at low exposure levels, asbestos produces a slight increase in the relative risk of lung cancer even in the absence of asbestosis." In contrast, Jones et al. (1996) concluded from their review that, "While the issue of whether asbestosis is a necessary precursor to asbestos-attributable lung cancer cannot at this time be considered settled, the weight of the available evidence strongly supports this proposition."
Deposition and Clearance of Inhaled Asbestos Fibers: Overview
Human and animal studies indicate that when asbestos fibers are inhaled, thick fibers (diameters greater than 2-5 μm) are deposited in the upper airways, whereas thinner fibers are carried deeper into the alveolar regions of the lung (ATSDR 2001a; Lippman 1994; Wylie et al. 1993). Absorption by epithelial cells and penetration through the epithelial layers of the respiratory tract are thought to be minimal, but some transport of inhaled fibers from the lung to the pleural cavity occurs (ATSDR 2001a; Wylie et al. 1993). Fiber width is a key determinant of access of fibers to the lung and pleural cavity, and thus of fiber toxicity. Wylie et al. (1993) reviewed available evidence from human epidemiology studies, human lung burden studies, and studies of animals implanted or injected with asbestos indicating that fibers with widths greater than 1 μm are unlikely to cause lung cancer or mesothelioma.
Fibers deposited in the respiratory tract are principally removed by mucociliary transport and swallowing, followed by elimination from the gastrointestinal tract via feces. Small numbers of fibers may reach the lymph system or be transported to the pleura and peritoneum. Dissolution of fibers by alveolar macrophages is also thought to play a role in eliminating asbestos fibers from the lung, especially for chrysotile fibers; interstitial macrophages, intravascular macrophages, and pleural macrophages also interact with deposited asbestos fibers (see Oberdorster 1994). In addition, some fibers are not cleared from the lung, leading to a gradual accumulation.
There is evidence in animals that long fibers are retained in the lungs for longer periods than short fibers (e.g., Coin et al. 1992; Davis 1989). This relationship may be associated with the inability of macrophages to engulf and remove fibers that are significantly larger than themselves (Bignon and Jaurand 1983), but analysis of autopsied human lung or parietal tissue for retained fibers often shows higher numbers of short (< 5 μm) fibers than long (> 5 μm ) fibers (Dodson et al. 1997, 1999; Sebastien et al. 1980).
There is also evidence that amphibole fibers are retained for longer periods than chrysotile fibers (Albin et al. 1994; Churg 1994; Churg et al. 1993; Davis 1989; Wagner et al. 1974). For example, amphibole retention in lungs of rats repeatedly exposed to airborne amphibole fibers for 24 months showed a continuous increase throughout exposure, whereas chrysotile lung retention reached a much lower maximum level within about 3 months in rats similarly exposed to chrysotile fibers (Wagner et al. 1974). Tremolite fibers in autopsied lung tissue from workers exposed to airborne chrysotile fibers contaminated with small amounts of tremolite (<1%) accounted for disproportionately large percentages (47-67%), and chrysotile fibers accounted for disproportionately small percentages (19-53%), of the total fibers detected (Churg and Wright 1994). The apparent longer retention of amphibole fibers in lung tissue has been proposed as a partial explanation of why amphibole asbestos appears to be more potent in producing mesothelioma than chrysotile (American Thoracic Society 1990; Mossman et al. 1990).
Mechanisms of Asbestos Fiber Toxicity: Overview
Identification of the molecular and cellular responses leading to the progressive development of asbestos-induced lung cancer, mesothelioma, pulmonary fibrosis, and pleural thickening and effusion has been the subject of extensive research within the past two decades. Published reviews of this work include those by Begin et al. (1992), Kamp and Weitzman (1997, 1999), Kamp et al. (1992), Luster and Simeonova (1998), Mossman and Churg (1998), Mossman et al. (1983, 1996), Rom et al. (1991), and Tanaka et al. (1998). In general, it is recognized that there are multiple cellular and molecular responses to asbestos fibers, that no single mechanism is likely to account for all asbestos-related diseases, that the precise steps in pathogenic pathways leading to asbestos-related disease are not fully established, and that fiber structural and chemical properties (e.g., length, width, iron content, durability, surface areas) are important variables that play a role in the development of lung and pleural injury.
A central working hypothesis proposes that the presence of asbestos fibers in the lung activates alveolar macrophages, pulmonary neutrophils, pulmonary epithelial cells, and pleural mesothelial cells to produce reactive oxygen species (such as hydrogen peroxide, the superoxide anion, and the hydroxyl radical) and/or reactive nitrogen species (such as nitric oxide and peroxynitrite) that can damage cellular macromolecules (e.g., deoxyribonucleic acid [DNA], ribonucleic acid [RNA], signal transduction proteins, and membrane lipids) and lead to cellular dysfunction, cytotoxicity, cellular transformation (to malignancy), and cellular proliferation (see the reviews cited in the previous paragraph for evidence in support of this hypothesis). In addition, iron cations associated with asbestos fibers may augment the production of hydroxyl radicals. The pathogenesis of asbestos-induced lung injury is also thought to involve altered expression of genes involved in oxidation protection (e.g., catalase and superoxide dismutase), other stress responses (e.g., heat shock proteins and ferritin), cellular proliferation (e.g., cytokines, cytokine binding proteins, and growth factors), and apoptosis in alveolar macrophages, pulmonary epithelial cells, and/or pleural mesothelial cells. Further understanding of how persistent production of reactive oxygen or nitrogen species and persistent inflammatory cellular responses precisely interact may be useful for developing better approaches to the diagnosis, prevention, and treatment of asbestos-related disease.
Health Effects from Tremolite Asbestos
As with other forms of asbestos, health effects of concern from exposure to inhaled tremolite asbestos are lung cancer, mesothelioma, and nonmalignant lung and pleural disorders. Evidence in humans comes from epidemiologic studies of tremolite asbestos-exposed groups of vermiculite miners and millers from Libby, Montana. This evidence is supported by reports of increased incidences of nonmalignant respiratory diseases, lung cancer, and mesothelioma in villages in various regions of the world that have traditionally used tremolite-asbestos whitewashes or have high surface deposits of tremolite asbestos and by results from animal studies.
Nonmalignant Respiratory Effects: Pulmonary Fibrosis and Pleural Changes. Studies of Libby, Montana, vermiculite workers chronically exposed to airborne tremolite asbestos provide evidence that exposure to tremolite asbestos increases the risk of interstitial pulmonary fibrosis, pleural calcification, and pleural wall thickening and the risk of death from these nonmalignant diseases. Supporting evidence comes from observations of 1) high prevalences of pleural calcification among residents of villages where whitewashes containing tremolite asbestos were used or where there are abundant surface deposits of tremolite asbestos and 2) pulmonary fibrogenic reactions in lungs of rats and mice after exposure to tremolite asbestos by inhalation or intratracheal instillation.
In response to a report of 12 cases of pleural effusion within a 12-year period in an Ohio fertilizer plant that processed Libby, Montana, vermiculite, 501 workers were surveyed for symptoms of respiratory distress, examined by chest radiography, and tested for pulmonary function (Lockey et al. 1984). Chest radiographs showed 479/501 (95.6%) workers with no significant radiographic changes, 1/501 (0.2%) workers with small irregular parenchymal opacities indicative of pulmonary fibrosis, 10/501 (2.0%) workers with significant pleural changes described as thickening, plaques, and/or calcification, and 11/501(2.2%) workers with costophrenic angle blunting only. Cumulative fiber exposures for the 11 employees with parenchymal or pleural changes ranged from 0.01 to 39.9 fiber-year/mL (mean = 12 fiber-year/mL). Cumulative fiber exposures for the 11 employees with costophrenic angle blunting ranged from 0.2 to 27.5 fiber-year/mL (mean = 5.4 fiber-year/mL). Increased prevalences of radiographic pleural changes, self-reported pleuritic chest pain, and self-reported shortness of breath were significantly associated with cumulative fiber exposure indices, but exposure-related changes in pulmonary function (spirometric variables and carbon dioxide diffusing capacity) were not found.
Chest radiographs of 184 men employed at the Libby, Montana, vermiculite mine and mill for at least 5 years during 1975-1982 were evaluated for parenchymal abnormalities indicative of pulmonary fibrosis (presence of small irregular parenchymal opacities with a profusion ≥ International Labor Organization [ILO] category 1/0 (3)) and pleural abnormalities including calcification and thickening on the wall (Amandus et al. 1987b). Prevalences for small parenchymal opacities ≥ ILO category 1/0, any pleural change, pleural calcification, and pleural wall thickening were 10, 15, 4, and 13%, respectively. Vermiculite workers who were smokers, were of age 45 or greater, and had cumulative fiber exposure indices >100 fiber-year/mL (but not those with exposures <100 fiber-year/mL) showed a significantly higher prevalence of small irregular parenchymal opacities (4/13, 30.8%) than several reference groups of workers of similar age and smoking habits without known fiber exposure (e.g., nonasbestos cement workers). Amandus et al. (1987b) suggested that the finding of higher prevalence of parenchymal changes in the Libby, Montana, vermiculite workers compared with the Ohio fertilizer plant workers (Lockey et al. 1984) may be explained by a higher average cumulative exposure index for the Montana workers.
Another study examined possible relationships between cumulative fiber exposure and chest radiographic findings for 173 workers employed in the Libby, Montana, mine and mill in July 1983, 80 of 110 former male employees who resided within 200 miles of Libby, and 47 local men without known exposure to dust (McDonald et al. 1986a). Age-standardized percentages of subjects with parenchymal opacities (small irregular opacities with ≥ ILO category 1/0) and pleural thickening of chest wall increased with increasing cumulative fiber exposure categories. For example, age-standardized percentages for small opacities were 10.6%, 18.4%, 15.4%, 31.3%, and 27.9% for subjects with mean cumulative exposures of 4.1, 17.5, 53.9, 144.4, and 495.8 fiber-year/mL, respectively. Logistic regression analysis indicated that the prevalence of small opacities (with profusion ILO category 1/0) was significantly affected by age, smoking, and cumulative exposure; prevalence for pleural thickening was significantly affected by age and cumulative exposure. The logistic regression analysis predicted that for current smokers at age 65, the risk for developing small parenchymal opacities ≥ ILO category 1/0 would increase by about 5-10% with each cumulative exposure increment of 100 fiber-year/mL. McDonald et al. (1986a) also concluded that at 0.1 fiber/mL, no detectable excess of radiological change should be detectable after a working life of 40 years. However, in a later discussion of their Libby, Montana, regression analysis, McDonald et al. (1988) noted that the increased risk of small radiographic opacities (≥ ILO category 1/0) was between 0.05 and 0.1% per fiber-year/mL.
There are two cohort mortality studies of tremolite-asbestos-exposed workers employed for at least 1 year at the Libby, Montana, vermiculite mine and mill. Causes of death were evaluated among 161 deaths that occurred by 1981 in 575 men who were hired before 1970 (Amandus and Wheeler 1987) and among 165 deaths that occurred by 1983 in 406 men who were hired before 1963 (McDonald et al. 1986b). Both studies assigned cumulative fiber exposure indices (fiber/year-cc = fiber-year/mL) to each subject based on individual work histories and estimated fiber concentrations in air at various job locations (fiber/mL). Workplace air concentrations were estimated from microscopic examination of membrane filter samples collected after 1968 and from dust concentrations from midget impinger samples collected before 1968 in the dry mill area (Amandus et al. 1987a; McDonald et al. 1986b). Fiber concentrations in periods before 1968 were adjusted to reflect higher fiber concentrations expected to have existed in these earlier periods at several job locations due to changes in production methods.
