Physical Activity Guidelines Advisory Committee Report
Part G. Section 3: Metabolic Health
List of Figures
Introduction
Metabolic syndrome and diabetes are highly significant public health
problems in the United States. Ford and colleagues (1)
estimate, based on government surveys, that 47 million people in the United
States have metabolic syndrome. It is also estimated that 20.8 million
Americans (about 7% of the US population) have type 1 diabetes (T1D) or type 2
diabetes (T2D), of whom only two thirds have been diagnosed and the remaining
one third are unaware of their condition (2;3). The great majority (estimated to be 90% or more) of these
individuals have T2D. The prevalence of diabetes is higher among persons of
Hispanic, African American, and Native American background than among persons
of non-Hispanic white origins. The majority of deaths in persons with diabetes
are caused by cardiovascular disease (CVD), including myocardial infarction and
stroke. People with diabetes not only have a high prevalence of manifestations
of atherosclerosis but also have increased prevalence of cardiovascular (CV)
risk factors, including hypertension and the dyslipidemias. Alarmingly, type 2
diabetes, once called adult-onset diabetes because it chiefly presented in
middle-aged persons, is now appearing in ever younger people, and its
prevalence in adolescents and children is increasing rapidly. The potential
ramifications of T2D in adolescents and children has yet to be determined.
Exercise and physical activity play a clear role in preventing and
treating metabolic syndrome and T2D as well as the macrovascular complications
of T2D. The importance of the role of exercise and physical activity is highly
important and is of increasing interest both in the United States and in other
countries as well, as the magnitude of the public health problems of metabolic
syndrome and diabetes continues to increase and as solutions are being sought.
The role of physical activity and exercise in treating T1D is less well
established than for T2D, although evidence suggests that benefits are likely,
perhaps most of all in the area of reducing mortality, CVD risk factors, and
microvascular complications. For both T1D and T2D, physical activity may
prevent the development of diabetic neuropathy and diabetic nephropathy.
Finally, it appears likely that physical activity and exercise may help prevent
and treat gestational diabetes although more research is needed to further
establish these findings.
Review of the Science
Overview of Questions Asked
This chapter considers 6 major questions dealing with the potential role
of physical activity and exercise in preventing and treating metabolic
syndrome, T1D and T2D, common complications of diabetes, and gestational
diabetes:
- Does physical activity have a role in preventing or treating
metabolic syndrome?
- Does physical activity have a role in preventing and treating type 2
diabetes?
- Does physical activity have a role in reducing macrovascular risks in
type 2 diabetes?
- Does physical activity have benefits for type 1 diabetes?
- Does physical activity have a role in preventing and treating
diabetic microvascular complications?
- Does physical activity and exercise have a role in preventing and
treating gestational diabetes?
Data Sources and Process Used To Answer
Questions
The Metabolic Health subcommittee used the Physical Activity
Guidelines for Americans Scientific Database as its primary source of
references for the topics covered in this section of the report (see Part F: Scientific Literature Search
Methodology, for a full description of the Database). The
Database contains studies published in 1995 and later. In its search, the
subcommittee used broad study selection criteria, which included: all age
groups; all study designs; all physical activity types as well as
cardiorespiratory fitness; disease conditions including T2D, T1D, diabetic
nephropathy/neuropathy/retinopathy, metabolic syndrome, gestational diabetes,
hypoglycemia, glucose, and insulin.
Studies were also identified through computerized searches of several
databases, including PubMed, CINAHL, Health Plan, Cochrane Collaboration, and
Best Evidence. Standard MESH terms often were only partially successful in
identifying relevant articles. Articles also were found through a combination
of searching published reference lists as well as references from meta-analyses
and systematic reviews.
Question 1. Does Physical Activity Have a
Role in Preventing or Treating Metabolic Syndrome?
Conclusions
Regular physical activity is associated with reduced risk of metabolic
syndrome (Tables G3.A1 [PDF - 102 KB],
G3.A2 [PDF - 112 KB],
G3.A3 [PDF - 125 KB], and
G3.A4 [PDF - 111 KB], which summarize
these studies). The available data demonstrate an inverse dose-response
association between level of activity and risk of metabolic syndrome, with the
minimal amount of activity to prevent metabolic syndrome ranging from 120 to
180 minutes per week of moderate-intensity physical activity, and many studies
supporting a goal of 150 minutes per week. The findings derived from studies
using self-report measures of physical activity are similar to those studies in
which cardiorespiratory fitness was measured. The dose-response association
between physical activity and prevention of metabolic syndrome is similar in
men and women. Although limited data support the use of exercise for the
treatment of metabolic syndrome, this is an area in great need of more work, as
is the role of physical activity in preventing and treating metabolic syndrome
in youth (Table G3.A5 [PDF - 118 KB],
which summarize these studies) and across ethnicities.
Introduction
A number of clinical criteria, such as those of the National Cholesterol
Education Program and World Health Organization (4), have
been developed to define the metabolic syndrome. These criteria are very
similar and share the following cluster of characteristics: abnormal levels of
lipids (low high-density lipoprotein and high triglycerides), elevated glucose,
hypertension, and excess abdominal obesity (5-8). This
review is not limited to any specific clinical definition of metabolic syndrome
but rather includes any report in which the definition of metabolic syndrome
was consistent with the above characteristics.
Rationale
In general both cross-sectional and longitudinal cohort studies
consistently show a lower incidence and prevalence, respectively, of metabolic
syndrome among physically active individuals as compared with their inactive
peers (9-45).
Dose-Response Relation
In the cross-sectional studies, which examined the prevalence of
metabolic syndrome across levels of physical activity and primarily used
questionnaires to obtain self-report data (Figure
G3.1), (Table
G3.A.3 [PDF - 125 KB], which summarize these studies), all found an inverse gradient
between amount of physical activity and metabolic syndrome (10;11;13;21;23;26;36).
Figure G3.1. Summary of Cross-Sectional
Physical Activity and Metabolic Syndrome Studies Using Categories of Physical
Activity That Could Be Used To Examine Dose-Response
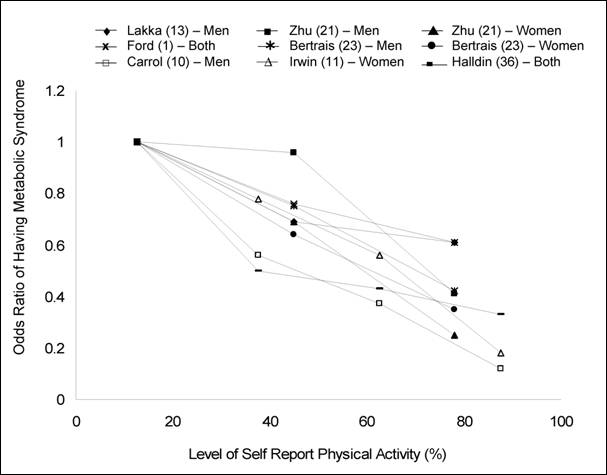
From the cross-sectional studies in which minutes per week of
moderate-intensity physical activity for each category were provided or could
be estimated, 120, 150, and 180 minutes or more per week of moderate intensity
activity have all been reported as minimum amounts associated with reduced
prevalence of metabolic syndrome (13;23;26;36). It
should be noted that these studies used different methods of activity
assessment, the activity categories have large ranges, and the cut-points for
the activity categories were not similar or generated using the same
statistical methods. None of the studies was designed or powered to analyze the
minimal dose of activity to prevent metabolic syndrome. However, the
cross-sectional data supports that obtaining at least 120 to 180 minutes per
week of moderate-intensity physical activity is consistently associated with a
lower prevalence of metabolic syndrome. Only the 2002 report from Laaksonen and
colleagues (Figure G3.2) provides data that could
be used to examine the dose-response between physical activity and the
development of metabolic syndrome (41).
