 |

NCI Technology Research Facility Gets Off the Ground
Ground was broken last week for an advanced technology research facility in Frederick, MD, marking the launch of an NCI research initiative to spur the development of new treatments and diagnostics. The facility will house a number of technology-based research programs currently based at NCI-Fredrick on the nearby Fort Detrick campus. Slated to open in early 2011, NCI will be the "anchor tenant" in the new facility, which is part of a larger research park under development. The majority of the existing NCI-Frederick programs will remain at Fort Detrick.
The work done in the laboratories and related offices relocating to the facility, NCI leaders explained, involves technologies such as biopharmaceutical manufacturing, proteomics, genomics, and nanotechnology, and will form the core of NCI's Advanced Technology Partnerships Initiative (ATPI).
Read more 


Bevacizumab May Increase Blood Clot Risk
Bevacizumab (Avastin), the first FDA-approved drug designed to inhibit the growth of new blood vessels to tumors, significantly increases the risk of venous thromboembolism (VTE) in cancer patients, according to a meta-analysis in the November 19 Journal of the American Medical Association.
Pooled results from nearly 8,000 patients with a variety of advanced solid tumors in 15 randomized trials published since 2003 showed that patients taking bevacizumab were
33 percent more likely to develop VTE than those who did not. Incidence among those taking bevacizumab was 11.9 percent for VTE of all grades, and 6.3 percent for high-grade VTE. Those taking the drug had a 38 percent greater risk of developing high-grade VTE.
A dosage as small as 2.5 mg/kg per week was enough to pose a risk, which the authors believe "suggests that the so-called low dose of bevacizumab may already be reaching the saturation level to induce thrombosis." Based on the greater risk found in patients with mesothelioma and aerodigestive malignancies such as non-small cell lung cancer, the authors advised that patients with these conditions receive concurrent prevention for VTE.
Read more 
 |
The NCI Cancer Bulletin is produced by the National Cancer Institute (NCI). NCI, which was established in 1937, leads the national effort to eliminate the suffering and death due to cancer. Through basic, clinical, and population-based biomedical research and training, NCI conducts and supports research that will lead to a future in which we can identify the environmental and genetic causes of cancer, prevent cancer before it starts, identify cancers that do develop at the earliest stage, eliminate cancers through innovative treatment interventions, and biologically control those cancers that we cannot eliminate so they become manageable, chronic diseases.

For more information on cancer, call 1-800-4-CANCER or visit http://www.cancer.gov.

NCI Cancer Bulletin staff can be reached at ncicancerbulletin@mail.nih.gov. |
|
 |
 |

NCI Technology Research Facility Gets Off the Ground
 Ground was broken last week for an advanced technology research facility in Frederick, MD, marking the launch of an NCI research initiative to spur the development of new treatments and diagnostics. The facility will house a number of technology-based research programs currently based at NCI-Fredrick on the nearby Fort Detrick campus. Slated to open in early 2011, NCI will be the "anchor tenant" in the new facility, which is part of a larger research park under development. The majority of the existing NCI-Frederick programs will remain at Fort Detrick.
Ground was broken last week for an advanced technology research facility in Frederick, MD, marking the launch of an NCI research initiative to spur the development of new treatments and diagnostics. The facility will house a number of technology-based research programs currently based at NCI-Fredrick on the nearby Fort Detrick campus. Slated to open in early 2011, NCI will be the "anchor tenant" in the new facility, which is part of a larger research park under development. The majority of the existing NCI-Frederick programs will remain at Fort Detrick.
The work done in the laboratories and related offices relocating to the facility, NCI leaders explained, involves technologies such as biopharmaceutical manufacturing, proteomics, genomics, and nanotechnology, and will form the core of NCI's Advanced Technology Partnerships Initiative (ATPI).
The ATPI, still in its early stages, was launched to expand NCI's ability to build the much-needed collaborations with the private sector that focus on technology-driven translational research, explained NCI Director Dr. John E. Niederhuber.
"This site will be the center of an intensive new effort to bring together government, industry, academic, and nonprofit partners, working side-by-side, utilizing technological resources second to none, to more rapidly translate our latest genetic and molecular discoveries about cancer into effective new treatments that benefit patients," he said.
Initially the facility will provide up to 330,000 square feet of space for offices and state-of-the-art laboratories, explained Dr. Craig Reynolds, director of the Office of Scientific Operations at NCI-Frederick.
The new facility, Dr. Reynolds said, "will provide the physical infrastructure to carry out the ATPI." Most of the NCI laboratories moving to the new facility are part of NCI's Advanced Technology Program or Biopharmaceutical Development Program. "They were chosen because they fit both of the criteria for the ATPI: they involve advanced technologies and are areas in which both industry and academia have strongly indicated they would like to partner."