Elevated standardized mortality ratios (SMRs) for nonmalignant respiratory disease, using mortality rates for U.S. males as reference, were calculated for both cohorts. Amandus and Wheeler (1987) reported an SMR of 2.43 (95% confidence interval [CI] = 1.48, 3.75; 20 observed deaths versus 8.2 expected), and McDonald et al. (1986b) reported an SMR of 2.55 (95% CI was not reported; 20 observed deaths, expected deaths not reported). For workers with cumulative exposure indices >399 fiber-year/mL, Amandus and Wheeler (1987) reported a statistically significantly elevated SMR of 4.00 (7 observed versus 1.8 expected). Deaths from nonmalignant respiratory disease expected to be directly related to tremolite fiber exposure (pulmonary fibrosis or pneumoconiosis) represented 50% (10/20; Amandus and Wheeler 1987) and 40% (8/20; McDonald et al. 1986b) of deaths from nonmalignant respiratory disease. Neither study was able to demonstrate consistent, statistically significant relationships between increasing exposure index and increasing risk for death from nonmalignant respiratory disease, but the statistical power was limited in both studies because of the small numbers of workers evaluated. Other limitations of the studies include the limited follow-up periods (only 28% and 40% of the cohorts had died when the studies were conducted) and the lack of information about individual smoking histories. Nevertheless, the results from these studies add considerable weight to the evidence that exposure to airborne asbestos, including tremolite asbestos, can lead to the development of nonmalignant respiratory disease and death.
Two studies of other groups of miners and millers at other vermiculite mines in South Africa and South Carolina did not find evidence for increased prevalence of diseases associated with asbestos exposure (Hessel and Sluis-Cremer 1989; McDonald et al. 1988). The vermiculite in these studies was reported to contain much lower levels of tremolite asbestos or other amphibole asbestos fibers than the Libby, Montana, vermiculite (Atkinson et al. 1982; Moatamed et al. 1986; McDonald et al. 1988). It is plausible that the lack of increased prevalences of diseases associated with asbestos exposure in these workers is primarily due to the very low levels of asbestiform amphibole minerals in these vermiculite deposits (Atkinson et al. 1982; Moatamed et al. 1986; Ross et al. 1993). In addition, such factors as lower levels of airborne fiber concentrations at the worksites, small numbers of subjects in the studies, and limitations in study design and exposure data may have contributed to this lack of evidence.
In a cross-sectional study by Hessel and Sluis-Cremer (1989), no increased prevalence of parenchymal or pleural abnormalities on chest radiographs, no excess of self-reported respiratory symptoms, and no lung function performance deficits were found in a group of 172 South African vermiculite workers (average duration of employment was 15.3 years) compared with a group of workers involved in mining and refining copper. Samples of unexpanded and expanded vermiculite from this mine showed 0.4% and 0.0% amphibole content (Moatamed et al. 1986). The amphibole was nonasbestiform with "rare, short fibrous structures" that were predominantly anthophyllite. From this analysis, Moatamed et al. (1986) concluded that the South Africa vermiculite samples were "essentially fiber free."
McDonald et al. (1988) evaluated causes of 51 deaths that occurred by the end of 1985 in 194 men who were employed for at least 6 months before the end of 1970 in the mining and milling of vermiculite from Enoree, South Carolina. Only 3 deaths were attributed to nonmalignant respiratory disease compared with 2.45 expected (not statistically significant); no deaths were attributed to pneumoconiosis. Chest radiographs of 83 current employees with expected dust exposure revealed no elevated percentage of subjects with parenchymal or pleural abnormalities compared with a group of 25 workers in another division of the company without exposure to dust. The vermiculite from South Carolina contains, at most, only trace amounts of tremolite asbestos (see Occurrence of Tremolite Asbestos section). Atkinson et al. (1982) reported that in samples of vermiculite from Patterson and Enoree, South Carolina, less than 1% of the weight was accounted for by asbestiform particles. Estimates of workplace air concentrations of particles with length ≥5 μm and aspect ratio > 3:1 were low, ranging from 0.4 fiber/mL in 1970 samples to 0.0 fiber/mL in 1985 in "wet zone" work areas and from 0.84 fiber/mL in 1970 to 0.02 fiber/mL in 1985 in "dry zone" areas (McDonald et al. 1988). Transmission electron microscopy and energy dispersive X-ray analysis of settled dust samples from dry zone locations showed four types of elongated particles: tremolite-actinolite (37.9%), vermiculite fragments (28.0%), talc/anthophyllite (15.9%), and iron-rich fibers (4.6%); 14% of the particles were not identified. McDonald et al. (1988) noted that the lack of observed respiratory effects in these vermiculite workers may have been due to a combination of the small number of subjects in the study (i.e., decreased detection power) and low airborne fiber concentrations. The mean cumulative fiber exposure of the Libby, Montana, mortality cohort studied by McDonald et al. (1986b) was 144.6 fiber-year/mL, whereas the mean of the South Carolina cohort was estimated at 0.75 fiber-year/mL.
High prevalences of pleural calcification have been noted in inhabitants of northwestern Greece villages who had no known occupational exposure to asbestos fibers. In a 1980 study of 408 subjects who represented 15% of the population of three villages (Metsovo, Anilio, and Milea) over the age of 10, chest radiographs showed very few small opacities indicative of pulmonary fibrosis, but an overall prevalence of pleural calcification in 34.7% of men and 21.5% of women examined (Bazas 1987; Bazas et al. 1985). Constantopoulos et al. (1985, 1987a) reported that radiographic screening detected pleural calcifications in up to 323/688 (46.9%) inhabitants of the same villages and another village (Votonossi) in this area (called Metsovo). The frequency of pleural calcification increased with age; about 70% of inhabitants of age >70 years had pleural calcification (Constantopoulos et al. 1985, 1987a). Constantopoulos et al. (1991) also found pleural calcifications in 24 of 101 (23.7%) examined inhabitants of another Greek village (Distrato) outside the Metsovo region.
Constantopoulos et al. (1985, 1987a, 1991) attributed the pleural calcifications to the domestic production and use of a tremolite-asbestos-containing whitewash ("luto") made from a local soil. Analysis of samples of the whitewash material by light microscopy, transmission electron microscopy, and X-ray dispersion analysis indicated that it contained predominantly asbestiform tremolite (Langer et al. 1987). The finding of tremolite fibers in transbronchial lung biopsy specimens from individuals diagnosed with pleural calcification supported the attribution of the effect to the use of tremolite-asbestos-containing whitewash; the amphibole fibers in the tissue were described as "tremolitic and asbestiform" (Constantopoulos et al. 1985). Furthermore, pleural calcifications were not observed in nearby villagers who did not use "luto" for whitewashing; these villagers used limestone (calcium oxide) (Constantopoulos et al. 1987a). Sakellariou et al. (1996) reported that domestic use of "luto" whitewash in the Metsovo area decreased from about 92% in 1950 to 71% in 1960, to 38% in 1970, and to 18% in 1980. Mineralogic analysis of Distrato whitewash also revealed chrysotile and tremolite asbestos fibers, but details of this analysis were not reported (Constantopoulos et al. 1991).
High incidences of pleural calcifications have also been reported for inhabitants of several rural regions of Turkey where tremolite-asbestos-containing whitewash has been used to cover interior walls (Baris et al. 1988a; Coplu et al. 1996; Dumortier et al. 1998; Metintas et al. 1999; Yazicioglu et al. 1980). For example, chest radiographs of 167 inhabitants (20 years or more of age) of the village of Caparkayi showed that 63 (37.7%) had radiological abnormalities. Interlobar fissure thickening (thickening in the regions between lobes of the lung), diffuse interstitial fibrosis, calcified pleural plaques, and pleural thickening were observed in 16.8%, 15.6%, 14.4%, and 7.8% of the 167 inhabitants, respectively (Baris et al. 1988a). The whitewash material used in this village was shown to be rich in tremolite asbestos fibers, both fine and coarse (Baris et al. 1988a). In a survey of 124 inhabitants of the village of Kureysler, 14% showed calcified pleural plaques and 4% showed noncalcified pleural plaques (Coplu et al. 1996). Tremolite asbestos fibers were abundant in the whitewash material and in soil from the roads of Kureysler. Indoor air fiber concentrations in samples from a Kureysler house were 0.14 and 0.94 fiber/mL, before and after the floor was swept, respectively (Coplu et al. 1996). Tremolite fibers represented the predominant fiber type in bronchoalveolar lavage fluid samples from 64 Turkish subjects with expected environmental exposure to asbestos fibers; concentrations of fibers in the samples were similar to concentrations in samples from subjects with known occupational exposure to asbestos (Dumortier et al. 1998).
Northeastern Corsica is another region where environmental exposure to tremolite asbestos fibers has been associated with radiographic pleural abnormalities (Boutin et al. 1989; Rey et al. 1993, 1994). A retrospective survey of 1,721 chest radiographs of subjects from northern Corsica found prevalences of pleural plaques in 3.7% and 1.1% of subjects from northeastern and northwestern Corsica, respectively (Boutin et al. 1989). Northeastern Corsica, unlike the northwest, contains surface deposits of chrysotile and tremolite asbestos. Rey et al. (1993, 1994) reported that the incidence of bilateral pleural plaques was 41% in nonoccupationally exposed inhabitants of a village in northeastern Corsica where tremolite fiber concentrations in air samples ranged from 6 to 72 ng/m3. In contrast, the incidence was 7.5% in inhabitants of a village with airborne tremolite concentrations <1 ng/m3. Rey et al. (1993) suggested that the presence of pleural plaques is an indicator of exposure to fibers, but is not a precancerous lesion. It was noted that concomitant pleural plaques were found in only 43% of 14 Corsican cases of mesothelioma attributed to environmental exposure to tremolite asbestos fibers.