Figure G3.2. Data Prospectively
Demonstrating That Both Higher Levels of Physical Activity and Fitness Protect
Against the Future Development of Metabolic Syndrome
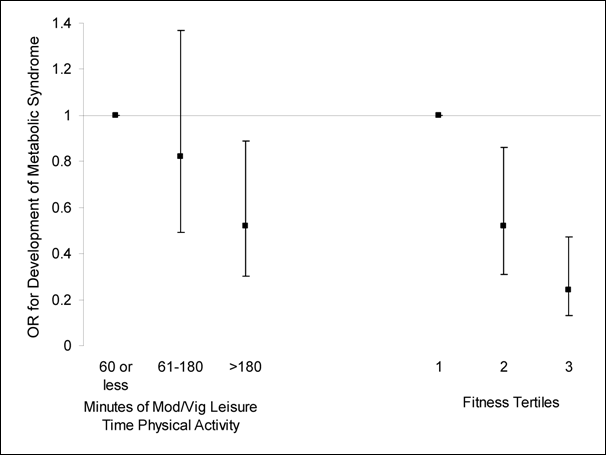
Source: Laaksonen et al. (41)
Figure G3.2. Data Points
| Development of Metabolic Syndrome |
Time Physical Activity
Low |
Time Physical
Activity
Middle |
Time Physical Activity
High |
Fitness Tertiles
Low |
Fitness Tertiles
Middle |
Fitness Tertiles
High |
| Odds Ratio |
1 |
0.66 |
0.55 |
1 |
0.59 |
0.36 |
The results were similar to those from the cross-sectional studies. A
dose-response relation exists between level of activity and risk of developing
metabolic syndrome, with 180 or more minutes per week of moderate intensity
physical activity being the minimal amount of time associated with reduced risk
of developing metabolic syndrome.
Physical Activity Level Versus Cardiorespiratory Fitness
Laaksonen and colleagues also measured cardiorespiratory fitness and, as
depicted in Figure G3.2 and
Table G3.A1 [PDF - 102 KB], the
inverse dose-response relationship associated with prevention of metabolic
syndrome, is even stronger than that seen with questionnaire-assessed
self-report of physical activity (41).
All available prospective studies that measured fitness and categorized
participants based on fitness level similarly show a strong inverse
dose-response between fitness and risk of developing metabolic syndrome (Figure G3.3) (39;41;46-48) .
Figure G3.3. Summary of Longitudinal
Fitness and Metabolic Syndrome Studies That Used Categories of Fitness To
Examine Dose-Response Relations
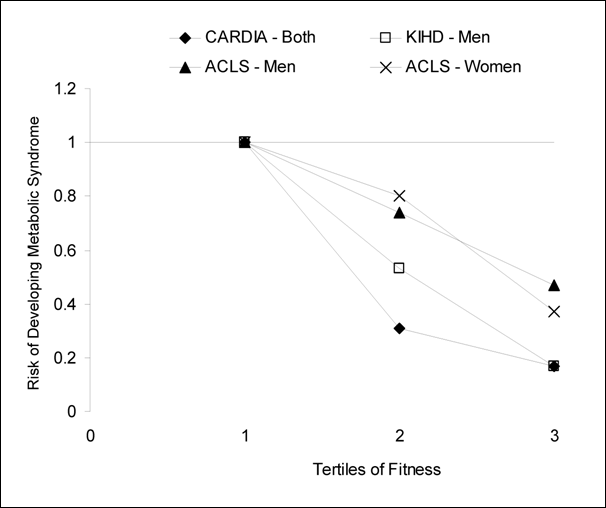
CARDIA, Coronary Artery Risk Development in Young
Adults; KIHD, Kuopio Ischemic Heart Disease Risk Factor Study; ACLS, Aerobic
Center Longitudinal Study
Thus, despite the methodological differences in assessing physical
activity through self-report (questionnaire) vs. measured cardiorespiratory
fitness, the association with the prevention of metabolic syndrome is similar
for these two modes of activity assessment.
Sex Differences
The available data are composed of men-only studies, women-only studies,
and combined-sex studies, with no one type of study comprising the
preponderance of the data. As demonstrated in Figure
G3.1, the physical activity-metabolic syndrome association is similar in
men and women, indicating that both men and women benefit from participating in
regular physical activity. As demonstrated in Figure
G3.3, the fitness-metabolic syndrome association also is similar in men and
women. Thus, no matter whether studies using self-reports of physical activity
or objective measures of fitness, it appears that no sex differences exist in
regard to the benefits of physical activity in preventing metabolic
syndrome.
Youth
Only very limited data are available for youth. These studies, using a
variety of methods to quantify physical activity and define metabolic syndrome,
are consistent with the findings in adults, namely that higher levels of
activity and fitness are associated with reduced risk of metabolic syndrome (Table G3.A5 [PDF - 118 KB], which
summarize these studies) (15;44;49;50;50-53).
However, this topic is deserving of future study and investigation.
Effect of Race and Ethnicity
The majority of studies with large sample sizes were conducted in Europe
or were composed of persons of American or European descent. Though some of the
better studies were conducted in populations composed of both African Americans
and whites, no studies have examined the physical activity-metabolic syndrome
association in an African American or Mexican American population only (11;26;46). Thus, the data on the
relationship between physical activity or fitness in terms of preventing
metabolic syndrome in non-white populations are limited, and this is clearly an
area that needs additional research. It should be noted that in the studies
that used study populations composed of both non-Hispanic whites and African
Americans, such as the National Health and Nutrition Examination Survey
(NHANES) and the Coronary Artery Risk Development in Young Adults (CARDIA)
Study, a strong dose-response relation between activity (or fitness) and
prevention of metabolic syndrome was evident (26;46).