As is the case with NCI-Frederick - one of only 38 Federally Funded Research and Development Centers, and the only one solely dedicated to biomedical research - this new research facility will be operated by SAIC-Frederick, a private contractor.
NCI operations currently housed in 33 different buildings on the NCI-Frederick campus and at satellite offices will move to the new facility. Many of these buildings, Dr. Reynolds stressed, are aging (most were built during World War II) and cannot provide the space or amenities needed to undertake the types of partnerships expected to emerge under the ATPI.
"We're proud of the work we've been able to do in these facilities," he said. "But the new facility will provide state-of-the-art space to work with collaborators from industry and academia, as well as to train the next generation of scientists on how to use advanced technologies."
The new facility should help free up space on the Fort Detrick campus to enhance other programs and core services that support both the intramural and extramural cancer research community, he added.
"The new facility," says Dr. Niederhuber, "embodies the vision of NCI as a cancer research campus where private sector research and development programs can
co-locate with other extramural institutions and join forces with federal labs to understand cancer and develop interventions that will prevent, diagnosis, and treat the disease."
—Carmen Phillips
|
 |
 |
Bevacizumab May Increase Blood Clot Risk
Bevacizumab (Avastin), the first FDA-approved drug designed to inhibit the growth of new blood vessels to tumors, significantly increases the risk of venous thromboembolism (VTE) in cancer patients, according to a meta-analysis in the November 19 Journal of the American Medical Association.
Pooled results from nearly 8,000 patients with a variety of advanced solid tumors in 15 randomized trials published since 2003 showed that patients taking bevacizumab were
33 percent more likely to develop VTE than those who did not. Incidence among those taking bevacizumab was 11.9 percent for VTE of all grades, and 6.3 percent for high-grade VTE. Those taking the drug had a 38 percent greater risk of developing high-grade VTE.
A dosage as small as 2.5 mg/kg per week was enough to pose a risk, which the authors believe "suggests that the so-called low dose of bevacizumab may already be reaching the saturation level to induce thrombosis." Based on the greater risk found in patients with mesothelioma and aerodigestive malignancies such as non-small cell lung cancer, the authors advised that patients with these conditions receive concurrent prevention for VTE.
"It may be appropriate to add a black box warning [to the package insert currently required by the FDA]," noted the study authors, led by Dr. Shobha Rani Nalluri and colleagues at Stony Brook University. Other angiogenesis inhibitors such as thalidomide and lenalidomide have also been shown to increase risk of VTE, and the authors warned that combining them with bevacizumab could compound the increased risk.
Number of Adult U.S. Smokers Drops, But So Do Quit Attempts
A new report released by the CDC shows that, for the first time in
3 years, the prevalence of cigarette smoking among adults in the
United States fell significantly, from 20.8 percent in 2006 to 19.8 percent in 2007. More than three in four
adult smokers reported smoking every day, while about one in four reported smoking only on some days. The article appeared in the November 14 Morbidity and Mortality Weekly Report.
Self-reported data were collected through the National Health Interview Survey (NHIS) from more than 23,000 randomly chosen people aged 18 years and over who completed in-person interviews.
Despite overall progress, only two groups of adults showed significant declines in smoking prevalence: blacks (from 23.0 percent to 19.8 percent) and people over the age of 65 (from 10.2 percent to 8.3 percent). Additionally, the percent of "everyday smokers" who attempted to quit in the previous 12 months was lower in 2007 than in 1993; those aged 18 to 24 years were more likely to make a quit attempt than older adults.
The authors suggest that the "lack of funding for comprehensive state tobacco-control programs" is a barrier to achieving greater progress in encouraging adults to quit. They also note that "Clinicians and health-care delivery systems need to consistently identify and document tobacco use status, treat every tobacco user seen in the health-care setting, and promote patients' use of quitlines," as part of a comprehensive tobacco control program. Quitlines are now available nationwide through the toll-free access number 1-800-QUIT-NOW.
PAX2 Protein Mediates Effect of Tamoxifen in Breast Cancer
The protein PAX2 plays an important role in the effectiveness of tamoxifen for the treatment of breast cancer. The presence of PAX2 in tumor cells blocks production of the protein ERBB2 (also called HER2), which fuels tumor growth, and a lack of PAX2 decreases the effectiveness of tamoxifen, British researchers reported online November 12 in Nature.