Results from a study of rats exposed repeatedly to high concentrations of tremolite asbestos confirm the capability of airborne tremolite to cause progressive pulmonary fibrosis (Davis et al. 1985a). Groups of 48 SPF male Wistar rats (AF/HAN strain) were exposed to a nominal concentration of 10 mg/m3 tremolite asbestos, 7 hours/day, 5 days/week for 12 months starting at 10 weeks of age. The test material from Korea was determined to be about 95% tremolite asbestos (termed " 95% pure fibrous tremolite" by the authors) as confirmed by scanning electron microscopy and X-ray diffraction analysis with only minor amounts of iron and minor contamination with other silicate materials. Phase contrast microscopy of air samples determined the average fiber concentration (with lengths ≥5 μm) at about 1,600 fiber/mL. At 12 and 18 months after the start of exposure, 3 and 4 rats, respectively, were sacrificed and lungs were examined histologically for nonmalignant and malignant lesions (other tissues were also examined for tumors). Other rats were allowed to live until spontaneous death. At 12 months, average percentages of areas with nonmalignant lesions were 23% for peribronchiolar fibrosis, 35.2% for irregular alveolar wall thickening, and 0% for interstitial fibrosis. At 18 months, percentages of areas affected by these lesions were 13.4%, 27.7%, and 3%, respectively. None of the 12 rats dying between 27 and 29 months showed peribronchiolar fibrosis or irregular alveolar wall thickening, but 14.5% of lung area showed interstitial fibrosis. The fibrogenic activity of tremolite was also demonstrated in mice given single intratracheal instillations of suspensions of 5 mg Indian tremolite asbestos in saline (250 mg/kg body weight) (Sahu et al. 1975). Examination of lung tissue from mice sacrificed at 1, 2, 7, 15, 30, 60, 90, 120, and 150 days after instillation showed signs of a progressive fibrogenic reaction consisting of moderate proliferation of alveolar macrophages starting at 30 days, phagocytosis at 60 days, and moderate reticulinosis by 90 days. The fibrosis was classified as "grade I," compared with a more severe "grade II" fibrosis from similar exposure to amosite fiber suspensions. The results show that exposure of rats and mice to tremolite asbestos leads to a progressive development of pulmonary interstitial fibrosis after exposure has ceased. They are consistent with results from human studies indicating a long latency of development of pulmonary fibrosis from exposure to high concentrations of asbestos fibers. Animal studies designed to characterize exposure-response relationships for pulmonary fibrosis and varying concentrations of airborne tremolite asbestos were not located; neither were studies examining nonmalignant pleural changes in animals and exposure to airborne tremolite asbestos.
Lung Cancer. Elevated incidences of lung cancer and respiratory cancers have been observed in Libby, Montana, vermiculite workers exposed to tremolite asbestos. Results from studies of animals exposed to tremolite asbestos by inhalation and intratracheal instillation confirm that tremolite asbestos can induce lung cancer.
Mortality studies of Libby, Montana, vermiculite workers exposed to tremolite asbestos found excess mortalities from lung cancer (SMR=2.23; 95% CI=1.36, 3.45; 20 observed versus 9.0 expected; Amandus and Wheeler 1987) and respiratory cancer (SMR=2.45; 95% CI not reported; 23 observed deaths; expected deaths not reported; McDonald et al. 1986b). The respiratory cancer category included malignant neoplasms of the larynx, trachea, bronchus, lung, pleura, and mediastinum. Both studies found statistically significant relationships between increased risk for lung or respiratory cancer and increasing cumulative exposure. A precise tobacco smoking adjustment of the data could not be made, and some portion of the excess lung cancer occurrence may be reasonably attributed to smoking (Amandus and Wheeler 1987). Tobacco smoking, a potential confounding factor, was not addressed in the study by McDonald et al. (1986b). Comparative analysis of exposure-response relationships with other studies of asbestos-exposed workers indicated that the slope of the exposure-response regression was steeper in the Libby workers than in other workers exposed predominantly to chrysotile or to chrysotile, amosite, and crocidolite, but was less steep than the slope for workers exposed in asbestos textile plants (Amandus and Wheeler 1987).
In a mortality study of South Carolina vermiculite workers (McDonald et al. 1988), no increased risk for lung cancer was found. McDonald et al. (1988) attributed the apparent absence of cancer effect in these vermiculite miners and millers to the small number of subjects in the study and the low levels of airborne fibers at the South Carolina workplace relative to the Libby, Montana, mine and mill. As discussed earlier, this source of vermiculite does not appear to contain significant quantities of amphibole asbestos (Atkinson et al. 1982).
Lung tumors were found in 18/39 SPF male Wistar rats (AF/HAN strain) exposed to 10 mg/m3 Korean tremolite asbestos for 12 months and allowed to live until spontaneous death occurred (Davis et al. 1985a). Rats with tumors included 2 with benign and 16 with malignant tumors. No lung tumors were found in a concomitant control group of 36 nonexposed rats (Davis et al. 1985a). Primary benign or malignant lung tumors were found in 1/38 (adenoma) and 3/37 (1 adenoma, 1 adenocarcinoma, and 1 squamous cell carcinoma) female Wistar rats given 10 or 20 twice weekly intratracheal instillations of suspensions of 0.5 mg "fibrous" tremolite in saline (7x107 or 30x107 total fibers with length >5 μm, diameter <2 m, and length:width ratio >5:1) (Pott et al. 1994). The test material in this study was not further characterized with respect to asbestiform or nonasbestiform habit. After treatment, the rats were allowed to live until spontaneous death. No lung tumors were found in 79 control rats instilled with saline. Pott et al. (1994) speculated that the lack of a marked lung carcinogenic response in their study was due to insufficient numbers of tremolite fibers instilled.
Mesothelioma. Elevated incidences of mesotheliomas have been observed in Libby, Montana, vermiculite workers exposed to tremolite asbestos and in inhabitants of rural villages in Greece, Corsica, and Turkey where tremolite-asbestos-rich surface deposits exist or where tremolite-asbestos-containing whitewashes were domestically produced and used to paint interior walls. Results from studies of animals exposed to tremolite asbestos by intrapleural implantation, intraperitoneal injection, and inhalation confirm that tremolite asbestos can induce mesothelioma.
In the cohort mortality studies of Libby, Montana, vermiculite workers exposed to tremolite asbestos, mesotheliomas were noted in 4 of the 165 deaths (proportionate mortality ratio [PMR] = 2.4%) studied by McDonald et al. (1986b) and 2 of the 161 deaths (PMR = 1.2%) studied by Amandus and Wheeler (1987). No mesotheliomas were identified among the 51 deaths in the cohort mortality study of vermiculite workers in a South Carolina mine and mill where airborne fiber concentrations were estimated to be much lower than in the Libby, Montana, workplaces (McDonald et al. 1988). Cohort mortality studies of other groups of vermiculite miners and millers were not located.
Cases of mesotheliomas have been reported among inhabitants of villages in the Metsovo region of Greece where whitewash containing tremolite asbestos was domestically produced and used to paint interior walls (Constantopoulos et al. 1987b, 1991; Langer et al. 1987; Sakellariou et al. 1996). Six pleural mesotheliomas were reported among 600 deaths (about 1%) that occurred in four of these villages between 1981 and 1985 (Constantopoulos et al. 1987b; Langer et al. 1987). Constantopoulos et al. (1987b) noted that the incidence of mesothelioma deaths in the Metsovo region between 1981 and 1985 was about 300 times greater than expected in a non-asbestos-exposed population. Sakellariou et al. (1996) later reported that eight cases were recorded in the Metsovo region between 1980 and 1984 and that six cases were recorded for the 1985-1994 period. Sakellariou et al. (1996) proposed that the incidence of pleural mesothelioma may be decreasing as the use of the tremolite-asbestos whitewash is diminishing.
Other regions in which cases of mesothelioma have been attributed to environmental exposure to tremolite asbestos (not occupational exposure) include northeastern Corsica, a region with abundant surface deposits rich in tremolite fibers (Magee et al. 1986; Rey et al. 1993), the island of Cyprus (McConnochie et al. 1987), regions of New Caledonia (Luce et al. 1994, 2001), and regions of rural Turkey where tremolite-asbestos-containing whitewashes have been used domestically (Baris et al. 1988a, 1988b; Erzen et al. 1991; Metintas et al. 1999; Schneider et al. 1998; Yazicioglu et al. 1980).
Increased incidences of pleural tumors resembling human mesotheliomas have been observed in rats (Stanton et al. 1981; Wagner et al. 1982) and hamsters (Smith et al. 1979) exposed to tremolite asbestos by intrapleural implantation, in rats exposed to tremolite asbestos or actinolite asbestos samples by intraperitoneal injection (Davis et al. 1991; Pott et al. 1989; Roller et al. 1996, 1997), and in rats exposed to airborne tremolite asbestos (Davis et al. 1985a). Increases in most of these studies were statistically significant.
For example, pleural fibrosarcomas resembling human mesotheliomas developed in 22/28 (78.6%) and 21/28 (75%) female Osborne-Mendel rats within 2 years of intrapleurally implanting 40 mg of 2 tremolite asbestos samples in gelatin, compared with 17/598 (2.8%) control rats implanted with gelatin (Stanton et al. 1981) (4). Percentages of fibers in the 2 tremolite asbestos samples with lengths >4 μm were 34% and 31% and diameters <2.5 m were 100% and 94%. In another study, mesotheliomas were found in 36/36, 35/36, 32/33, and 24/36 rats given single 10-mg intraperitoneal doses of four samples of tremolite asbestos and allowed to live until spontaneous death (Davis et al. 1991). Respective median survival time for these groups of AF/HAN rats were 301, 365, 428, and 755 days, indicating some variance in tumor-development period. Numbers of fibers (x105) with length ≥8 μm and diameter <0.25 μm in 1 mg of these samples were 121, 8, 48, and 1, respectively. Mesotheliomas developed in only 4/33 and 2/36 rats given similar injections of two samples of tremolite that did not have as distinct an asbestiform morphology (no fibers with length ≥8 μm and diameter <0.25 μm were detected, although some fibers were detected with length ≥8 μm and diameter >0.25 μm) (Davis et al. 1991). Mesotheliomas also were found in 2/39 SPF male Wistar (AF/HAN strain) rats exposed by inhalation to 10 mg/m3 Korean tremolite asbestos for 12 months, but none were found in 36 control rats (Davis et al. 1985a).
Overall Health Effects
Weight of Evidence Studies of workers exposed to
airborne dusts of Libby, Montana, vermiculite containing tremolite
asbestos provide strong evidence that exposure to high levels of
airborne tremolite asbestos can lead to increased risk of structural
changes in the lung and pleura including pulmonary fibrosis, pleural
calcification, and pleural wall thickening (Amandus et al. 1987b;
Lockey et al. 1984; McDonald et al. 1986a) and of death from
nonmalignant respiratory disease (Amandus and Wheeler 1987; McDonald
et al. 1986b). Additional observations adding to the evidence that
long-term exposure to airborne tremolite fibers can lead to the
development of nonmalignant changes in the lung and pleura include:
- high prevalences of pleural calcification among residents of villages in Greece (Bazas 1987; Bazas et al. 1985; Constantopoulos et al. 1985, 1987a, 1991), Turkey (Baris et al. 1988a; Coplu et al. 1996; Dumortier et al. 1998; Metintas et al. 1999; Yazicioglu et al. 1980), and Corsica (Boutin et al. 1989; Rey et al. 1993, 1994) where whitewashes containing tremolite asbestos have been used domestically or where there are abundant surface deposits rich in tremolite asbestos, and
- progressive pulmonary fibrogenic reactions in the lungs of rats and mice after exposure to tremolite asbestos by inhalation or intratracheal instillation (Davis et al. 1985a; Sahu et al. 1975).
Evidence that repeated exposure to airborne tremolite asbestos can lead to increased risk for the development of lung cancer includes observations of statistically significantly increased rates of mortality from lung cancer in groups of Libby Montana vermiculite workers compared with rates for the general population (Amandus and Wheeler 1987; McDonald et al. 1986b), statistically significant relationships between cumulative fiber exposure measures and prevalence of lung or respiratory cancer among Libby vermiculite workers (Amandus and Wheeler 1987; McDonald et al. 1986b), and increased incidences of lung tumors in rats exposed to tremolite asbestos by inhalation (Davis et al. 1985a) or intratracheal instillation (Pott et al. 1994). The weight of the human evidence for tremolite asbestos-induced lung cancer is limited by the inability to adjust for likely confounding factors from smoking in the Libby vermiculite workers.