Prolonged Sitting and Other Sedentary Behaviors
Although regularly participating in physical activity and not leading a
sedentary lifestyle may appear to be synonymous, evidence suggests that these
two behaviors should be treated as different dimensions of the same pubic
health issue. In other words, it is important not only to obtain adequate
amounts of aerobic exercise but also to avoid extreme sedentary behaviors, such
as prolonged sitting. This is obviously of great importance in today's
environment, in which the typical work day is characterized by long bouts of
sitting and most non-work hours are spent watching television. Available data
suggest a direct relationship between the prevalence of metabolic syndrome and
the time spent watching television or using the computer (23;25;26). For
example, using NHANES data (n=1,626 men and women), Ford and colleagues
observed that individuals who reported watching television or using the
computer 4 or more hours a day had a 2 times greater risk of having metabolic
syndrome compared to individuals who reported less than 1 hour a day of
television or computer use (26). Given that the current
environment in the United States promotes sedentary behavior both within and
outside the work place, strategies for reducing sedentary behavior, in addition
to promoting exercise, have great potential public health impact.
Role of Physical Activity in Treating Metabolic Syndrome
Numerous studies have examined the benefits of exercise training on
individual components of metabolic syndrome, such as blood pressure or fasting
glucose. In general, improvements to the variables of interest are noted with
exercise training. However, no published studies have been specifically
designed to examine the efficacy of exercise training in the reversal of the
clinical diagnosis of metabolic syndrome. Two reports have conducted post-hoc
analyses to examine the role of exercise in reversing metabolic syndrome. Using
data from the HERITAGE study, Katzmarzyk and colleagues report that 20 weeks of
aerobic training were associated with improvements in triglycerides, blood
pressure, fasting glucose, and waist circumference among 105 participants who
had metabolic syndrome at baseline (54). Further, the
prevalence of metabolic syndrome decreased 30.5% in this sub-set of
participants who received exercise training. However, this study was not
controlled, which makes the interpretation of this data challenging. In a
recent manuscript using data from the dose-response STTRIDE study, Johnson and
colleagues observed an improvement in waist circumference, triglycerides, and
blood pressure when the included exercise groups (walking or jogging exercise
in varying intensities) (n=130) were combined. None of these variables changed
in the control group (n=41) (55). The prevalence of
metabolic syndrome also decreased in the combined exercise group from 41% to
27%, with no change in prevalence of metabolic syndrome in the control group
(39% to 46%). Although these preliminary data generated from post hoc analyses
suggest that exercise training may be an important therapeutic option for the
treatment of metabolic syndrome, this area needs additional research. In
particular, clinical exercise trials prospectively designed and powered to
examine the efficacy of exercise in treating metabolic syndrome are needed.
Resistance Training
Very few studies have examined the role of resistance training or
quantified muscular strength in preventing or treating metabolic syndrome (56-58). In both a cross-sectional and longitudinal report
from the Aerobic Center Longitudinal Database, greater muscular strength was
associated with lower risk of metabolic syndrome (56;57). However, in the report using longitudinal data, the
degree of risk reduction associated with greater levels of strength was
attenuated (from −34% to −24%) when cardiorespiratory fitness was
adjusted for (57). Given the important role of skeletal
muscle in insulin sensitivity, developing a better understanding of the role of
resistance training in the prevention and treatment of metabolic syndrome is an
area of great interest.
Question 2. Does Physical Activity Have a
Role in Preventing and Treating Type 2 Diabetes?
Conclusions
Increased levels of physical activity are associated with significantly
decreased risks of developing T2D. Most of the studies addressing T2D
prevention have focused on vigorous activity, but a number have included
walking at moderate intensity, which has proven efficacious as well.
Importantly, two randomized controlled trials (RCTs) and results of
observational studies provide empiric evidence to support 150 minutes per week
of moderate intensity physical activity for T2D prevention. Several studies
have shown that 30 minutes per day of moderate intensity exercise 5 days per
week are effective in preventing T2D. Available data do not enable minimal
recommendations, although some of the large observational studies show that any
amount of increased physical activity is associated with T2D prevention.
Recommendations are valid for both men and women. Data are insufficient to
clearly show that the benefits are uniform across all ethnicities and racial
groups but no data support a lack of benefit and available data do support the
benefit in these groups.
Introduction
As noted at the beginning of this chapter, diabetes is a highly
significant public health problem in the United States. Available data reveal
that physical activity has a strong role in the prevention and treatment of
T2D. These data include results from observational studies, and RCTs as well as
physiological studies related to physical activity and/or exercise. The
relationship between T2D and cardiovascular fitness also is important because
population studies reveal a direct correlation between all-cause mortality and
reduced fitness in persons with T2D (59;60). Following are data that support the importance of
physical activity and exercise in the prevention and treatment of T2D as well
as a discussion of the safety of exercise for persons with T2D.
Rationale
Observational Studies of Physical Activity in Preventing Type 2
Diabetes
Large prospective cohort and cross-sectional observational studies that
assessed physical activity through the use of questionnaires all show that
increased physical activity levels are associated with reduced risk for
developing T2D. As with the assessments looking at the relationship between
metabolic syndrome and physical activity, it should be noted that these studies
used different methods of activity assessment, the activity categories have
large ranges, and the cut-points for the activity categories were not generated
using the same statistical methods. In addition, none of the studies was
designed or powered to analyze the minimal dose of activity to prevent T2D.
Importantly though, however the studies were conducted, the benefit of physical
activity in preventing T2D is consistently present. Major prospective cohort
studies are described here to illustrate the range of methods used and results
obtained. Meta-analyses and structured reviews on this topic are summarized in
Table G3.A6 [PDF - 117 KB], which
summarize these studies. These studies reveal that both moderate and vigorous
physical activity can prevent T2D. Dose-response summary information is
provided separately below.