In experiments using breast cancer cell lines, the researchers identified several new DNA binding sites for the estrogen receptor (ER), including one in the ERBB2 gene. The researchers found that when exposed to tamoxifen, PAX2 forms a complex with the ER and binds to ERBB2, blocking the gene's expression. In normal cells, treatment with estrogen or tamoxifen therefore reduced the levels of ERBB2. But when the researchers blocked the expression of PAX2, the amount of ERBB2 in the cells increased, which subsequently caused an increase in cell growth.
Another protein called AIB-1, which is known to promote tumor formation, was found to bind to the same ERBB2 gene site as the ER-PAX2 complex; AIB-1 and PAX2 appear to compete for this binding site. When PAX2 protein levels fell, AIB-1 preferentially bound to the site, reversing the effects of tamoxifen. In a breast cancer cell line that had reduced levels of PAX2 and was resistant to tamoxifen, the investigators were able to restore sensitivity to the drug by introducing PAX2 into the cells.
The researchers, led by Dr. Antoni Hurtado of Cancer Research UK, confirmed their findings in 109 tissue samples taken from primary breast tumors (from patients who had all been treated with tamoxifen). Patients with PAX2-positive tumors had a significantly improved recurrence-free interval compared to patients with PAX2-negative tumors.
In a press conference accompanying the release of the paper, the researchers explained their hope that this work will eventually be used to help identify women who would not benefit from tamoxifen and who would require an alternate therapy such as an aromatase inhibitor.
Burden of Cervical Cancer Prior to HPV Vaccine Assessed
An estimated 25,000 cancers associated with the human papillomavirus (HPV) occurred annually in 38 states and the District of Columbia between 1998 and 2003, new research suggests. The study is one of 22 new reports coordinated by the CDC to assess the burden of HPV-associated cancers in the United States prior to the introduction of the HPV vaccine Gardasil. The vaccine protects against the two HPV types responsible for 70 percent of cervical cancers and the two types that cause 90 percent of genital warts.
The studies appear in the November 15 supplement
of Cancer.
Cervical cancer is the most common HPV-associated cancer, with nearly 11,000 cases in the United States per year. But HPV, which includes more than 100 different types, is also associated with cancers of the vulva, vagina, penis, anus, oral cavity, and oropharynx. (FDA recently expanded its approval of Gardasil to include prevention of vaginal and vulvar cancers.)
The estimates of HPV-associated cancers in the period before the HPV vaccine will give researchers baseline data for measuring the impact of the vaccine and cervical-cancer screening programs in reducing the incidence of cervical cancer and other HPV-associated cancers and precancers, according to Dr. Mona Saraiya of the CDC, who coordinated the studies.
Dozens of investigators authored the 22 articles for project ABHACUS (Assessing the Burden of Human Papillomavirus-Associated Cancers). A range of HPV-cancer related issues, including racial disparities, behavioral risk factors, and cancer mortality, are addressed in the studies.
The results, for instance, confirm recent reports showing higher rates of cervical cancer among black and Hispanic women and women in parts of the South. The mortality data on cervical cancer, one study concludes, are a reminder of the importance of screening and prevention programs, because during each year of the study more than 4,000 women died from this largely preventable disease.
|
 |
 |

Building an Advanced Technology Research Initiative
I have participated in the symbolic turning of a shovel of dirt to begin some important projects during my academic career, but none more meaningful to me than one this past Wednesday. I had the distinct honor of participating in the ground breaking ceremony for a new research facility, not just a laboratory construction project, but the manifestation of an Advanced Technology Partnerships Initiative (ATPI) and the beginning of a new era to expand drug and technology development using public, private, and academic partnerships. For NCI and the larger cancer community, this research park represents an opportunity to co-locate private sector research and development programs, biotechnology partners, and academic collaborators on a research campus dedicated to reducing the cancer burden.
As I said at the ceremony, during a recent visit and tour of NCI, a leader of a prominent cancer advocacy group was impressed by the exciting work supported by NCI and the individuals leading these various projects. He remarked how they clearly come to work each day committed to "owning the cure." That's just what the ATPI and the construction of
this new facility are about: Taking a proactive approach to accelerate progress against cancer, not just by funding and conducting the research, but also by establishing the platforms - in this case, state-of-the-art technology and drug development platforms - to turn that research into effective interventions as quickly and efficiently as possible.

Indeed, while this will be a building constructed to facilitate cancer science, the ATPI will take advantage of NCI-Frederick's capabilities as one of only 38 Federally Funded Research and Development Centers (FFRDC). Using the FFRDC capabilities, the ATPI will expand collaborations with a variety of private companies and institutes to develop new agents, new diagnostics, and new ways of monitoring response to therapy and then carry them forward to first-in-human studies; and to find ways to work together to create new ideas. Even before the first thrust of the shovel, NCI had already established the first collaborative agreement under this program with GE Global Research to develop nanoparticles for use as diagnostic imaging agents.