There is a causal relationship between long-term exposure to airborne tremolite asbestos and mesothelioma, which is a rare fatal cancer accounting for 2.87 deaths per million within the U.S. white male general population in 1996 (NIOSH 1999). The evidence includes elevated prevalences of mesothelioma deaths (of about 1/100 to 2/100) among groups of Libby, Montana, vermiculite workers (Amandus and Wheeler 1987; McDonald et al. 1986b), among residents of Greek (Constantopoulos et al. 1987b, 1991; Langer et al. 1987; Sakellariou et al. 1996), Turkish (Baris et al. 1988a, 1988b; Erzen et al. 1991; Metintas et al. 1999; Schneider et al. 1998; Yazicioglu et al. 1980), and New Caledonia (Luce et al. 1994, 2001) villages that used tremolite-asbestos whitewashes on interior walls, and in regions of northeastern Corsica and Cyprus that have abundant surface deposits of tremolite asbestos (Magee et al. 1986; McConnochie et al. 1987; Rey et al. 1993). Strong supporting evidence comes from animal studies showing increased incidences of pleural tumors resembling human mesotheliomas in rats (Stanton et al. 1981; Wagner et al. 1982) and hamsters (Smith et al. 1979) exposed to tremolite asbestos by intrapleural implantation, in rats exposed to tremolite asbestos or actinolite asbestos samples by intraperitoneal injection (Davis et al. 1991; Pott et al. 1989; Roller et al. 1996, 1997), and in rats exposed to airborne tremolite asbestos (Davis et al. 1985a).
Clinical Aspects of
Diseases Associated with Exposure to Asbestos
Exposure to tremolite asbestos or other forms of asbestos can increase risks for developing pleural plaques, pleural thickening (i.e. pleural fibrosis), pleural effusions, interstitial lung fibrosis, lung cancer, and mesothelioma.
Asbestos-related pleural abnormalities have been commonly associated with asbestos-related lung parenchyma lesions, but the American Thoracic Society (1986) noted that they should be diagnosed separately because "there are differences between pleural and parenchymal fibrosis in epidemiology, clinical features, and prognosis." Asbestos-related pleural plaques have been described as "fibrohyaline nodular lesions, most often on the parietal pleura, but also on the diaphragmatic pleura and less frequently on the pericardium" (Mossman and Gee 1989).
Unlike people with pleural plaques alone, who do not have impaired pulmonary functions or symptoms such as chest pain, persons with asbestos-related pleural thickening commonly experience symptoms and have impaired pulmonary function (American Thoracic Society 1986). Studies of groups of modern asbestos workers, who likely were exposed to lower airborne concentrations of asbestos fibers than workers in the first half of the twentieth century, found that the prevalence of pleural abnormalities (most often plaques) is often as high as 10 times higher than the prevalence of parenchymal abnormalities (Becklake 1994; Mossman and Gee 1989; Orlowski et al. 1994). Pleural effusions are early manifestations of inhalation exposure to high concentrations of asbestos; the fluid contains varying amounts of red blood cells, macrophages, lymphocytes, and mesothelial cells (American Thoracic Society 1986; Mossman and Gee 1989). Pleural effusions may be an early indication of mesothelioma and warrant further evaluation. Early identification of mesothelioma and intervention may increase chances of survival (ATSDR 2000).
The American Thoracic Society (1986) defines asbestosis as interstitial fibrosis of the lung parenchyma from exposure to asbestos. Studies of occupationally exposed patients who develop asbestosis have shown that latency periods of at least 15 years are common between the time of initial exposure to asbestos fibers and the onset of respiratory symptoms (American Thoracic Society 1986; Kamp and Weitzman 1997; Mossman and Gee 1989). These symptoms include shortness of breath during physical exertion (i.e., exertional dyspnea), pleuritic chest pain, phlegm production, wheezing, and end-inspiratory crackles. Lung functions that can be decreased are lung volumes, pulmonary compliance, and diffusing capacity for carbon monoxide (DLCO) (Becklake 1994; Kamp and Weitzman 1997).
Clinical diagnosis of asbestosis is accomplished by a reliable exposure history; a latency period of at least 15-20 years since first exposure; chest radiographic evidence of parenchymal abnormalities (small, irregular opacifications of a profusion of 1/1 or greater); a restrictive pattern of lung impairment with a reduced forced vital capacity; reduced diffusing capacity; and bilateral late or pan inspiratory crackles (American Thoracic Society 1986). Chest radiography is the most important clinical tool for the diagnosis of asbestosis. Supplemental use of high resolution computerized tomography improves the sensitivity and accuracy of detecting parenchymal and pleural changes that can account for symptoms of respiratory distress and lung function deficits in patients (Aberle et al. 1988a, 1988b; Becklake 1994; Begin et al. 1992; Harkin et al. 1996; Klaas 1993). When clinically indicated, detection of asbestos bodies (fibers surrounded by a coat of iron and protein) in surgically removed lung parenchymal tissue with diffuse interstitial fibrosis confirms the diagnosis of asbestosis (American Thoracic Society 1986).
People who repeatedly inhale dusts with tremolite asbestos also are expected to have increased risk for lung cancer and malignant mesothelioma. Several studies of asbestos workers have found that smoking increases the risk of lung cancer in a greater than additive manner, but does not appear to increase the risk for mesothelioma (Berry et al. 1985; Hammond et al. 1979; McDonald et al. 1980; Selikoff et al. 1980).
Eighty to ninety percent of patients diagnosed with mesothelioma report a history of occupational or environmental exposure to some form of asbestos (Attanoos and Gibbs 1997; Bianchi et al. 1997; Colt 1997; Roggli et al. 1997). Malignant mesothelioma is an aggressive and fatal cancer that is most often located in the pleura (90%) and sometimes in the peritoneum (6%-10%) (Attanoos and Gibbs 1997; Kelley 1998). In a review of 1,690 cases of mesothelioma associated with occupational exposure to asbestos, the authors reported that the median period of latency between initial exposure and detection was 32 years; 99% and 96% of the cases had latency periods of more than 15 and 20 years, respectively (Lanphear and Buncher 1992).
Treatment options are few for patients diagnosed with asbestos-related nonmalignant lung or pleural disease. Preventing further exposure and ceasing any tobacco smoking activities are the most important steps individuals can take to minimize development of health problems. Once developed, these diseases may remain stable or progress in severity in the absence of further exposure (Becklake 1994). The diseases rarely regress. Treatment options for patients diagnosed with asbestos-related cancer of the lung or pleura are restricted to resection and/or chemotherapy. One study suggests that subjects who stop smoking after already having been exposed to asbestos show some improvement in lung health (Waage et al. 1996), but long-term data for the effectiveness of cessation of smoking in large cohorts of asbestos-exposed individuals are not available.
- Tremolite is an amphibole
mineral that most commonly exists in the earth's crust in forms
that are nonasbestiform. Tremolite asbestos has only rarely been
found in amounts sufficient for commercial use, but has been
reported to occur at various sites throughout the world.
- Vermiculite deposits in the
region of Libby, Montana, contain fibrous amphibole that is
popularly called tremolite asbestos. Although scientists have
called this mineral by various names, there is agreement that
exposure to the mineral increased the risk of nonmalignant
respiratory and pleural disorders, lung cancer, and mesothelioma
in Libby mine and mill workers. The mine has been closed since
1990, and access to the sites is restricted.
- Exposure to all types of
asbestos can increase the risk of developing lung cancer,
malignant mesothelioma, and nonmalignant respiratory and pleural
effects, including pulmonary interstitial fibrosis (asbestosis),
pleural plaques, pleural calcification, and pleural thickening.
Asbestos-exposed smokers have greater than additive risks for
lung cancer and asbestosis than do asbestos-exposed nonsmokers.
- Important determinations of
asbestos toxicity include exposure concentration, duration,
fiber dimensions, and fiber durability. There is animal and
human evidence that long fibers are retained in the lungs for
longer periods than short fibers and that amphibole fibers, such
as tremolite asbestos, are retained longer than chrysotile
fibers. Short and long fibers may contribute to the pathogenesis
of inflammation, fibrosis, and cancer in humans, but their
relative importance is uncertain.
- Latency periods for the
development of asbestos-related nonmalignant respiratory effects
are usually 15-40 years from the time of initial exposure to
asbestos.
- The latency periods are
generally 20 years or more for lung cancer and 30 years or more
for mesothelioma due to asbestos exposure.
- Occupational exposure to
asbestos may occur in workers involved in mining, milling, and
handling of certain sources of chrysotile and vermiculite ores;
in exfoliating vermiculite that contains tremolite asbestos; and
in mining, milling, and handling of other ores and rocks that
may contain tremolite asbestos. Residents who live close to
mining, milling, or manufacturing sites that involve tremolite
asbestos-containing-material may be potentially exposed to
higher levels of airborne tremolite asbestos than levels in
general ambient air.
- Asbestos may be released to
indoor or outdoor air as a result of the disturbance of
asbestos-containing building materials such as insulation,
fire-proofing material, dry wall, and ceiling and floor tile.
Amphibole asbestos has been found in some sources of vermiculite
that has been used as home and building insulation. Workers or
homeowners involved in demolition work or asbestos removal, or
in building or home maintenance, repair, and remodeling,
potentially can be exposed to higher levels of airborne asbestos
than levels in general ambient air. In general, exposure may
occur only when the asbestos-containing material is disturbed in
some way to release asbestos fibers into the air. When
asbestos-containing materials are solidly embedded or contained,
exposure will be negligible.
- Recently, small amounts of
amphibole asbestos have been found in some samples of
vermiculite-containing consumer garden products and in some
talc-containing crayons. Consumers can reduce possible exposure
by limiting the production of dusts when using the garden
products. The risk that children might be exposed to asbestos
fibers through inhalation or ingestion of crayons containing
asbestos and transitional fibers is extremely low. The U.S.
manufacturers of these crayons, however, have agreed to
eliminate talc from their products in the near future.
- The combined use of light
microscopy, electron microscopy (transmission and scanning), and
X-ray dispersive methods in analyzing air and/or bulk material
samples offers the most accurate approach to estimating airborne
asbestos concentrations.
- Clinical diagnostic methods
for determining exposure and effects of asbestos include chest
radiography, pulmonary function tests, and high resolution
computerized tomography. Microscopic detection of asbestos
bodies in autopsied or biopsied lung tissue can be used to
confirm exposure when tissue is available.
- Pleural effusions are early
manifestations of inhalation exposure to high concentrations of
asbestos. Pleural effusions may be an early indication of
mesothelioma and warrant further evaluation. Early
identification of mesothelioma and intervention may increase
chances of survival.
- Additional research may help to develop therapeutic methods to interfere with the development of nonmalignant lung and pleural disorders, and to cause the disorders to regress once they are established. Such research may include studies on the mechanism of asbestos-related disease to provide further understanding of how persistent production of reactive oxygen or nitrogen species and persistent inflammatory cellular responses precisely interact.
Prevention of exposure and cessation of any tobacco smoking activities are the most important steps that individuals can take to prevent or minimize the development of asbestos-related health problems.