In a study by Helmrich and colleagues (61) in 5,990
male alumni of the University of Pennsylvania, incidence rates of T2D decreased
as energy expenditure in leisure time physical activity in kilocalories per
week increased from less than 500 to 3,500. They found that for each 500
kilocalorie increment in leisure-time physical activity, the age-adjusted risk
of T2D was reduced by 6% (relative risk [RR]=0.94, 95% CI= 0.90-0.98) (61). In a study by Manson and colleagues (62) in the Nurses' Health Study cohort (87,252 US women aged
34 to 59 years), the investigators found that women who engaged in vigorous
exercise at least once per week had an age-adjusted RR of 0.67 when compared to
women who did not exercise (P <0.0001). This significant benefit
persisted even after adjustment for body mass index (BMI) although results were
somewhat attenuated by this measure (62). Hu and
colleagues (63) compared the benefits of walking with
benefits of vigorous physical activity on risk of developing T2D in the Nurses'
Health Study. Physical activity was divided into quintiles in this study. The
authors found that walking (considered a moderate intensity form of exercise)
as well as vigorous activity were associated with decreased risk of T2D, with
greater physical activity levels providing the most benefit. A study of 5,159
British men revealed a decreased risk for developing T2D that progressively
decreased with increasing levels of physical activity (64). Participants were sorted into one of 6 defined levels of
physical activity ranging from inactive to vigorously active based on frequency
and intensity of the physical activities of each participant. The authors found
that the age-adjusted relative risk of T2D decreased progressively with
increasing levels of physical activity with even moderate physical
activity having a significant effect. In a study of 6,013 Japanese men , Okada
and colleagues (65) found that those who engaged in
regular physical exercise at least once a week had a relative risk of T2D of
0.75 (95% CI, 0.61-0.93) compared with men not engaging in exercise. In a
cohort of 34,257 women aged 55 to 69 years, Folsom and colleagues determined
that any level of physical activity was associated with a decreased risk of
developing T2D (RR=0.69, 95% CI=0.63, 0.77) when compared with sedentary
behavior (66). In a study assessing the effects onT2D of
physical activity in 37,918 healthy men where activity levels were classified
in metabolic equivalent (MET)-hours per week and considered either moderate or
vigorous, relative risks for T2D across increasing quintiles of
MET-hours per week were 1.00, 0.78, 0.65, 0.58, and 0.51 (P for trend
<.001) (67). Walking pace also was assessed in this
study, and walking was found to be efficacious for preventing T2D. Hu and
colleagues (68) assessed data from 6,898 Finnish men and
7,392 women ranging in age from 35 to 64 years to evaluate the relationship of
occupational, commuting, and leisure-time physical activity with the incidence
of T2D. After adjustment for potential confounders, the hazards ratios of
diabetes associated with light, moderate, and active work were 1.00, 0.70, and
0.74 respectively (P=0.020 for trend) and the authors concluded that
high or moderate levels of activity were associated with a reduced risk of T2D
(68). In a prospective cohort study of 37,878 women, a
participant was considered active if she expended more than 1,000 kilocalories
on recreational activities per week, with activity levels being divided into
quartiles (69). Physical activity was an independent
predictor of T2D in this study although BMI was a more powerful predictor. In
the Women's Health Initiative Observational Study, Hsia and colleagues (70)
found that physical activity across exercise quintiles was associated with a
decreased risk of T2D particularly in non-Hispanic white women. This was true
for walking (multivariate-adjusted hazard ratios 1.00, 0.85, 0.87, 0.75, 0.74;
P for trend <0.001 across exercise quintiles) and total physical
activity score (hazard ratios 1.00, 0.88, 0.74, 0.80, 0.67;
P=0.002).
These data demonstrate a strong inverse relationship of physical
activity across quintiles with diabetes risk in non-Hispanic white women and
men. Associations in women of other races and ethnicites are less clear, but
the authors of one study (70) note that the study may not
have been adequately powered to fully assess data from particular race or
ethnic subgroups or possibly that physical activity levels among these groups
may not have been intense enough to allow for analyses (see section below).
Physical Activity Level Versus Cardiorespiratory Fitness
Similar to the questionnaire studies, observational studies that
assessed physical activity levels using objective measures of cardiorespiratory
fitness reported that better fitness is associated with a reduced risk of
developing T2D (71-73). Lynch and colleagues (71) found that in a population-based sample of 897
middle-aged Finnish men, higher cardiorespiratory fitness was associated with
lower risk of developing T2D compared to sedentary persons. Wei and colleagues
(60;72) found that low
cardiorespiratory fitness (measured during a maximal exercise test) and
physical inactivity (measured by self-report) were associated with risk of
impaired fasting glucose and T2D as well as all-cause mortality in men with
T2D. In the former study, after adjusting for potential confounders, men in the
low-fitness group (the least fit 20% of the cohort) at baseline had a 1.9-fold
risk (95% CI, 1.5- to 2.4-fold) of impaired fasting glucose and a 3.7-fold risk
(CI, 2.4- to 5.8-fold) of T2D compared with those in the high-fitness group. In
another study, in which cardiorespiratory fitness was measured during an
exercise test and the 6,249 female participants were divided into thirds by
level of fitness, Sui and colleagues (73) found that
compared with the least fit third, the adjusted hazard ratio was 0.86 (95%
CI=0.59-1.25) for the middle third and 0.61 (95% CI=0.38-0.96) for the upper
third of cardiorespiratory fitness. Similar to results from studies using
self-report data, results from these studies overall suggest a benefit for
achieving and maintaining increased levels of physical activity (64;66;74;75).
Randomized Controlled Trials of Type 2 Diabetes Prevention
The difficulty of evaluating many of the large RCTs looking at the
effects of physical activity or exercise on diabetes prevention has been to
sort out the effects of diet versus physical activity, as these treatments are
commonly combined in large trials. Three large RCTs have assessed the role of
physical activity independently, either using trial design or by analytic means
(Table G3.A7 [PDF - 123 KB], which
summarize these studies). The Da Qing Impaired Glucose Tolerance and Diabetes
Study in China (76) included an exercise-only treatment
arm and found that even modest changes in exercise, without change in diet,
reduced the risk of developing diabetes. The exercise prescription in this
study was 1 or 2 units of exercise a day, with units defined in terms of
intensity and duration. One unit was equal to 20 minutes of "mild" exercise
(e.g., slow walking, shopping, housekeeping), 20 minutes of "moderate" exercise
(e.g., fast walking, cycling), or 10 minutes of "strenuous" exercise (e.g.,
slow running, stair climbing) or 5 minutes of very strenuous exercise (e.g.,
skipping, basketball). In this trial, which was randomized by clinic rather
than by participant, diabetes risk was reduced 46% in the
exercise group, 42% in the diet and exercise group, and 31% in the
diet-treated group.
The Diabetes Prevention Study in Finland (77;78) and the Diabetes Prevention Program in the United States
(79) have provided clear evidence that intensive lifestyle
modifications, including strong diet and physical activity interventions,
reduce the risk of developing T2D. Importantly, the role of physical activity
is independently beneficial to preventing diabetes. In the Diabetes Prevention
Study, 522 middle-aged, overweight men and women with impaired glucose
tolerance (IGT) were randomized to either lifestyle modification or a control
group (77;78). The physical activity
prescription portion of the lifestyle modification (which included a strong
dietary component) was for 30 minutes a day of moderate exercise for a total of
more than 4 hours per week. Incidence of diabetes was very significantly
reduced in the intervention group.
In the Diabetes Prevention Program, 3,234 men and women with IGT and
impaired fasting glucose were randomized into control, medication (i.e.,
metformin, a drug commonly used to treat T2D), or lifestyle modification
groups. The physical activity prescription portion of the lifestyle arm (which
also had a strong dietary component) was 150 minutes of activity per week. The
lifestyle component reduced incident diabetes by 58% and had a more powerful
effect than metformin (by 39%). In the Diabetes Prevention Program and Diabetes
Prevention Study, weight loss was the dominant predictor of a reduced incidence
of diabetes. However, recent analyses from these studies showed that increased
levels of physical activity prevented diabetes even after adjusting for
confounders (80-82).