We know well from discussions with both large and small private-sector companies and academic investigators that what NCI can provide under the ATPI is access to cutting-edge, often costly technologies that are not readily available in the research community. Partnerships will flow from access to these technologies and the expertise required to operate them.
Under the ATPI, for example,
NCI will collaborate with partners to test the optimal use of new technologies in cancer research, or to aid start-up biotechnology and pharmaceutical companies that have received small business grants by providing the expertise and equipment needed to characterize their investigational agents or manufacture pharmaceutical-grade agents for use in human trials.
What the new facility, in particular, will provide is a tremendous opportunity to expand training capabilities. With the space and expertise available in a single facility, we can significantly enhance our ability to teach the next generation of scientists how to use new and emerging technologies, a process that they can then repeat at their home institutions.
NCI is already making inroads in working with the private sector to facilitate clinical trials involving multiple targeted therapies, using phase 0 and phase I trials to help the most promising agents move forward into later-stage clinical trials, and developing new funding mechanisms to help small businesses survive the often tumultuous road of commercializing their products.
We are answering the challenges of 21st century biomedical research, of how we think of competition, of cooperation, of collaboration, of intellectual property, and even the language of contracts. The ATPI and the new research facility will greatly enhance NCI's ability to attract partners to work together with our institute by sharing scientific resources and tools - creating a Brookings Institution-like entity that brings together today's scientific minds as a "think tank" for innovation.
Collectively, the shovel full of dirt and the many expectations it symbolizes are based on the premise that we cannot wait for scientific discovery to occur. Rather, we have to provide avenues and novel opportunities across the research and development spectrum to ensure that it does occur. The American people expect nothing less.
Dr. John E. Niederhuber
Director, National Cancer Institute |
 |
 |

New Option Approved for Indolent NHL
The FDA has granted marketing approval for the chemotherapy agent bendamustine (Treanda) to be used in patients with indolent B-cell non-Hodgkin lymphoma (NHL), the drug's manufacturer Cephalon announced on October 31. The approval covers the use of bendamustine in patients whose disease has progressed during or within 6 months of treatment with the monoclonal antibody rituximab (Rituxan) or a treatment regimen that includes rituximab. In March, the FDA approved bendamustine for use in patients with chronic lymphocytic leukemia.
The approval was based on the results of a phase III, 100-patient, single-arm clinical trial. The overall response rate - that is, the combination of complete or partial responses - was 75 percent and the median duration of the response was 9.2 months. Results from the trial were presented in December 2007 at the American Society of Hematology annual meeting. Side effects from the treatment included fatigue, nausea, and vomiting, among others, the company reported.
In 2007, there were approximately 30,000 cases of indolent, or
slow-growing, NHL in the United States. "Because most patients
with indolent NHL eventually become resistant to existing treatments, new treatment options like bendamustine are needed to improve patient outcome," said Dr. Bruce Cheson, director of hematology at the Lombardi Comprehensive Cancer Center in Washington, D.C., in a news release.
The drug is also being tested in a clinical trial in combination with rituximab as a first-line therapy
for NHL.
|
 |
 |

A Cancer Genome is Sequenced, Revealing Rare Mutations
To find genetic mutations involved in acute myeloid leukemia (AML), researchers have sequenced the genomes of normal cells and cancer cells from a woman with the disease. They identified 10 mutations that were found only in the cancer cells, including 8 affecting genes not previously associated with AML. Further research is needed to determine what role, if any, the mutations play in the disease, the study authors said.
The study marks the first time that researchers have decoded the entire DNA sequence of a person with cancer. Researchers at the Washington University School of Medicine in St. Louis launched the study after targeted-sequencing approaches had largely failed to produce new insights into the genetics of AML, a cancer of white blood cells that has been difficult both to study and to treat.
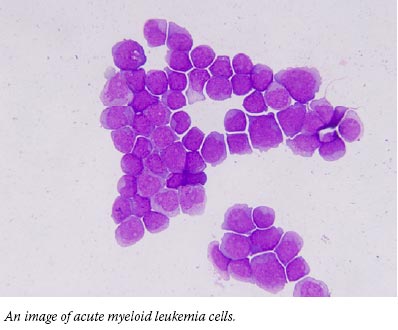
With "next-generation" whole-genome sequencing tools becoming available and costs dropping, the team undertook a side-by-side comparison of normal and cancer genomes from the same patient. The researchers detected mutations that would have been missed by other methods, such as Sanger sequencing.