People who were exposed to asbestos and who smoke are expected to be unusually susceptible to asbestos-related lung cancer and asbestosis and are encouraged to cease smoking. Studies of asbestos workers indicate that asbestos-exposed smokers have greater than additive risks for lung cancer and asbestosis than asbestos-exposed nonsmokers. Although the mechanism of this interaction is poorly understood, one possible mechanism that has received some support from research is that smoking can decrease the clearance of asbestos fibers from the lung by impairing mucociliary action and macrophage activity (see ATSDR 2001a for review).
Individuals residing or working in buildings with insulation or other building materials that may potentially contain asbestiform minerals (for example, vermiculite from the Libby Montana mine) are encouraged to ensure that the insulation material is solidly contained and not able to be disturbed and become airborne. If the material is to be removed, special procedures must be followed that minimize the generation of dust and specify appropriate locations for disposal. Individuals can obtain information about asbestos removal and disposal procedures from the 10 regional offices of the EPA.
Further evaluation of the progression of disease associated with exposure to Libby, Montana, vermiculite contaminated with asbestos is warranted. EPA, ATSDR, and other agencies currently are investigating exposure levels that Libby, Montana, residents (including children) who were not employed in the vermiculite mines and mills may have and are experiencing. In addition, ATSDR is currently conducting medical testing of individuals potentially exposed to fibrous amphibole associated with vermiculite in Libby, Montana.
References (* indicates cited in text)
Aalto M, Heppleston AG. 1984. Fibrogenesis by mineral fibres: an in-vitro study of the roles of the macrophage and fibre length. Br J Exp Pathol 65:91-99.
*Aberle DR, Gamsu G, Ray CS, et al. 1988a. Asbestos-related pleural and parenchymal fibrosis: Detection with high-resolution CT. Radiology 166:729-734.
*Aberle DR, Gamsu G, Ray CS. 1988b. High-resolution CT of benign asbestos-related diseases: Clinical and radiographic correlation. AJR Am J Roentgenol 151:883-891.
ACGIH. 1992. Asbestos. In: Documentation of threshold limit values. American Conference of Governmental Industrial Hygienists. Cincinnati, OH, 89-94.
*ACGIH. 1996. Particulates (insoluble) Not Otherwise Classified. In: Documentation of the threshold limit values and biological exposure indices. Supplement. American Conference of Governmental Industrial Hygienists. Cincinnati, OH.
*ACGIH. 1998. Asbestos, all forms. In: Documentation of threshold limit values. American Conference of Governmental Industrial Hygienists. Cincinnati, OH.
*Addison J, Davies LST. 1990. Analysis of amphibole asbestos in chrysotile and other minerals. Ann Occup Hyg 34(2):159-175.
*Albin M, Pooley FD, Stromberg U, et al. 1994. Retention patterns of asbestos fibres in lung tissue among asbestos cement workers. Occup Environ Med 51:205-211.
*Amandus HE, Wheeler R. 1987. The morbidity and mortality of vermiculite miners and millers exposed to tremolite-actinolite: Part II. Mortality. Am J Ind Med 11:15-26.
*Amandus HE, Wheeler R, Jankovic J, et al. 1987a. The morbidity and mortality of vermiculite miners and millers exposed to tremolite-actinolite: Part I. Exposure estimates. Am J Ind Med 11:1-14.
*Amandus HE, Althouse R, Morgan WKC, et al. 1987b. The morbidity and mortality of vermiculite miners and millers exposed to tremolite-actinolite: Part III. Radiographic findings. Am J Ind Med 11:27-37.
*American Thoracic Society. 1986. The diagnosis of nonmalignant diseases related to asbestos. Am Rev Respir Dis 134: 363-368.
*American Thoracic Society. 1990. Health effects of tremolite. Prepared by a subcommittee of the American Thoracic Society Scientific Assembly on Environmental and Occupational Health. Am Rev Respir Dis 142(6):1453-1458.
*Amethyst Galleries. 1999. The mineral tremolite. http://mineral.galleries.com/minerals/silicate/tremolit/tremolit.htm
Andrion A, Bosia S, Paoletti L, et al. 1994. Malignant peritoneal mesothelioma in a 17-year-old boy with evidence of previous exposure to chrysotile and tremolite asbestos. Hum Pathol 25:617-622.
Athanasiou K, Constantopoulos SH, Rivedal E, et al. 1992. Metsovo-tremolite asbestos fibres: in vitro effects on mutation, chromosome aberration, cell transformation and intercellular communication. Mutagenesis 7(5):343-347.
*Atkinson GR, Rose D, Thomas K, Jones D, Chatfield EJ, Going JE. 1982. Collection, analysis and characterization of vermiculite samples for fiber content and asbestos contamination. MRI report for EPA, project No. 4901-A32 under EPA contract 68-01-5915. F. Kutz, EPA Project officer.
*ATSDR. 2000. ATSDR/NCI workshop on asbestos-related therapies: summary report. May 8, 2000, Washington, DC.
*ATSDR. 2001a. Toxicological profile for asbestos. U.S. Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry. Atlanta, GA.
*ATSDR 2001b. Preliminary findings of medical testing of individuals potentially exposed to asbestiform minerals associated with vermiculite in Libby, Montana: an interim report for community health planning. February 22, 2001. U.S. Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry. Atlanta, GA.
*Attanoos RL, Gibbs AR. 1997. Pathology of malignant mesothelioma. Histopathology 30:403-417.
*Baris YI, Bilir N, Artvinli M, et al. 1988a. An epidemiological study in an Anatolian village environmentally exposed to tremolite asbestos. Br J Ind Med 45:838-840.
*Baris YI, Artvinli M, Sahin AA, et al. 1988b. Non-occupational asbestos related chest diseases in a small Anatolian village. Br J Ind Med 45:841-842.
*Bazas T. 1987. Pleural effects of tremolite in north-west Greece. Lancet 1(8548):1490-1491.
Bazas T, Bazas B, Kitas D, et al. 1981. Pleural calcification in north-west Greece. [Letter]. Lancet II:254.
*Bazas T, Oakes D, Gilson JC, et al. 1985. Pleural calcification in northwest Greece. Environ Res 38:239-247.
*Becklake MR. 1994. Symptoms and pulmonary functions as measures of morbidity. Ann Occup Hyg 38(4):569-580.
*Begin R, Gauthier J-J, Desmeules M, et al. 1992. Work-related mesothelioma in Quebec, 1967-1990. Am J Ind Med 22:531-542.
*Berman DW, Crump KS, Chatfield EJ, et al. 1995. The sizes, shapes, and mineralogy of asbestos structures that induce lung tumors or mesothelioma in AF/HAN rats following inhalation. (Errata attached). Risk Anal 15:181-195.
*Berry G, Newhouse ML, Antonis P. 1985. Combined effect of asbestos and smoking on mortality from lung cancer and mesothelioma in factory workers. Br J Ind Med 42:12-18.
*Bianchi C, Brolo A, Ramani L, et al. 1997. Pleural plaques as risk indicators for malignant pleural mesothelioma: a necropsy-based study. Am J Ind Med 32:445-449.
*Bignon J, Jaurand MC. 1983. Biological in vitro and in vivo responses of chrysotile versus amphiboles. Environ Health Perspect 51:73-80.
*BOHS. 1968. Hygiene standards for chrysotile asbestos dust. Ann Occup Hyg 11:47-69.
*Boutin G, Viallat JR, Steinbauer J, et al. 1989. Bilateral pleural plaques in Corsica: a marker of non-occupational asbestos exposure. IARC Sci Publ 90:406-410.
Brown DP, Dement JM, Wagoner JK. 1979. Mortality patters among miners and millers occupationally exposed to asbestiform talc. In: Lemen R, Dement JM, eds. Dust and disease: proceedings of the conference on occupational exposures to fibrous and particulate dust and their extension into the environment, 1977, Washington, DC. Park Forest South, IL: Pathotox Publishers, Inc., 317-324.
Brown GM, Cowie H, Davis JMG, et al. 1986. In vitro assays for detecting carcinogenic mineral fibres: a comparison of two assays and the role of fibre size. Carcinogenesis 7(12):1971-1974.
*Camus M, Siemiatycki J, Meek B. 1998. Nonoccupational exposure to chrysotile asbestos and the risk of lung cancer. N Engl J Med 338(22):1565-1571.
*Case BW. 1991. Health effects of tremolite: now and in the future. Ann N Y Acad Sci 643:491-504.
*Case BW. 1994. Biological indicators of chrysotile exposure. Ann Occup Hyg 38:503-518.
Case BW, Dufresne A. 1997. Asbestos, asbestosis, and lung cancer: observations in Quebec chrysotile workers. Environ Health Perspect Suppl 5:1113-1119.
*Case BW, Dufresne A, McDonald AD, et al. 2000. Asbestos fiber type and length in lungs of chrysotile textile and production workers: fibers longer than 18 m. Inhal Toxicol 12:411-418.
Case BW, Sebastien P. 1987. Environmental and occupational exposures to chrysotile asbestos: a comparative microanalytic study. Arch Environ Health 42(4):185-191.
*Churchill RK, Higgins CT, Hill RL. 2001. A pilot project to map areas likely to contain natural occurrences of asbestos - El Dorado County, California. Poster presentation. 2001 Asbestos Health Effects Conference. Sponsored by U.S. Environmental Protection Agency. May 24-25, 2001. San Francisco, CA.
*Churg A. 1988. Chrysotile, tremolite, and malignant mesothelioma in man. Chest 93:621-628.
*Churg A. 1994. Deposition and clearance of chrysotile asbestos. Ann Occup Hyg 38(4):625-633.
Churg A, DePaoli L. 1988. Clearance of chrysotile asbestos from human lung. Exp Lung Res 14:567-574.
Churg A, Wiggs B. 1986. Fiber size and number in workers exposed to processed chrysotile asbestos, chrysotile miners, and the general population. Am J Ind Med 9:143-152.
*Churg A, Wright JL. 1989. Fibre content of lung in amphibole- and chrysotile-induced mesothelioma: implications for environmental exposure. IARC Sci Publ 90:314-318.
*Churg A, Wright JL. 1994. Persistence of natural mineral fibers in human lungs: an overview. Environ Health Perspect Suppl 102(5):229-233.
*Churg A, Wright J, Wiggs B, et al. 1990. Mineralogic parameters related to amosite asbestos-induced fibrosis in humans. Am Rev Respir Dis 142:1331-1336.
*Churg A, Wright JL, Vedal S. 1993. Fiber burden and patterns of asbestos-related disease in chrysotile miners and millers. Am Rev Respir Dis 148:25-31.
Chuwers P, Barnhart S, Blanc P, et al. 1997. The protective effect of beta-carotene and retinol on ventilatory function in an asbestos-exposed cohort. Am J Respir Crit Care Med 155:1066-1071.
*Coin PG, Roggli VL, Brody AR. 1992. Deposition, clearance, and translocation of chrysotile asbestos from peripheral and central regions of the rat lung. Environ Res 58:97-116.
*Colt HG. 1997. Mesothelioma: epidemiology, presentation, and diagnosis. Semin Respir Med 18:353-361.
*Constantopoulos SH, Goudevenos JA, Saratzis N, et al. 1985. Metsovo lung: pleural calcification and restrictive lung function in northwestern Greece. Environmental exposure to mineral fiber as etiology. Environ Res 38:319-331.
*Constantopoulos SH, Saratzis NA, Kontogiannis D, et al. 1987a. Tremolite whitewashing and pleural calcifications. Chest 92:709-712.