Physiological Data Showing Benefits of Exercise in Treating Type 2
Diabetes and Elucidating the Role of Cardiorespiratory Fitness
Type 2 Diabetes is associated with reduced exercise capacity (83;84). Maximal oxygen consumption was
approximately 20% lower compared to nondiabetic persons of similar weight and
physical activity levels in these studies. These exercise abnormalities are
present even in the absence of diabetes-related complications and even in
persons with recently diagnosed T2D. The abnormalities are likely associated
with cardiac and hemodynamic abnormalities (85-87).
It has been well established that a single bout of moderate exercise has
a profound effect on glucose metabolism that may last up to about 18 hours (88). In addition, repeated bouts of exercise appear to have a
cumulative beneficial effect on glucose metabolism. A meta-analysis (89) including 14 studies, provides evidence that regular
moderate-intensity exercise improves metabolic control in T2D. This
meta-analysis shows that exercise significantly improves glycemic control and
reduces visceral adipose tissue and plasma triglycerides, although not plasma
cholesterol, in people with T2D, even in the absence of weight loss. Exercise
training in persons with T2D also has a very significant effect in terms of
improving maximal oxygen consumption, measures of submaximal exercise
performance, and other measures of fitness (e.g., 90;91). Available data suggest that these findings are true for
African American women (92) as well as white women. These
findings are further discussed in the section on preventing macrovascular
complications of T2D.
Dose-Response Relation
Data on exactly how much physical activity is needed in order to prevent
T2D are limited because such studies have not been prospectively designed. Data
from observational studies indicate that the amounts of effective physical
activity range from any increase over sedentary levels to moderate and vigorous
activity levels. It appears, therefore, that any physical activity may be
better than none in terms of preventing diabetes, but better results are
achieved if individuals engage in higher intensity and more frequent physical
activity. Data from several studies support that approximately 30 minutes of
moderate intensity exercise at least 5 days per week provides a substantial
(25% to 36%) reduction in the risk of T2D according to the Nurses' Health Study
(63), the Iowa Women's Health Study (66), the Study of Eastern Finns (68),
and the Diabetes Prevention Program (79). Importantly,
several of the prospective cohort studies discussed above included walking as a
specific modality of physical activity and all of these found that walking was
beneficial in terms of preventing T2D compared to sedentary behavior (61;63;67;69;70). Thus, data from observational
studies and RCTs support the current recommendation that 2.5 hours per week or
typically 30 minutes a day for 5 days a week be performed to prevent T2D. Jeon
and colleagues (75) performed a meta-analysis on the
prospective cohort studies that assessed the preventive effects of
moderate-intensity physical activity that could be analyzed independent of
vigorous-intensity physical activity. Moderate-intensity physical activity was
defined as an activity requiring 3.0 to 6.0 METs (75).
They identified 10 cohort studies that met these criteria. These studies in
total included 301,121 participants and 9,367 incident cases. Five of the
studies specifically included walking. The summary RR of T2D was 0.69 (95% CI
0.58-0.83) among participants who regularly participated in moderate-intensity
exercise compared to sedentary counterparts. The RR for T2D was 0.70
(0.58-0.84) for walking on a regular basis (typically briskly for 2.5 hours per
week or more) compared to no walking. However, no data are available to support
a specific recommendation for a minimal or even a lesser dose of exercise. In
addition, it is not clear how much additional risk reduction is obtained with
higher levels of physical activity.
Sex and Race/Ethnicity Differences
In observational studies that included women only, 3 large US cohort
studies (67-70) all found that greater physical activity
was associated with a lower incidence of diabetes. However, in one study, this
relationship was present only in non-Hispanic white women and not in women of
African American, Hispanic or Asian descent (70). These
findings await confirmation in further studies because the study may not have
been powered to detect differences across all race or ethnic groups. Results
were based on self report of diabetes in the total population but were
confirmed in a subset who also provided blood samples and physician
reports.
Data from RCTs as well as observational studies suggest clearly that
overall, increased levels of physical activity play a beneficial role in
preventing T2D for both women and men. In the Diabetes Prevention Program (93), treatment effects did not differ significantly according
to sex, race, or ethnic group. Lifestyle factors addressed in the Program
included diet and physical activity, and both had an independent effect on
preventing T2D. Although participant numbers became too small for clear results
when grouped by ethnicity, it appears that risk reduction compared with placebo
was greater for the lifestyle group than for the metformin group in
non-Hispanic whites (50% versus 12%, respectively) and Hispanics (57% versus
2%, respectively) (94). African Americans (42% versus 29%)
and Native Americans (43% versus 42%), showed similar efficacy for the
lifestyle and metformin groups. However, for Asian Americans, metformin showed
a nonsignificantly greater reduction than intensive lifestyle intervention (62%
versus 30%). Neither lifestyle nor metformin showed significant heterogeneity
across the 5 ethnic groups in terms of efficacy. Subsequent studies in India
and Japan (95;96), as well as the Da
Qing study in Chinese people (76), similarly found an
independent effect of physical activity in preventing T2D, and the findings
were true for men and women and appeared to be true for all ethnic groups
involved.
Thus, overall, acknowledging the limited data available to date, no
strong evidence is available to negate the data suggesting that physical
activity prevents T2D in men and women of different race and ethnic groups,
although further research should explore this important issue.
Youth
Type 2 Diabetes is growing in prevalence in children and adolescents.
Alarmingly, unlike youth who do not have T2D, youth with this condition often
have CV risk factors, such as hypertension and dyslipidemias as well. Thus,
potentially, youth who have T2D may develop CVD at relatively young ages (97;98). Data from RCTs show that
increased physical activity improves insulin sensitivity in obese youth,
although longitudinal data are limited (99-101) and the
effects on CV risk factors are not well established because trials are lacking.
A recent review has highlighted the efforts of different interventions to
address obesity in youth of various ethnic and racial groups. These
interventions focused on lifestyle changes including increased physical
activity (102), and several had a physical activity-only
component (103;104). Overall
findings were encouraging. The studies of both Sallis and colleagues (103) and Pangrazi and colleagues (104)
showed that school-based programs promoting increased physical activity were
effective at increasing the physical activity level or cardiorespiratory
endurance (although not in reducing BMI) of girls especially.
No RCTs have been completed that show that physical activity or exercise
prevents T2D in youth although it is likely give results in aduls. To date, the
limited intervention and observation studies suggest that to prevent and manage
T2D, daily goals for youth should include less than 60 minutes of daily screen
(television, computer or video game) time and 60 to 90 minutes of daily
physical activity (105-107). A large multicenter trial
(the TODAY study) is currently underway to assess the role of physical activity
in preventing T2D in youth (108).
