Past Updates from the Clinician Registry Listserv
Update Sent June 07, 2007
NOTE: This document is provided for historical purposes only and may not provide our most accurate and up-to-date information. The most current Clinician's information can be found on the Clinician Home Page.
The following is a summary of clinical guidance for evaluation and management of persons potentially exposed to XDR TB on two recent transatlantic flights.
If you have any questions on these or other clinical issues, please write to us at coca@cdc.gov.
Introduction
Summary of Post-exposure TB Evaluation & Testing
Evaluation Algorithm for Non-immunocompromised Contacts
Management of Non-immunocompromised Contacts with Positive TST or QFT-G Results
Evaluation Algorithm for Immunocompromised Contacts
Management of Immunocompromised Contacts with Positive TST or QFT-G Results
Notes for Algorithms
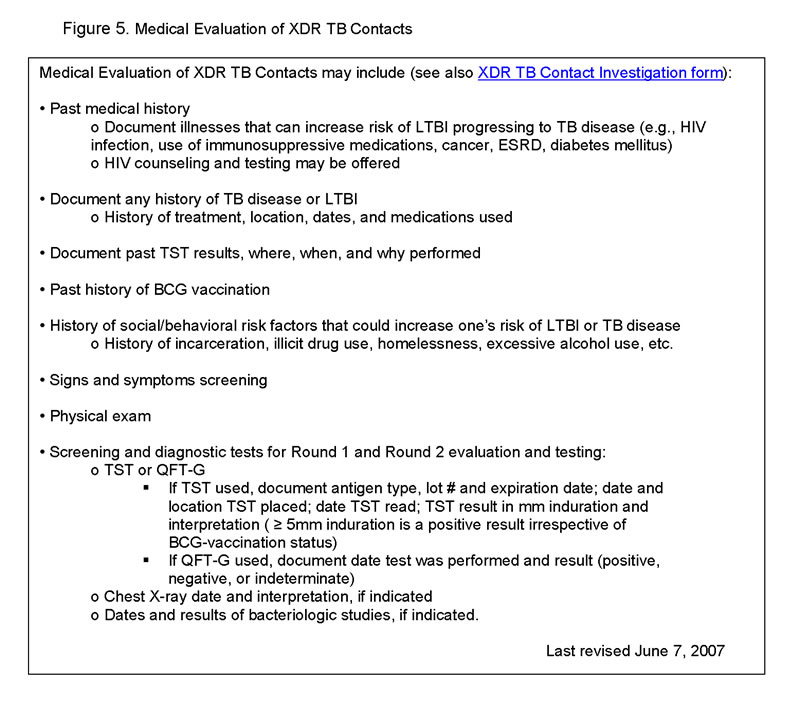
Medical Evaluation of XDR TB Contacts
Introduction
Dear Healthcare Professional,
A person with recently diagnosed culture-confirmed, extensively drug-resistant pulmonary tuberculosis (XDR TB) traveled on the following two extended flights (more than 8 hours in duration) in May 2007:
Date Depart Arrive Airline/Flight#
May 12Atlanta, GA (ATL) Paris, France (CDG)Air France #385 / Delta #8517
May 24 Prague, Czech Republic Montreal, CanadaCzech Air #0104
For purposes of this XDR TB contact investigation, all U.S. residents and citizens who were on the flights listed above are to be considered contacts of the XDR TB patient.
If a person identifies themselves as a passenger on one of these flights, we are requesting your assistance to perform TB evaluation and testing, or to refer the passenger to the appropriate state or local TB Control office so TB testing, evaluation and follow-up can be performed.
TB Controller Officers - State: http://www.cdc.gov/tb/xdrtb/pdf/tbcontrol-state.pdf
TB Controller Officers - City: http://www.cdc.gov/tb/xdrtb/pdf/tbcontrol-city.pdf
Specific information must be collected on each passenger as part of this contact investigation.
Please access the XDR TB Contact Investigation Form (PDF) and enter the requested information.
Please keep a copy of this completed form for your records, give a copy to the person tested, and also please contact your State or Local TB Control office.
For any inquiries related to this investigation, please call your State or Local TB Control office:
TB Controller Officers - State: http://www.cdc.gov/tb/xdrtb/pdf/tbcontrol-state.pdf
TB Controller Officers - City: http://www.cdc.gov/tb/xdrtb/pdf/tbcontrol-city.pdf
Below on this update are the latest algorithms for evaluation and management of both non-immunocompromised and immunocompromised contacts of this XDR TB patient.
We greatly appreciate your assistance on this important international XDR TB contact investigation.
Summary of Post-exposure TB Evaluation & Testing
As noted above, for purposes of this XDR TB contact investigation, all U.S. residents and citizens who traveled on either of the flights listed above are to be considered contacts of the XDR TB patient. Among persons who are infected with M. tuberculosis (i.e., latent tuberculosis infection), it can take 8 to 10 weeks following exposure until the tuberculin skin test (TST) result or QuantiFERON®-TB Gold (QFT-G) becomes positive. A second-round of TB evaluation and testing should be done because a negative TST or QFT-G result obtained <8 weeks after exposure may be considered unreliable for excluding latent tuberculosis infection (LTBI). |
Evaluation Algorithm for Non-immunocompromised Contacts
Management of Non-immunocompromised Contacts
with Positive TST or QFT-G Results
Evaluation Algorithm for Immunocompromised Contacts
Management of Immunocompromised Contacts
with Positive TST or QFT-G Results
Notes for Algorithms
Medical Evaluation of XDR TB Contacts
The CDC and HHS logos are the exclusive property of the Department of Health and Human Services and may not be used for any purpose without prior express written permission.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of Health and Human Services. Links to non-Federal organizations are provided solely as a service to our users. Links do not constitute an endorsement of any organization by CDC or the Federal Government, and none should be inferred. The CDC is not responsible for the content of the individual organizations.
Please visit the COCA web page for additional information: http://www.bt.cdc.gov/coca/.
Our Clinician Communication Team is committed to excellence in reporting our weekly updates. Please e-mail coca@cdc.gov should you note any written errors or discrepancies.
If you need to unsubscribe or update your information, please go to our website: http://www.bt.cdc.gov/clinregistry
If you need further information or technical help, please send an e-mail message to: coca@cdc.gov
- Page last updated June 07, 2007
- Content source: CDC Emergency Communication System (ECS), Division of Health Communication and Marketing (DHCM), National Center for Health Marketing (NCHM)
Get email updates
To receive email updates about this page, enter your email address:
Contact Us:
- Centers for Disease Control and Prevention
1600 Clifton Rd
Atlanta, GA 30333 - 800-CDC-INFO
(800-232-4636)
TTY: (888) 232-6348
24 Hours/Every Day - cdcinfo@cdc.gov