The results demonstrate the power of sequencing entire genomes to discover novel cancer-related mutations, said Dr. Richard K. Wilson, who directs the Genome Sequencing Center at Washington University.
Reporting their results in the November 6 Nature, the researchers caution that hundreds, if not thousands, more normal and AML genomes will need to be sequenced
to put the findings in context
and advance the development of clinical tools.
"Sequencing one cancer genome doesn't tell you how to treat patients," noted lead investigator Dr. Timothy J. Ley. "The real significance of this study is that we've learned how to sequence the human genome to find all of the mutations in the genes. This has never been done before, and technologically it's extremely challenging."
Dr. Brian Druker, who led the development of imatinib (Gleevec) for another type of leukemia, called the study a "tour-de-force." The discovery of mutations in a small number of genes that no one predicted sets the stage for large-scale sequencing of cancer genomes, he said in a statement. Dr. Druker directs the Oregon Health and Science University Cancer Institute and was not involved in the research.
The cells were from a woman who developed AML in her 50s and died of the disease after a relapse. (She was also the first woman to have her genome sequence published.) Nine of the 10 gene mutations were present in all of the cancer cells examined, and this suggests that "they are all likely to be relevant," said Dr. Ley.
The mutations were also present when the disease recurred. "It's reasonably clear to us that basically the same tumor recurred in this patient," Dr. Ley noted. A population of cells in the original tumor may have survived the initial treatment.
The 8 newly identified mutations appear to be rare, as they were not detected in 187 additional patients.
The current analysis was limited to genes, but the investigators are now focusing on other parts of the genome. Genes make up a tiny fraction of the genome, and there is growing evidence that other regions which harbor elements that regulate genes are important in cancer.
As with all genome projects, generating and analyzing the sequence are really just the beginning. But having a complete sequence ensures that in the future, as more is learned about how the genome functions, researchers can go back and ask new questions, said co-lead investigator Dr. Elaine Mardis.
The investigators are sequencing
the genome of a second patient
with AML, a man who has been in remission, and they have plans to start sequencing a third patient.
The hope is that integrating information about the biological pathways involved in AML will start to benefit patients, perhaps through the development of diagnostic and prognostic tools and eventually targeted therapies.
"At the end of the day the purpose of this research is to improve patient care," said Dr. Mardis.
—Edward R. Winstead
|
 |
 |

New Drug for Patients with Advanced Thyroid Cancer
Name of the Trial
Phase II Study of 17-N-Allylamino-17-Demethoxygeldanamycin (17-AAG) in Patients with Inoperable Locoregionally Advanced or Metastatic Medullary or Differentiated Thyroid Carcinoma. See the protocol summary at http://www.cancer.gov/clinicaltrials/MAYO-MC0476.
Principal Investigators
Dr. Jeffrey Moley, Washington University in St. Louis, and Dr. Robert Smallridge, Mayo
Clinic Cancer Center
Why This Trial Is Important
Most thyroid gland cancers, including follicular and papillary carcinomas, are well differentiated, slow growing, and highly treatable, often by administering radioactive iodine131 both before and after surgery to remove the thyroid. However, some of these differentiated tumors eventually stop taking up iodine, start behaving aggressively, and metastasize. Medullary thyroid cancers (MTC) arise from a different type of thyroid cell and are generally more aggressive. Many patients with MTC will see their cancer return and metastasize after initial treatment.
Surgery is the only curative treatment for most patients with iodine-resistant differentiated thyroid cancer or MTC. Some patients, however, have tumors that cannot be surgically removed (unresectable), and, in other patients, cancer cells have spread to lymph nodes in the neck or to distant sites, such as the lungs, liver, or bones. No effective treatment has been found for these patients, and new treatments are needed.
In this phase II trial, researchers are studying the effects of treatment with 17-AAG, an antitumor antibiotic drug that targets a protein called heat shock protein 90 (HSP90). Many proteins implicated in cancer development need HSP90, a member of a class of proteins called molecular chaperones, to help them achieve their correct functional shape and cellular location. Cancer cells often contain higher levels of HSP90 than normal cells, helping them to grow uncontrollably. In laboratory experiments with MTC, papillary and follicular thyroid carcinomas, and other cancer types, 17-AAG has proven effective in inhibiting cancer cell growth by interfering with the chaperone function of HSP90.
"While most thyroid cancers are
very slow-growing and survival rates can be good at 15 years or longer, this trial is for that subset of patients with aggressively metastatic disease," said Dr. Moley. "These patients face a difficult challenge, and there haven't been many trials mounted to address their plight."