Resistance Training
Resistance training has shown promise as a modality for
treating diabetes (109;110). Sigal and colleagues (111)
found, in a group of 251 individuals with T2D, that both aerobic and resistance
training individually improved glycemic control, but improvements were greatest
with combined aerobic and resistance training. However, this exercise modality
has not been explored for its role in prevention of T2D in large trials, and no
data currently exist showing that resistance training plays a role in
preventing T2D. Future studies should further investigate the role of
resistance training in preventing T2D given the beneficial effects of such
training on the metabolism of persons with T2D.
Safety of Physical Activity and Exercise for Persons With Type 2
Diabetes
The consensus is that the benefits of exercise for persons with T2D far
outweigh the risks. However, safety concerns about exercise in this group have
been voiced. These concerns range from cardiovascular risks associated with
physical activity and exercise to caution about hypoglycemia and foot care
concerns. The American Diabetes Association (ADA) guidelines on safety (112;113) provide a comprehensive review of safety issues and
measures, although the recommendations lack supporting data although the
recommendations lack supporting data in some cases.
Question 3. Does Physical Activity Have a
Role in Reducing Macrovascular Risks in Type 2 Diabetes?
Conclusions
Strong data support the benefits of physical activity and fitness for
CVD protection in T2D and IGT. The data are stronger for hard outcomes, such as
CVD events and mortality, than for known CVD risk factors, but this may be an
artifact of the relatively short duration of risk factor studies and the
potential for small changes in risk factors to have a large cumulative impact
on outcomes. These data suggest that a minimum of moderate-intensity aerobic
activity for more than 2 hours per week is necessary to achieve significant
benefit, and that near maximum benefit may be achieved with moderately vigorous
aerobic activity, such as brisk to very brisk walking, for 3 to 7 hours per
week (about 12 to 21 MET-hours per week). Combined aerobic and resistance
activity appears to have greater benefits than either type alone when CVD risk
factors (and non-CV effects) are considered, but CVD outcome data for activity
other than aerobic activity are lacking. In general, the existing data for CVD
risk reduction in persons with T2D are consistent with a recommendation of an
aerobic activity program with a goal of at least 120 minutes per week and
preferably more than 180 minutes per week of moderate to moderately vigorous
activity.
Rationale
Several studies have specifically considered the effects of physical
activity on CVD risk factors and outcomes in T2D. Observational studies have
shown that, among persons with this condition, those who exercise or are more
fit have a reduced risk of CV morbidity and mortality than do less active or
less fit individuals (67;114-118)
(Tables G3.A8 [PDF - 114 KB] and
G3.A9 [PDF - 140 KB], which summarize
these studies). A study of more than 3,000 Finns with T2D found that all types
of physical activity (e.g., recreational and occupational) are beneficial in
reducing CV events and mortality (117). Following is a
review of the evidence for benefits, dosage, and type of physical activity
specifically for reduction of CVD risk and outcomes in T2D.
Cardiovascular Disease Risk Factor Reduction
Many cross-sectional studies have found inverse correlations between
physical activity level and various CVD risk factors in T2D populations. Two
meta-analyses of these studies have been performed (119;120). One focused on lipid effects
and hemoglobin A1c (HbA1c) and found a small (5%) but significant decrease in
low-density lipoprotein (LDL) cholesterol (−6.4 mg/dl, range =
−11.8 to −1.1) and a strong trend toward improved HbA1c
(−0.4%, range = −0.8 to 0.0), but no change in total cholesterol or
triglycerides (120). This section focuses on a recent
meta-analysis of controlled intervention studies in subjects with T2D that
compared different exercise interventions for their effects on CVD risk factors
(119). The meta-analysis covers about 1,000 subjects,
aged 48-62 years. Exercise interventions were of aerobic, resistance, or
combined types. Overall conclusions from the analysis were that all forms of
exercise improved insulin sensitivity, with combined types having the greatest
effect (especially in men) and resistance alone the least. Combined exercise
also had small and moderate benefits on systolic and diastolic blood pressure,
respectively, and a small benefit on raising high-density lipoprotein (HDL)
levels. Aerobic exercise also benefited triglyceride levels and systolic blood
pressure. Resistance exercise did not show significant benefit on any CVD risk
factor. Another recent prospective trial with a 6-month, twice weekly,
progressive, supervised aerobic program in a population with T2D also
demonstrated improved HDL levels (12%) and marked decreases in markers of
endothelial dysfunction (ICAM-1 and P-selectin), but no changes in inflammatory
markers (hsCRP and TNF-alpha) or LDL levels (121).
Cardiovascular Disease Outcomes
Only one intervention study and no randomized trials have addressed the
effect of activity or fitness on hard CVD outcomes. The ongoing Look AHEAD
(Action for HEAlth in Diabetes) trial, currently underway, is a randomized
long-term study addressing hard CV outcomes after an intervention (122-124). However, the intervention is targeted at weight
loss by a combined program of diet and physical activity and thus will not
address the effect of physical activity in isolation. In the one existing
interventional trial looking at physical activity alone, Shinji and colleagues
followed a small group (n=102) of T2D adults for 17 months after institution of
a single, modest, home-based exercise program (walking 20 to 30 minutes, 4 to 6
times per week, at anaerobic threshold) (125). Incident
CVD was much higher in "dropouts" than in "completers" even after adjustment
for multiple parameters with a RR for incident CVD of 16.5 (95%CI, 1.19-228)
for dropouts versus completers. This study suggests that low-level physical
activity is beneficial for primary CVD prevention in people with T2D. However,
no data were reported or adjustments made for smoking or diet, the "dropouts
versus completers" study comparison was nonrandomized, the number of events was
very small (n=8), and the confidence interval was very large.
Several prospective cohort studies have found that CV fitness (60;126-128) (Table G3.A8 [PDF - 114 KB]) and physical
activity level (60;67;115-118;129;130)
(Table G3.A9 [PDF - 140 KB]) are
inversely correlated with mortality (all-cause and CVD) and/or CVD event rates
in subjects with T2D. Some of these studies have evaluated the effect of
frequency, duration, and/or intensity of physical activity on the protective
effect. A follow-up of the National Health Interview Survey of 2,896 adults
with T2D (115) found that walking for more than 2 hours
per week (but not more than 0 hours to 1.9 hours) was associated with a
significantly decreased hazard ratio (HR) for CVD mortality (HR = 0.59, 95% CI
0.40 to 0.87, P for trend 0.03 after exclusion of disabled subjects,
and after adjusting for age, sex, race, BMI, self-rated health, smoking, weight
loss approaches, hospitalizations, hypertension or medications, physician
visits, limitations caused by CVD or cancer, and extent of functional
limitation).