*Constantopoulos SH, Malamou-Mitsi VD, Goudevenos JA, et al. 1987b. High incidence of malignant pleural mesothelioma in neighboring villages of northwestern Greece. Respiration 51:266-271.
*Constantopoulos SH, Theodoracopoulos P, Dascalopoulos G, et al. 1991. Metsovo lung outside Metsovo: endemic pleural calcifications in the ophiolite belts of Greece. Chest 99:1158-1161.
*Constantopoulos SH, Dalavanga YA, Sakellariou K, et al. 1992. Lymphocytic alveolitis and pleural calcifications in nonoccupational asbestos exposure. Am Rev Respir Dis 146:1565-1570.
*CPSC. 2000. CPSC staff report on asbestos fibers in children's crayons. U.S. Consumer Product Safety Commission. Washington DC. http://www.cpsc.gov/LIBRARY/FOIA/Foia00/os/crayons.pdf
*Coplu L, Dumortier P, Demir AU, et al. 1996. An epidemiological study in an Anatolian village in Turkey environmentally exposed to tremolite asbestos. J Environ Pathol Toxicol Oncol 15(2-4):177-182.
*Crane DT. 2000. Background information regarding the analysis of industrial talcs. June 12, 2000 Report. U.S. Department of Labor, Occupational Safety and Health Administration, Salt Lake Technical Center, Salt Lake City, UT.
Davis JMG. 1983. Carcinogenic effect of mineral fibers in inhalation studies. VDI-Ber 475:241-246.
*Davis JMG. 1989. Mineral fibre carcinogenesis: experimental data relating to the importance of fibre type, size, deposition, dissolution and migration. IARC Sci Publ 90:33-45.
Davis JMG, Beckett ST, Bolton RE, et al. 1980. The effects of intermittent high asbestos exposure (peak dose levels) on the lungs of rats. Br J Exp Pathol 61:272-280.
*Davis JMG, Addison J, Bolton RE, et al. 1985a. Inhalation studies on the effects of tremolite and brucite dust in rats. Carcinogenesis 6(5):667-674.
Davis JM, Bolton RE, Cowie H, et al. 1985b. Comparisons of the biological effects of mineral fibre samples using in vitro and in vivo assay systems. NATO ASI Ser G 3:405-411.
*Davis JMG, Addison J, McIntosh C, et al. 1991. Variations in the carcinogenicity of tremolite dust samples of differing morphology. Ann N Y Acad Sci 643:473-490.
De Klerk NH, Musk AW, Ambrosini GL, et al. 1998. Vitamin A and cancer prevention II: comparison of the effects of retinol and beta-carotene. Int J Cancer 75:362-367.
Dement JM, Brown DP. 1994. Lung cancer mortality among asbestos textile workers: a review and update. Ann Occup Hyg 38:525-532.
Dement JM, Brown DP. 1998. Cohort mortality and case-control studies of white male chrysotile asbestos textile workers. J Clean Technol Environ Toxicol Occup Med 7(4):413-419.
Dement JM, Brown DP, Okun A. 1994. Follow-up study of chrysotile asbestos textile workers: cohort mortality and case-control analyses. Am J Ind Med 26:431-447.
*De Vuyst P, Dumortier P, Jacobovitz D, et al. 1994. Environmental asbestosis complicated by lung cancer. Chest 105(5):1593-1595.
*Dodson RF, O'Sullivan M, Corn CJ, et al. 1997. Analysis of asbestos fiber burden in lung tissue from mesothelioma patients. Ultrastruct Pathol 21:321-336.
*Dodson RF, Williams MG, Huang J, et al. 1999. Tissue burden of asbestos in nonoccupationally exposed individuals from east Texas. Am J Ind Med 35:281-286.
*DOL. 1980. Asbestiform and/or fibrous minerals in mines, mills, and quarries. Washington, DC: U.S. Department of Labor, Mine Safety and Health Administration. IR 1111.
Dufresne A, Harrigan M, Masse S, et al. 1995. Fibers in lung tissues of mesothelioma cases among miners and millers of the township of Asbestos, Quebec. Am J Ind Med 27:581-592.
Dufresne A, Begin R, Masse S, et al. 1996. Retention of asbestos fibres in lungs of workers with asbestosis, asbestosis and lung cancer, and mesothelioma in Asbestos township. Occup Environ Med 53:801-807.
*Dumortier P, Coplu L, de Maertelaer V, et al. 1998. Assessment of environmental asbestos exposure in Turkey by bronchoalveolar lavage. Am J Respir Crit Care Med 158:1815-1824.
Elmes P. 1994. Mesotheliomas and chrysotile. Ann Occup Hyg 38(4):547-553.
*EPA. 1986. Airborne asbestos health assessment update. Washington, DC: U.S. Environmental Protection Agency, Office of Health and Environment Assessment. EPA/600/8-84/003F.
*EPA. 2000. Sampling and analysis of consumer garden products that contain vermiculite. U.S. Environmental Protection Agency, Office of Prevention, Pesticides and Toxic Substances. EPA 744-R-00-010. http://www.epa.gov/opptintr/asbestos/verm.htm
*Erzen C, Eryilmaz M, Kalyoncu F, et al. 1991. CT findings in malignant pleural mesothelioma related to nonoccupational exposure to asbestos and fibrous zeolite (erionite). J Comput Assist Tomogr 15(2):256-260.
Finkelstein MM, Dufresne A. 1999. Inferences on the kinetics of asbestos deposition and clearance among chrysotile miners and millers. Am J Ind Med 35:401-412.
*Frank AL, Dodson RF, Williams MG. 1998. Carcinogenic implications of the lack of tremolite in UICC reference chrysotile. Am J Ind Med 34:314-317.
Gamble JF, Fellner W, Dimeo MJ. 1979. An epidemiologic study of a group of talc workers. Am Rev Respir Dis 119:741-753.
*Hammond EC, Selikoff IJ, Seidman H. 1979. Asbestos exposure, cigarette smoking and death rates. Ann N Y Acad Sci 330:473-490.
*Harkin TJ, McGuinness G, Goldring R, et al. 1996. Differentiation of the ILO boundary chest roentgenograph (0/1 to 1/0) in asbestosis by high-resolution computed tomography scan, alveolitis, and respiratory impairment. J Occup Environ Med 38:46-52.
*HEI. 1991. Health Effects Institute. Asbestos in public and commercial buildings: a literature review and synthesis of current knowledge. Report of the asbestos literature review panel. Cambridge, MA: Health Effects Institute.
*Henderson DW, de Klerk NH, Hammar SP, et al. 1997. Asbestos and lung cancer: is it attributable to asbestosis or to asbestos fiber burden? In: Corrin B, ed. Pathology of lung tumors. New York, NY: Churchill Livingstone, 83-118.
*Hessel PA, Sluis-Cremer GK. 1989. X-ray findings, lung function, and respiratory symptoms in black South African vermiculite workers. Am J Ind Med 15:21-29.
*Hillerdal G, Henderson GW. 1997. Asbestos, asbestosis, pleural plaques and lung cancer. Scand J Work Environ Health 23:93-103.
*Hodgson JT, Darnton A. 2000. The quantitative risks of mesothelioma and lung cancer in relation to asbestos exposure. Ann Occup Hyg 44(8):565-601.
*Hughes JM. 1994. Human evidence: lung cancer mortality risk from chrysotile exposure. Ann Occup Hyg 38(4):555-560.
*Hughes JM, Weill H. 1991. Asbestosis as a precursor of asbestos related lung cancer: results of a prospective mortality study. Br J Ind Med 48:229-233.
*IARC. 1987a. Asbestos and certain asbestos compounds. In: IARC monographs on the evaluation of the carcinogenic risk of chemicals to humans. Chemicals, industrial processes and industries associated with cancer in humans. IARC monographs, Vols 1 to 42. IARC monographs, supplement 7. Lyon, France: World Health Organization, International Agency for Research on Cancer, 29-33, 56-58.
IARC. 1987b. Talc. In: IARC monographs on the evaluation of the carcinogenic risk of chemicals to humans. Silica and some silicates. IARC monographs, volume 42. Lyon, France: World Health Organization, International Agency for Research on Cancer, 185-224.
ILO. 1989. International Labour Office. Guidelines for the use of the ILO international classification of radiographs of pneumoconiosis, revised edition. Geneva, Switzerland: ILO Occupational Safety and Health Series. No 22.
*Jackson JA. 1997. Glossary of Geology. Fourth Edition. American Geological Institute, Alexandria, VA.
*Jolicoeur CR, Alary JF, Sokov A. 1992. Asbestos. In: Kroschwitz JI, Howe-Grant M, ed. Kirk-Othmer encyclopedia of chemical technology. New York: John Wiley & Sons, 659-688.
*Jones RN , Hughes JM, Weill H. 1996. Asbestos exposure, asbestosis, and asbestos-attributable lung cancer. Thorax 51: S9-S15.
*Kamp DW, Weitzman SA. 1997. Asbestosis: clinical spectrum and pathogenic mechanisms. Proc Soc Exp Biol Med 214:12-26.
*Kamp DW, Weitzman SA. 1999. The molecular basis of asbestos induced lung injury. Thorax 54:638-652.
*Kamp DW, Graceffa P, Pryor WA, et al. 1992. The role of free radicals in asbestos-induced diseases. Free Radic Biol Med 12:293-315.
Kamp DW, Dunne M, Dykewicz MS, et al. 1993. Asbestos-induced injury to cultured human pulmonary epithelial-like cells: role of neutrophil elastase. J Leukoc Biol 54:73-80.
*Kelley J. 1998. Occupational lung disease caused by asbestos, silica, and other silicates. In: Baum GL, Crapo JD, Celli BR, et al., eds. Textbook pulmonary diseases. Philadelphia, PA: Lippincott-Raven, 659-682.
*Klaas VE. 1993. A diagnostic approach to asbestosis, utilizing clinical criteria, high resolution computed tomography, and gallium scanning. Am J Ind Med 23:801-809.
Kleinfeld M, Messite J, Kooyman O, et al. 1967a. Mortality among talc miners and millers in New York State. Arch Environ Health 14:663-667.
Kleinfeld M, Messite J, Kooyman O. 1967b. Mortality experience in a group of asbestos workers. Arch Environ Health 15:177-180.
Kleinfeld M, Messite J, Langer AM. 1973. A study of workers exposed to asbestiform minerals in commercial talc manufacture. Environ Res 6:132-143.
Kleinfeld M, Messite J, Zaki MH. 1974. Mortality experiences among talc workers: a follow-up study. J Occup Med 16:345-349.
*Landrigan PJ. 1998. Asbestos-still a carcinogen. N Engl J Med 338(22):1618-1619.
*Langer AM, Nolan RP. 1998. Asbestos in the lungs of persons exposed in the USA. Monaldi Arch Chest Dis 53(2):168-180.
*Langer AM, Nolan RP, Constantopoulos SH, et al. 1987. Association of Metsovo lung and pleural mesothelioma with exposure to tremolite-containing whitewash. Lancet 1(8539):965-967.
*Lanphear BP, Buncher CR. 1992. Latent period for malignant mesothelioma of occupational origin. J Occup Med 34(7):718-721.
*Lash TL, Crouch EAC, Green LC. 1997. A meta-analysis of the relation between cumulative exposure to asbestos and relative risk of lung cancer. Occup Environ Med 54:254-263.