For More Information
See the lists of entry criteria and trial contact information at http://www.cancer.gov/clinicaltrials/MAYO-MC0476 or call the NCI's Cancer Information Service at 1-800-4-CANCER (1-800-422-6237). The call is toll free and confidential.
An archive of "Featured Clinical Trial" columns is available at http://www.cancer.gov/clinicaltrials/ft-all-featured-trials. |
 |
 |

Cancer Disparities: A Biological and Psychosocial Perspective
 |
 |
Also in the Journals |
 |
|
A study by researchers at Ohio State University's Comprehensive Cancer Center suggests that a psychological intervention may improve survival for women diagnosed with stage II or III breast cancer.
The year-long intervention consisted of small group meetings during which clinical psychologists taught patients stress reduction techniques, exercise and diet tips, how to find support from family and friends, and how to deal with fatigue and other treatment side effects. After a median of 11 years of follow-up of the 227 women in the trial, those receiving the intervention were found to have a 56 percent lower risk of dying from breast cancer; were less likely to die from causes other than cancer; and those who eventually died from breast cancer, did so later than women in the control group.
Study findings appeared November 17 in Cancer. |
|
While it is clear that there is a relationship between poverty, poor health, and mortality, a possible corollary to this pattern has emerged: Regardless of socioeconomic status, minorities have worse health outcomes than their white counterparts when it comes to diseases like breast cancer, prostate cancer, and diabetes. In trying to understand these disparities, researchers can find themselves veering toward complex issues that stray from biomedical science and into the social sciences.
In 2003, four institutes at NIH, including NCI, funded eight Centers for Population Health and Health Disparities (CPHHD) to address this type of cross-disciplinary research, the cancer-related portions of which are overseen by staff in NCI's Division of Cancer Control and Population Sciences (DCCPS).
"These interrelated projects are scientifically ambitious because they address a more comprehensive set of pathways than traditional biomedical studies," says DCCPS Director Dr. Robert Croyle. "If the evidence suggests that looking at behavioral, environmental, and economic variables is an essential adjunct to biology, we may be on the way to resolving long-standing debates over which factors are necessary to account for health outcome disparities."
Four studies underway through the CPHHD initiative at the University of Chicago focus on the experience of 230 African American women living on Chicago's South Side who were newly diagnosed with breast cancer.
"Black women in the United States are twice as likely as whites to die from breast cancer that occurs before menopause," said Dr. Sarah Gehlert, who directs the CPHHD project at the University of Chicago. "This mortality gap has been known for years, but we haven't discovered any racial disparity in germ-line mutations for breast cancer - mutations that, in any case, don't explain even 10 percent of breast cancers," she adds.
Instead, since cancer is a multi-step process, she reasons that numerous health behaviors and environmental factors are likely to cause changes that are not inherited but can persist as cells divide. "Ultimately the CPHHD initiative and other trans-disciplinary studies will begin to point us to interventions that truly affect group health differences," she says.
In 2000 Dr. Suzanne Conzen, a colleague of Dr. Gehlert's at the University of Chicago, was looking into apoptosis - or induced cell suicide - in breast cancer. She reasoned that if mammary epithelial cells were proliferating abnormally, they must have found a pathway that enabled them to evade apoptosis and survive.
Research in her lab eventually showed that this survival pathway may be tied to glucocorticoids (GCs), neuroendocrine hormones produced when a person is under stress. "This surprised us," said Dr. Conzen, because GCs, which flood the body in response to stress, induce apoptosis in some cell types, an observation that has been exploited in the treatment of lymphomas.
However, in experiments with breast epithelial cancer cell lines, Dr. Conzen's lab found that prolonged exposure to elevated GC concentrations inhibited apoptosis through the activation of GC receptors, which was then associated with the expression of several "survival genes" - SGK1 and MKP1, for example - that interfered with apoptosis.
But further insight may be gained by looking at social factors, such as the neighborhoods the women live in, explained Dr. Gehlert. "We're only beginning to understand the causal pathways through which social context contributes to health disparities," she said.
At the community level, many studies have documented an association between neighborhood context - including the rates of violent crimes, the lack of, or availability of, social supports/networks, and factors in the built environment - and specific health outcomes, such as depression and cancer. "People engage, form relationships, and leverage resources based on how they perceive and fit into surrounding social structures, as well as economic realities," said Dr. Gehlert.