In the Nurses' Health Study of more than 5,000 diabetic women followed
for 14 years, subjects were placed in 5 groups based on hours of total
moderate-vigorous activity per week, including non-leisure activities (67). RR for CVD events (fatal and nonfatal myocardial
infarction or stroke) decreased progressively with increasing weekly volume of
moderate to vigorous activity (less than 1, 1 to 1.9, 2 to 3.9, 4 to 6.9, and 7
or more hours per week). Age-adjusted relative risks were 1.0, 0.93 (95% CI,
0.69 to 1.26), 0.82 (95% CI, 0.61 to 1.10), 0.54 (95% CI, 0.39 to 0.76), and
0.52 (95% CI, 0.25 to 1.09) (P for trend <0.001). This relationship
did not change appreciably after adjustment for smoking, BMI, and other CV risk
factors. Among women who primarily walked for exercise, both increased pace
(easy pace: 1.0, average pace: 0.52, brisk pace: 0.47, P for trend
0.001) and weekly MET walking score were inversely associated with CVD event
risk. Among women who did not exercise vigorously in addition to walking,
multivariate relative risks across quartiles of MET scores for walking were
1.0, 0.85 (0.62-1.34), 0.63 (0.36-1.10), 0.56 (0.31‑1.00) (P for
trend 0.03) for 0 to 0.5, 0.6 to 2.7, 2.8 to 7.5, and more than 7.5 MET hours
per week of walking.
In the Health Professionals follow-up study, Tanasescu and colleagues
followed about 2,800 men with T2D for 14 years and assessed incident CVD (fatal
or nonfatal MI or stroke) (116). Risk of total and fatal
CVD events showed a statistically significant improvement with increasing
physical activity after age-adjustment (P for trend 0.02, 0.03,
respectively) and a strong trend after multivariate analysis (adjusted for
alcohol intake; smoking; family history of myocardial infarction; use of
vitamin E supplements; duration of T2D; diabetes medication; quintiles of
dietary intake of trans fat, saturated fat, fiber, and folate; history of
angina and coronary artery bypass graft; and baseline presence of hypertension
and high serum cholesterol; P for trend 0.07, 0.13,
respectively). Additional adjustment for BMI further attenuated the trend (for
total CVD events: 1.0, 0.91 [0.63-1.31], 0.68 [0.45-1.02], 0.76 [0.51-1.14],
and 0.72 [0.47-1.09] by quintile; P for trend 0.14). Their results
suggest that physical activity protects from CVD events, especially fatal
events, and that for T2D, moderate energy expenditure (3rd quintile, 12 to 22
MET-hours per week, corresponding to about 3 to 5 hours per week of brisk
walking) provides the most protection. The authors state that this was not the
case in the non-diabetic cohort where a more continuous dose-response was seen.
A separate walking intensity multivariate analysis suggests that for those who
walked for exercise, the higher the walking speed, the greater the protection.
After adjustment for CVD risk factors, walking time, and other vigorous
activity, the relative risks for normal pace (2 to 2.9 miles per hour), brisk
pace (3 to 3.9 miles per hour), and very brisk pace (more than 4 miles per
hour) were 0.82, 0.58, and 0.17 (95% CI 0.04 to 0.71; P for trend
<0.001) compared to an easy pace (less than 2 miles per hour).
The studies described above suggest that maximum benefit may be achieved
with substantial volumes of moderately vigorous exercise, such as brisk to very
brisk walking, for 3 to 7 hours per week. It is interesting to speculate that
subjects with T2D may differ from non-diabetic subjects in their response to
very vigorous exercise, but further studies are needed to fully address the
intensity response of CVD risk reduction with physical activity in T2D.
In the Whitehall Study, Batty and colleagues performed a comparative
study of the benefits of physical activity in men with T2D or IGT (Table G3.A9 [PDF - 140 KB]) compared to
men with normal glucose tolerance (131). After adjustment
for other factors, physical activity remained an independent predictor of
all-cause, CHD, and other CVD mortality. The gradient for benefit with
increasing physical activity was much steeper for the IGT/T2D subjects than for
those with normal glucose tolerance, suggesting a greater benefit for
metabolically impaired subjects than for the general population. A plot adapted
from this data illustrates that the highest level of physical activity actually
eliminated the excess CHD mortality associated with IGT and T2D (132) (Figure G3.4).
Others have also found a steeper response of CVD risk to physical
activity in diabetic subjects, but most studies have found that CVD risk
remains greater in diabetic than non-diabetic subjects even in the most active
subgroups (116).
Figure G3.4. Physical Activity/Exercise and
Macrovascular Risk Reduction in Type 2 Diabetes
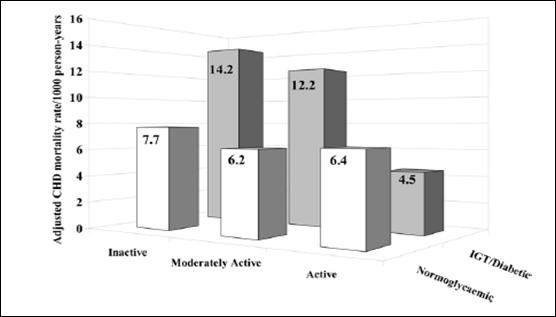
Note: Age-adjusted cardiovascular disease mortality rates
by leisure time activity in normoglycemic men (n=6,056) versus men with
impaired glucose tolerance/diabetes (n=352) in the Whitehall Study (Adapted by
Gill and Malakova 2006, (132) from data from the
Whitehall Study). P=0.006 for trend in normoglycemic men, P=0.003
for trend in men with IGT/diabetes.
Source: Gill JM, Malkova D. Physical activity, fitness
and cardiovascular disease risk in adults: interactions with insulin resistance
and obesity. Clin Sci (Lond). 2006 Apr;110(4):409-425. Review. Reproduced with
permission.
Physical Activity, Cardiovascular Fitness,
and Type 2 Diabetes
A recent meta-analysis evaluated the benefits of physical activity for
CV fitness in persons with T2D (133). The overall
analysis of 9 randomized, controlled, prospective interventional studies had
mean exercise characteristics of 3.4 sessions per week and 49 minutes per
session for 20 weeks. Mean baseline maximal oxygen consumption of 22.4
ml/kg/min increased 11.8% in the exercise arms and decreased 1.0% in the
control arms. Magnitude of improvement in maximal oxygen consumption and in
HbA1c correlated better with exercise intensity than with exercise volume.
Because fitness and glycemic control appear to benefit overall and CVD
mortality, this suggests that more intense exercise would have greater
mortality benefits. However, the possibility of a mortality impact of intense
exercise in diabetic people cannot be ruled out and is, in fact, suggested by
some outcome studies (discussed above). Furthermore, overt nephropathy,
peripheral neuropathy, and retinopathy present in many diabetic individuals may
be contraindications to very vigorous activity, prolonged stepping activities,
and weight-lifting or high-impact activities, respectively, though these
recommendations appear to be based on little experimental evidence (see
Question 5. Does Physical Activity Have a Role in
Preventing and Treating Diabetic Microvascular Complications?).
Question 4. Does Physical Activity Have
Benefits for Type 1 Diabetes?