*Leake BE. 1978. Nomenclature of amphiboles. Am Mineral 63: 1023-1052.
*Leake BE, Wooley AR, Arps CES, et al. 1997. Nomenclature of amphiboles: report of the subcommittee on amphiboles of the International Mineralogical Association, Commission on New Minerals and Mineral Names. Am Mineral 82: 1019-1037.
*Lee RJ, Van Orden DR, Corn M, et al. 1992. Exposure to airborne asbestos in buildings. Regul Toxicol Pharmacol 16:93-107.
*Lippmann M. 1994. Deposition and retention of inhaled fibres: effects on incidence of lung cancer and mesothelioma. Occup Environ Med 51:793-798.
*Lockey JE, Brooks SM, Jarabek AM, et al. 1984. Pulmonary changes after exposure to vermiculite contaminated with fibrous tremolite. Am Rev Respir Dis 129:952-958.
*Luce D, Brochard P, Quenel P, et al. 1994. Malignant pleural mesothelioma associated with exposure to tremolite. Lancet 344:1777.
*Luce D, Billon-Galland MA, Bugel I, et al. 2001. Environmental exposure to tremolite and respiratory cancer in New Caledonia (South Pacific). Poster presentation, 2001 Asbestos Health Effects Conference. Sponsored by U.S. Environmental Protection Agency. May 24-25, 2001. San Francisco, CA.
*Luster MI, Simeonova PP. 1998. Asbestos induces inflammatory cytokines in the lung through redox sensitive transcription factors. Toxicol Lett 102-103:271-275.
*Magee F, Wright JL, Chan N, et al. 1986. Malignant mesothelioma caused by childhood exposure to long-fiber low aspect ratio tremolite. Am J Ind Med 9:529-533.
*Mansinghka BK, Ranawat PS. 1996. Mineral economics and occupational health hazards of the asbestos resources of Rajathan. J Geol Soc India 47: 375-382.
McConnell EE, Rutter HA, Ulland BM, et al. 1983. Chronic effects of dietary exposure to amosite asbestos and tremolite in F344 rats. Environ Health Perspect 53:27-44.
*McConnochie K, Simonato L, Mavrides P, et al. 1987. Mesothelioma in Cyprus: the role of tremolite. Thorax 42:342-347.
McDonald AD, Case BW, Churg A, et al. 1997. Mesothelioma in Quebec chrysotile miners and millers: epidemiology and aetiology. Ann Occup Hyg 41(6):707-719.
*McDonald JC. 1998. Mineral fibre persistence and carcinogenicity. Ind Health 36:372-375.
*McDonald JC, McDonald AD. 1997. Chrysotile, tremolite and carcinogenicity. Ann Occup Hyg 41(6):699-705.
*McDonald JC, Liddell FDK, Gibbs GW, et al. 1980. Dust exposure and mortality in chrysotile mining, 1910-75. Br J Ind Med 37:11-24.
*McDonald JC, Sebastien P, Armstrong B. 1986a. Radiological survey of past and present vermiculite miners exposed to tremolite. Br J Ind Med 43:445-449.
*McDonald JC, McDonald AD, Armstrong B, et al. 1986b. Cohort study of mortality of vermiculite miners exposed to tremolite. Br J Ind Med 43:436-444.
*McDonald JC, McDonald AD, Sebastien P, et al. 1988. Health of vermiculite miners exposed to trace amounts of fibrous tremolite. Br J Ind Med 45:630-634.
*McDonald JC, Armstrong B, Case B, et al. 1989. Mesothelioma and asbestos fiber type. Evidence from lung tissue analysis. Cancer 63:1544-1547.
*McDonald JC, McDonald AD, Hughes JM. 1999. Chrysotile, tremolite and fibrogenicity. Ann Occup Hyg 43(7):439-442.
*Meeker GP, Brownfield IK, Clark RN, et al. 2001. The chemical composition and physical properties of amphibole from Libby, Montana: a progress report. Poster presentation, 2001 Asbestos Health Effects Conference. Sponsored by U.S. Environmental Protection Agency. May 24-25, 2001. San Francisco, CA.
*Metintas M, Ozdemir N, Hillerdal G, et al. 1999. Environmental asbestos exposure and malignant pleural mesothelioma. Respir Med 93:349-355.
*Moatamed F, Lockey JE, Parry WT. 1986. Fiber contamination of vermiculites: a potential occupational and environmental health hazard. Environ Res 41:207-218.
Mossman BT. 1990. In vitro studies on the biologic effects of fibers: correlation with in vivo bioassays. Environ Health Perspect 88:319-322.
*Mossman BT, Churg A. 1998. Mechanisms in the pathogenesis of asbestosis and silicosis. Am J Respir Crit Care Med 157:1666-1680.
*Mossman BT, Gee JBL. 1989. Asbestos-related diseases. N Engl J Med 320(26):1721-1730.
*Mossman B, Light W, Wei E. 1983. Asbestos: mechanisms of toxicity and carcinogenicity in the respiratory tract. Annu Rev Pharmacol Toxicol 23:595-615.
*Mossman BT, Bignon J, Corn M, et al. 1990. Asbestos: scientific developments and implications for public policy. Science 247:294-301.
*Mossman BT, Kamp DW, Weitzman SA. 1996. Mechanisms of carcinogenesis and clinical features of asbestos-associated cancers. Cancer Invest 14(5):466-480.
NIOSH. 1980. Occupational exposure to talc containing asbestos, morbidity, mortality, and environmental studies of miners and millers. Cincinnati, OH: U.S. National Institute for Occupational Safety and Health. NTIS PB80-193352.
NIOSH. 1986. Occupational respiratory diseases. Washington, DC: U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Institute for Occupational Safety and Health. DHHS (NIOSH) Publication No. 86-102.
*NIOSH. 1989. Fibers-method 9002. In: Manual of analytical methods, 3rd edition. Supplement. Cincinnati, OH: U.S. Department of Health and Human Services, National Institute for Occupational Safety and Health.
*NIOSH. 1994a. Asbestos and other fibers by PCM. In: Manual of analytical methods, 4th edition. Cincinnati, OH: U.S. Department of Health and Human Services, National Institute for Occupational Safety and Health.
*NIOSH. 1994b. Asbestos by TEM. In: Manual of analytical methods, 4th edition. Cincinnati, OH: U.S. Department of Health and Human Services, National Institute for Occupational Safety and Health.
*NIOSH. 1999. Work-related lung disease surveillance report 1999. Washington, DC: U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Institute for Occupational Safety and Health, Division of Respiratory Disease Studies. DHHS (NIOSH) Publication No. 96-134.
*NRC. 1984. National Research Council. Asbestiform fibers: nonoccupational health risks. Washington, DC: National Academy Press.
NTP. 1990. National Toxicology Program. Technical report on the toxicology and carcinogenesis studies of tremolite (CAS no. 14567-73-8) in Fischer 344 rats (feed study). Research Triangle Park, NC: U. S. Department of Health and Human Services, Public Health Service, National Institutes of Health. NIH Publication No. 90-2531. NTP TR 277.
*NTP. 1993. National Toxicology Program. Toxicology and carcinogenesis studies of talc (CAS no. 14807-96-6) in Fischer 344/N rats and B6C3F1 mice. (Inhalation studies). Research Triangle Park, NC: U. S. Department of Health and Human Services, Public Health Service, National Institutes of Health. NIH Publication No. 93-3152. NTP TR 421.
*NTP. 2001. National Toxicology Program. Asbestos: CAS No. 1332-21-4. In: Report on carcinogenicity, ninth edition. Revised January 2001. Research Triangle Park, NC: U.S. Department of Health and Human Services.
*Oberdorster G. 1994. Macrophage-associated responses to chrysotile. Ann Occup Hyg 38(4):601-615.
Okayasu R, Wu L, Hei TK. 1999. Biological effects of naturally occurring and man-made fibres: in vitro cytotoxicity and mutagenesis in mammalian cells. Br J Cancer 79(9/10):1319-1324.
Omenn GS, Goodman GE, Thornquist MD, et al. 1996a. Risk factors for lung cancer and for intervention effects in CARET, the beta-carotene and retinol efficacy trial. J Natl Cancer Inst 88(21):1550-1559.
Omenn GS, Goodman GE, Thornquist MD, et al. 1996b. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med 334:1150-1155.
*Orlowski E, Pairon JC, Ameille J, et al. 1994. Pleural plaques, asbestos exposure, and asbestos bodies in bronchoalveolar lavage fluid. Am J Ind Med 26:349-358.
Osgood C, Sterling D. 1991. Chrysotile and amosite asbestos induce germ-line aneuploidy in drosophila. Mutat Res 261:9-13.
*OSHA. 1986. U.S. Department of Labor, Occupational Safety and Health Administration. Federal Register 51:22612-22790.
*OSHA. 1992. U.S. Department of Labor, Occupational Safety and Health Administration. Federal Register 57:7877-7878, 24310-24331, 49657-49661.
*OSHA. 1994. Occupational exposure to asbestos. U.S. Department of Labor, Occupational Safety and Health Administration. Federal Register 59(153):40964-41162.
*Pang TWS, Schonfeld-Starr RA, Patel K. 1989. An improved membrane filter technique for evaluation of asbestos fibers. Am Ind Hyg Assoc J 50(3):174-180.
*Paoletti L, Caiazza S, Donelli G, et al. 1984. Evaluation by electron microscopy techniques of asbestos contamination in industrial, cosmetic, and pharmaceutical talcs. Regul Toxicol Pharmacol 4:222-235.
Peto J, Seidman H, Selikoff IJ. 1982. Mesothelioma mortality in asbestos workers: implications for models of carcinogenesis and risk assessment. Br J Cancer 45:124-135.
Pooley FD. 1981. Mineralogy of asbestos: the physical and chemical properties of the dusts they form. Semin Oncol 8(3):243-249.
Pott F, Ziem U, Reiffer F-J, et al. 1987. Carcinogenicity studies on fibres, metal compounds, and some other dusts in rats. Exp Pathol 32:129-152.
*Pott F, Roller M, Ziem U, et al. 1989. Carcinogenicity studies on natural and man-made fibres with the intraperitoneal test in rats. IARC Sci Publ 90:173-179.
*Pott F, Dungworth DL, Heinrich U, et al. 1994. Lung tumours in rats after intratracheal instillation of dusts. Ann Occup Hyg 38(Suppl. 1):357-363.
*Renner R. 2000. Asbestos in the air. Sci Am Feb:34.
*Rey F, Boutin C, Steinbauer J, et al. 1993. Environmental pleural plaques in an asbestos exposed population of northeast Corsica. Eur Respir J 6:978-982.
*Rey F, Boutin C, Viallat JR, et al. 1994. Environmental asbestotic pleural plaques in northeast Corsica: correlations with airborne and pleural mineralogic analysis. Environ Health Perspect 102(Suppl 5):251-252.
*Rödelsperger K, Woitowitz H-J, Brückel B, et al. 1999. Dose-response relationship between amphibole fiber lung burden and mesothelioma. Cancer Detect Prev 23(3):183-193.
*Rogers AJ, Leigh J, Berry G, et al. 1991. Relationship between lung asbestos fiber type and concentration and relative risk of mesothelioma. Cancer 67:1912-1920.