Building on these community level studies, the researchers in Chicago measured features in a four-block area around women's homes that might impede or enhance social interactions; for example, vacant buildings and lots, fences, and deteriorating infrastructure, or conversely, neighborhood watches or block clubs. "We gathered data on how people respond to these neighborhood contexts" in order to move down a level to the psychosocial, she explained. Interviewing the neighborhood women, the researchers looked for successful coping strategies.
"The connection between psychosocial factors and biological consequences makes enormous intuitive sense, despite the fact that it turns the reigning biology-centric paradigm on its head," said Dr. Gehlert. "But when you get to know the women and the stress-generating neighborhoods they live in, it's hard to maintain the belief that a biomedical approach alone is going to provide a meaningful solution to the problem of understanding health disparities."
"The CPHHD initiative highlights an increasingly sophisticated approach to the study of social context and its influence on cancer and cancer disparities," said Dr. Paige McDonald, chief of NCI's Basic and Biobehavioral Research Branch. "While preliminary, these studies are promising. However, more research is needed to understand how the macroenvironment may influence tumor biology in biologically relevant ways that contribute to differences in cancer outcomes."
—Addison Greenwood
|
 |
 |

Researchers Consider "NCI Translates" Approach
Last week nearly 800 cancer researchers, patient advocates, and NCI staff convened in Washington, D.C., for "NCI Translates: the NCI Translational Science Meeting." Attendees convened to discuss a new approach for accelerating early translational research that was recommended by NCI's Translational Research Working Group (TRWG). "This approach allows NCI to become a connectivity platform enabling researcher to collaborate to rapidly move discoveries to patients," said NCI Director Dr. John Niederhuber.
The meeting continues a process intended to identify promising basic science discoveries that can be expedited to the clinic and marketplace. It began in early 2008 when NCI program directors were asked to nominate exemplary NCI-funded translational research grants. About 500 researchers submitted abstracts describing their work, which were "self-coded" by population, organ site, and relevant developmental pathways.
These developmental pathways, proposed by the TRWG and recently described in Clinical Cancer Research, provide a template onto which scientific projects can be mapped and coordinated to accelerate translational progress.
"By the end of this first phase, we hope to demonstrate that this broadly collaborative approach can identify strong and compelling candidates for a prioritization and funding process that will be presented to NCI leadership in the spring," said Dr. Lynn Matrisian, who co-chaired the TRWG and now leads the TRWG implementation team.
—Addison Greenwood
|
 |
 |
NCI Advisors Approve Major Cancer Research Initiatives
Earlier this month NCI's Board of Scientific Advisors (BSA) reviewed and endorsed several of the institute's major, cutting-edge scientific endeavors. Board members approved a proposal that provides 5-year funding for a new network of 4 to 6 physical sciences oncology centers. These centers, which were conceptualized during a series of "think tank" meetings NCI convened this year, would formally engage scientific teams from the fields of physics, mathematics, chemistry, and engineering, to examine cancer using new and often nontraditional approaches.
The BSA also recommended a 5-year renewal of The Cancer Genome Atlas, NCI's joint pilot project with the National Human Genome Research Institute. The pilot has already provided valuable insights into the biology of and potential new treatment approaches for brain cancer. NCI's Division of Cancer Biology's Integrative Cancer Biology Program also received strong support from the BSA, which recommended a 5-year renewal of that program.
Understanding NCI Teleconference: Translational Research Working Group
NCI's Office of Advocacy Relations' next "Understanding NCI" teleconference, titled "Transforming Translation: Implementation of the Translational Research Working Group (TRWG) Report," will take place December 16 from 1:00–2:00 p.m., ET. The featured speakers will be Dr. Lynn Matrisian, TRWG Co-Chair and special assistant to the NCI Director, and Ms. Gwen Darien, editor-in-chief of CR magazine and director of the Survivor and Patient Advocacy Program at the American Association for Cancer Research. A question-and-answer session for participants will follow the presentations.
No registration is required for participation. To join the teleconference, dial 1-800-857-6584; the passcode is: TRWG. Toll-free playback will be available through January 16, 2009 at 1-866-431-2907. A recording of the previous teleconference on P.L.A.N.E.T.: 5-Step Guide to Cancer Control Planning and Programming is available through December 12 at 1-866-434-5243.
| |
 |
 |
In Memoriam: Dr. Ronald M. Davis
 After a 10-month battle with pancreatic cancer, Dr. Ronald M. Davis passed away November 6 at his home in East Lansing, MI. After a 10-month battle with pancreatic cancer, Dr. Ronald M. Davis passed away November 6 at his home in East Lansing, MI.
Dr. Davis served as the 162nd president of the American Medical Association from June 2007 to June 2008. As the first preventive medicine specialist ever elected to that position, he focused on improving access to health care and the importance of prevention and sound public policy. He also helped lead the AMA's efforts to analyze its past history of racial inequality, which culminated in the organization's formal apology towards African American physicians in July 2008.
Dr. Davis' career as a public health official began as director of the CDC's Office on Smoking and Health, where he oversaw the publication of three landmark Surgeon General's reports on the health consequences of smoking and the health benefits of smoking cessation. He later served as medical director for the Michigan Department of Public Health and was most recently the director of the Center for Health Promotion and Disease Prevention at the Henry Ford Health System in Detroit.
From 1992-1998, Dr. Davis served as the founding editor of Tobacco Control, the first journal dedicated to tobacco control research, and later served as the North American editor of the British Medical Journal.
Dr. Davis was a key expert witness in many trials against the tobacco industry, and he testified before Congress and other legislative bodies on numerous occasions. His NCI grant, titled "Analysis of Tobacco Depositions and Trial Testimony," analyzed the sworn testimony of tobacco industry executives, researchers, and consultants.
He received many awards and honors, including the Surgeon General's Exemplary Service Medal and the Surgeon General's Medallion.
"He will be remembered by his colleagues and friends around the world for his seminal contributions to tobacco control and public health, as well as his kindness, integrity, and dedication to helping others," said Dr. Michele Bloch of NCI's Tobacco Control Research Branch.
|
 |
 |

Memorial Sloan-Kettering Cancer Center
President: Dr. Harold Varmus • 1275 York Avenue, New York, NY 10065 • Phone: 212-639-2000 • Web site: http://www.mskcc.org
 Background Background
Founded in 1884, Memorial Sloan-Kettering Cancer Center (MSKCC) is the world's oldest and largest private cancer center, with more than a century devoted to patient care and innovative research, making significant contributions to better understand, diagnose, and treat cancer. MSKCC is one of only 41 NCI-designated Comprehensive Cancer Centers and it has more than 10,000 employees. In 2007, approximately 21,870 patients were admitted to Memorial Hospital, and approximately 443,830 outpatient visits were accommodated at its Manhattan and regional facilities combined.
Patient Care
MSKCC's experts have established standards of care and treatment protocols for each type and stage of cancer. Its physicians have an extraordinary depth and breadth of experience in diagnosing and treating all forms of the disease, from the most common to the very rare. Each year, they treat more than 400 different subtypes of cancer. This level of specialization can have an often dramatic effect on patients' chances for a cure or control of their disease.
MSKCC offers a full range of
programs to help patients and families throughout all phases of treatment, including support groups, genetic counseling, help managing cancer pain and symptoms, rehabilitation, integrative medicine services, and assistance in navigating life after treatment.
Research
MSKCC's physicians and scientists have pioneered many novel therapeutic regimens that have made possible the remarkable advances in cancer treatment. Physicians at MSKCC are currently leading approximately 500 clinical trials for pediatric and adult cancer patients.
The Sloan-Kettering Institute (SKI) maintains one of the world's most dynamic programs of cancer research dedicated to understanding the biology of cancer through programs in cancer biology and genetics, cell biology, computational biology, developmental biology, immunology, molecular biology, molecular pharmacology and chemistry, and structural biology. The SKI research staff includes more than 90 laboratory investigators, 404 research fellows, and 150 graduate students (both Ph.D. and M.D./Ph.D. candidates). The institute boasts nine National Academy of Sciences members and seven Howard Hughes Medical Institute investigators.
Expansion
In recent years, MSKCC has undergone a major expansion, with new construction and renovations designed to meet the growing needs of both its patients and its scientific initiatives. The Center's newly opened Zuckerman Research Center has more than 300,000 square feet of laboratory space and houses many cancer research programs and laboratories. MSKCC has also developed a network of community-based, state-of-the-art outpatient cancer treatment facilities that bring the Center's expert care closer to patients living throughout its geographic region. MSKCC's new Breast and Imaging Center, which is currently under construction, will be ready for occupancy in 2009. The 16-story facility will accommodate the increasing number of breast cancer patients who come to MSKCC for treatment while also expanding services for cancer screening and diagnostic services. In 2006, MSKCC opened a new 72,000-square-foot surgical center that includes 21 state-of-the-art operating rooms, all of which are equipped for both minimally invasive and more traditional open surgery.
An exciting new dimension to MSKCC is the recent establishment of the Louis V. Gerstner, Jr. Graduate School of Biomedical Sciences. The novel program, which offers a Ph.D. degree in cancer biology, trains gifted young laboratory scientists to work in research areas directly related to cancer and other human diseases. |
 |
|