Roggli VL, Pratt PC, Brody AR. 1993. Asbestos fiber type in malignant mesothelioma: an analytical scanning electron microscopic study of 94 cases. Am J Ind Med 23:605-614.
Roggli VL, Pratt PC, Brody AR. 1994. Fiber potency vs. importance. Am J Ind Med 25:611-612.
*Roggli VL, Oury TD, Moffatt EJ. 1997. Malignant mesothelioma in women. In: Anatomic pathology. Chicago, Ill: American Society of Clinical Pathologists, 2:147-163.
Rohl AN, Langer AM, Selikoff IJ, et al. 1976. Consumer talcums and powders: mineral and chemical characterization. J Toxicol Environ Health 2:225-284.
Rohl AN, Langer AM, Selikoff IJ. 1977. Environmental asbestos pollution related to use of quarried serpentine rock. Science 196:1319-1322.
*Roller M, Pott F, Kamino K, et al. 1996. Results of current intraperitoneal carcinogenicity studies with mineral and vitreous fibres. Exp Toxicol Pathol 48:3-12.
*Roller M, Pott F, Kamino K, et al. 1997. Dose-response relationship of fibrous dusts in intraperitoneal studies. Environ Health Perspect 105 (Suppl 5):1253-1256.
*Rom WN, Travis WD, Brody AR. 1991. Cellular and molecular basis of the asbestos-related diseases. Am Rev Respir Dis 143:408-422.
Ross D, McDonald JC. 1995. Occupational and geographical factors in the epidemiology of malignant mesothelioma. Monaldi Arch Chest Dis 50(6):459-462.
*Ross M. 1981. The geologic occurrences and health hazards of amphibole and serpentine asbestos. In: Veblen DR, ed. Reviews in mineralogy. Chelsea, MI: Bookcrafters, Inc., 279-323.
Ross M, Kuntze RA, Clifton RA. 1984. A definition for asbestos. ASTM Spec Tech Publ 834:139-147.
*Ross M, Nolan RP, Langer AM, and Cooper WC. 1993. Health effects of mineral dusts other than asbestos. In: Guthrie GD, Mossman BT, eds. MSA Reviews in Mineralogy Vol 28: 361-407.
*Sahu AP, Dogra RKS, Shanker R, et al. 1975. Fibrogenic response in murine lungs to asbestos. Exp Pathol 11:21-24.
*Sakellariou K, Malamou-Mitsi V, Haritou A, et al. 1996. Malignant pleural mesothelioma from nonoccupational asbestos exposure in Metsovo (north-west Greece): slow end of an epidemic? Eur Respir J 9:1206-1210
*Schneider J, Rodelsperger K, Bruckel B, et al. 1998. Environmental exposure to tremolite asbestos: pleural mesothelioma in two Turkish workers in Germany. Rev Environ Health 13(4):213-220.
Seaborg GT. 1991. Actinides and transactinides. In: Kroschwitz J, Howe-Grant M, ed. Kirk-Othmer encyclopedia of chemical technology. New York, NY: John Wiley and Sons, Inc., 456-488.
*Sebastien P, Janson X, Gaudichet A. et al. 1980. Asbestos retention in human respiratory tissues: comparative measurements in lung parenchyma and in parietal pleura. IARC Sci Publ 30: 237-246.
Sebastien P, McDonald JC, McDonald AD, et al. 1989. Respiratory cancer in chrysotile textile and mining industries: exposure inferences from lung analysis. Br J Ind Med 46:180-187.
*Selikoff IJ, Seidman H, Hammond C. 1980. Mortality effects of cigarette smoking among site asbestos factory workers. J Natl Cancer Inst 65(3):507-513.
Selevan SG, Dement JM, Wagoner JK, Froines JR. 1979. Mortality patterns among miners and millers of non-asbestiform talc: preliminary report. In: Lemen R, Dement JM, eds. Dust and disease: proceedings of the conference on occupational exposures to fibrous and particulate dust and their extension into the environment, 1977, Washington, DC. Park Forest South, IL: Pathotox Publishers, Inc., 379-388.
*Skinner HCW, Ross M, Frondel C. 1988. Asbestos and other fibrous materials: mineralogy, crystal chemistry, and health effects. New York, NY: Oxford University Press.
Sluis-Cremer GK. 1988. Linking chrysotile asbestos with mesothelioma. [Letter]. Am J Ind Med 14:631-632.
Sluis-Cremer GK, Liddell FDK, Logan WPD, et al. 1992. The mortality of amphibole miners in South Africa, 1946-80. Br J Ind Med 49:566-575.
*Smith WE, Hubert DD, Sobel HJ, et al. 1979. Biologic tests of tremolite in hamsters. In: Lemen R, Dement JM Eds. Proc. Conf. Occup. Exp. Fibrous Part. Dust. Ther. Ext. Environ. Park Forest South IL: 335-339.
Smith WE, Hubert DD, Sobel HJ. 1980. Dimensions of fibres in relation to biological activity. In: Biological effects of mineral fibres. Lyon, France: International Agency for Research on Cancer, 357-360.
Srebro SH, Roggli VL. 1994. Asbestos-related disease associated with exposure to asbestiform tremolite. Am J Ind Med 26:809-819.
*Stanton MF, Layard M, Tegeris A, et al. 1981. Relation of particle dimension to carcinogenicity in amphibole asbestoses and other fibrous minerals. J Natl Cancer Inst 67(5):965-975.
*Stayner LT, Dankovic DA, Lemen RA. 1996. Occupational exposure to chrysotile asbestos and cancer risk: a review of the amphibole hypothesis. Am J Public Health 86(2):179-186.
*Stayner L, Smith R, Bailer J, et al. 1997. Exposure-response analysis of risk of respiratory disease associated with occupational exposure to chrysotile asbestos. Occup Environ Med 54:646-652.
Stille WT, Tabershaw IR. 1982. The mortality experience of Upstate New York talc workers. J Occup Med 24(6):480-484.
Streib WC. 1978. Asbestos. In: Grayson M, Eckroth D, eds. Kirk-Othmer encyclopedia of chemical technology. New York, NY: John Wiley & Sons, Inc., 269-278.
Suzuki K, Hei TK. 1996. Induction of heme oxygenase in mammalian cells by mineral fibers: distinctive effect of reactive oxygen species. Carcinogenesis 17(4):661-667.
*Tanaka S, Choe N, Hemenway DR, et al. 1998. Asbestos inhalation induces reactive nitrogen species and nitrotyrosine formation in the lungs and pleura of the rat. J Clin Invest 102:445-454.
USGS. 1998a. Talc and pyrophyllite. In: Minerals yearbook. U.S. Geological Survey. http://minerals.usgs.gov/minerals/pubs/commodity/talc/650498.pdf
*USGS. 1998b. Vermiculite. In: Minerals handbook. U.S. Geological Survey. http://minerals.usgs.gov/minerals/pubs/commodity/vermiculite/index.htm
USGS. 1998c. Directory of companies mining talc and pyrophyllite in the United States in 1997. In: Minerals industry surveys. U.S. Geological Survey. http://minerals.usgs.gov/minerals/pubs/commodity/talc/650297.pdf
*USGS. 1999. Talc and pyrophyllite. In: Minerals commodity summaries. U.S. Geological Survey. http://minerals.usgs.gov/minerals/pubs/commodity/talc/650399.pdf
Vacek PM, McDonald JC. 1991. Risk assessment using exposure intensity: an application to vermiculite mining. Br J Ind Med 48:543-547.
*Verkouteren JR, Wylie AG. 2000. The tremolite-actinolite-ferro-actinolite series: systematic relationships among cell parameters, composition, optical properties, and habit, and evidence of discontinuities. Am Mineral 85: 1239-1254.
Vianna NJ, Pola AK. 1978. Nonoccupational exposure to asbestosis and malignant mesothelioma in females. Lancet 1:1061.
*Veblen DR, Wylie AG. 1993. Mineralogy of amphiboles and 1:1 layer silicates. In: Guthrie GD, Mossman BT, eds. MSA Reviews in Mineralogy Vol 28: 61-137.
*Vu V. 1993. Regulatory approaches to reduce human health risks associated with exposures to mineral fibers. In: Guthrie GD, Mossman BT, eds. MSA Reviews in Mineralogy Vol 28: 545-554.
*Waage HP, Vatten LJ, Opedal E, and Hilt B. 1996. Lung function and respiratory symptoms related to changes in smoking habits in asbestos-exposed subjects. J Occup Environ Med 38: 178-183.
*Wagner JC, Berry G, Skidmore JW, et al. 1974. The effects of the inhalation of asbestos in rats. Br J Cancer 29:252-269.
*Wagner JC, Chamberlain M, Brown RC, et al. 1982. Biological effects of tremolite. Br J Cancer 45:352-360.
*WHO. 1998. Chrysotile asbestos: Environmental health criteria 203. Geneva, Switzerland: World Health Organization.
*Wilkinson P, Hansell DM, Janssens J, et al. 1995. Is lung cancer associated with asbestos exposure when there are no small opacities on the chest radiograph? Lancet 345:1074-1078.
*Wylie AG, Bailey KF, Kelse JW, et al. 1993. The importance of width in asbestos fiber carcinogenicity and its implications for public policy. Am Ind Hyg Assoc J 54(5):239-252.
*Wylie AG, Skinner HCW, Marsh J, et al. 1997. Mineralogical features associated with cytotoxic and proliferative effects of fibrous talc and asbestos on rodent tracheal epithelial and pleural mesothelial cells. Toxicol Appl Pharmacol 147:143-150.
*Wylie AG and Verkouteren R. 2000. Amphibole asbestos from Libby, Montana: aspects of nomenclature. Am Mineral 85: 1540-1542.
*Yazicioglu S, Ilcayto R, Balci K, et al. 1980. Pleural calcification, pleural mesotheliomas, and bronchial cancers caused by tremolite dust. Thorax 35:564-569.
*Zazenski R, Ashton WH, Briggs, D, et al. 1995. Talc: occurrence, characterization, and consumer applications. Regul Toxicol Pharmacol 21:218-229.
*Zoltai, T. 1979. Asbestiform and acicular mineral fragments. Ann N Y Acad Sci 330: 621-643.
*Zoltai, T. 1981. Amphibole asbestos mineralogy. In: Veblen DR, ed Amphiboles and other hydrous particles. MSA Reviews in Mineralogy. Vol 9A: 235-278.
1. Epidemiologic studies of Libby, Montana, miners and millers are discussed later in this document.
2. Called a solid state solution series by mineralogists.
3. The ILO classification system (ILO 1989) for profusion of opacities in chest radiographs establishes four categories of profusion of increasing severity, each with three subcategories noted in parentheses: 0 (0/-, 0/0, 0/1); 1 (1/0, 1/1, 1/2); 2 (2/1, 2/2, 2/3); 3 (3/2, 3/3, 3/4).
4. In the Stanton et al. (1981) experiments, seven samples of refined talc from different sources were tested. No malignancies were found in 6 of the talc-exposed groups of rats, including one group exposed to talc containing significant concentrations of particles with structures having lengths > 8 μm and diameters ≤0.25 μm. The incidence of rats with pleural sarcomas in the other talc-exposed group (1/26) was not significantly elevated compared with the incidence in a combined control group that included untreated rats and rats implanted with noncarcinogenic material.
This page last updated on October 30, 2008