Conclusions
Data are more limited for type 1 diabetes (T1D) than for T2D, but
generally support benefits of exercise for T1D in reducing mortality, CVD risk
factors, and microvascular complications. Data are weaker for benefits for
glycemic control, and CVD outcomes have not been studied. Data regarding the
optimal exercise prescription also are limited. This may still include limiting
exercise appropriately in proliferative retinopathy. However, any exercise
prescription in T1D also must address the issue of avoiding exercise-induced
hypoglycemia. This requires an individualized approach that includes modifying
insulin dosing, ingesting additional carbohydrates, and ensuring appropriate
details of the exercise prescription.
Rationale
Though T1D is less prevalent than T2D, it remains among the most
prevalent chronic, serious diseases of childhood affecting about 1.5/1,000
children in the United States (134). Overall prevalence
estimates are increasing now that it has been recognized that a quarter to a
half of all T1D develops in adults. Although the metabolic abnormalities
associated with insulin resistance have not been considered major factors in
this autoimmune form of diabetes, CVD has long been known to be a major cause
of morbidity and mortality in T1D. It is now becoming recognized that insulin
resistance is also present in T1D and that this may contribute to the
associated excess CVD risk. As T1D individuals spend a longer portion of their
lives with absolute endogenous insulin deficiency and relative insulin
sensitivity, hypoglycemia is a greater safety concern in T1D than in T2D.
Effects of physical activity on CVD risk factors and glycemic control and
safety concerns are addressed in this section. Microvascular complication
effects are addressed in a later section (see Question 5. Does Physical
Activity Have a Role in Preventing and Treating Diabetic Microvascular
Complications?).
As with T2D and non-diabetic populations, exercise has been shown to be
inversely correlated with mortality in T1D. In a cohort study of 548 T1D
subjects followed for 7 years in the Pittsburgh Insulin-dependent Diabetes
Morbidity and Mortality Study, sedentary males were 3 times as likely to die as
active males (135). The relationship did not achieve
statistical significance in women.
Physical Activity and Type 1 Diabetes Prevention
No data exist to show that habitual physical activity or exercise plays
a role in preventing T1D.
Physical Activity and Type 1 Diabetes Treatment
Glycemic Control
Exercise increases insulin sensitivity and induces non-insulin dependent
skeletal muscle glucose uptake. Overweight or otherwise insulin resistant T1D
individuals will derive benefit from the improvement in insulin sensitivity
that accompanies exercise in the same way that T2D individuals do (see Question
1. Does Physical Activity Have a role in Preventing or Treating Metabolic
Syndrome?). Recent evidence suggests that even apparently insulin sensitive
diabetic individuals are insulin resistant compared to non-diabetic controls
(136;137). Theoretically,
therefore, most or all T1D patients might be expected to improve insulin
sensitivity with physical activity. As such, it would seem that exercise could
improve glycemic control. However, for a T1D patient on a regular dose of
insulin, this improved sensitivity comes at the cost of an increased risk of
hypoglycemia and resultant hyperglycemia. Furthermore, high-intensity exercise
increases catecholamine release and can cause post-exercise hyperglycemia.
Thus, studies have had mixed results. Nevertheless, the largest studies have
demonstrated improved glycemic control with physical activity in T1D.
Interventional studies, most from the 1980s, have all been small (Table G3.A10 [PDF - 144 KB], which
summarize these studies). Most have used a moderate aerobic exercise program
and have had mixed results, with some negative (138-144)
and some modestly positive (145-148) trials. One of the
positive trials included a "carbohydrate control" diet intervention in addition
to exercise (145). Thus, the improved glycemic control in
this study cannot clearly be attributed to exercise. Other positive studies did
not include any dietary change or monitoring. Some negative trials followed
caloric intake and noted an increase in calories in the exercise group (139).
Few studies have looked at resistance training. Two studies with resistance
interventions were split, one with improvement in HbA1c (148), the other without (143). Larger
cross-sectional studies have also been split (Table G3.A11 [PDF - 125 KB], which
summarize these studies). Ligtenberg studied 200 subjects and found no
correlation between self-reported activity and HbA1c (149). The FinnDiane study of 1,030 T1D subjects found a
sex-based difference in that self-reported physical activity did correlate with
improved HbA1c in women, but not in men (150). The effect
on HbA1c in women was an 0.5% decrease in both the moderately active (10 to 40
MET-hours per week) and active groups (more than 40 MET-hours per week). In
contrast, in men, insulin doses were decreased to a greater extent in the more
active populations. In the largest study to date, Herbst and colleagues studied
more than 23,000 subjects with T1D and found a small, but highly significant
improvement in HbA1c (0.3%) in the 2 active groups (exercise 1 to 2 times a
week and 3 or more times a week) compared to the sedentary group (151). Only one study compared resistance to aerobic training
and found no benefit for glycemic control in either arm (143). Overall, good evidence for a significant role for
exercise alone in glycemic control is limited. Existing evidence suggests that
a modest improvement in glycemic control occurs with small amounts of activity
and does not increase with more frequent or more intense exercise. More studies
are needed to further clarify the role of physical activity in T1D because many
of the studies are relatively old.
Macrovascular Complications
CVD risk factors. The FinnDiane study found
that low physical activity correlated with the presence of metabolic syndrome
in TID, especially the waist circumference component (152). Lehman and colleagues found significant improvements
in insulin sensitivity, LDL, HDL, blood pressure, and waist-to-hip ratio with a
self-monitored increase in physical activity of about 150 minutes per week
without an increase in severe hypoglycemic events (153).
Few studies have investigated the effect of different doses or types of
exercise on CVD risk factors in TID. In one 12-week intervention study, Ramalho
and colleagues compared the effects of thrice weekly 40 minutes of moderate
aerobic training to resistance training (143). Neither
group improved lipid profiles, but the aerobic group had improved waist
circumference while the resistance group did not.
CVD outcomes. No data
exist on the effect of physical activity on actual CV outcomes specifically in
T1D.
Physical Activity, Type 1 Diabetes, and Risk of Hypoglycemia
Whatever the benefits of exercise in T1D, it is clear that they come at
the expense of an increased risk of hypoglycemia, both during and up to 30
hours after exercise. However, the ADA Position Statement on Physical Activity
and Exercise states the "all levels of physical activity, including leisure
activities, recreational sports, and competitive professional performance, can
be performed by people with T1D who do not have complications and are in good
glucose control (154, p.61). This is because it is
possible, with a good understanding of the physiologic responses to exercise,
to manage exercise and post-exercise blood sugars. Guidelines for hypoglycemia
control have been published, although they are not always strongly data-based
and therefore are outside the scope of this section. (155-162).
Question 5. Does Physical Activity Have a
Role in Preventing and Treating Diabetic Microvascular Complications?
Conclusions
Physical activity may prevent the development of diabetic neuropathy and
diabetic nephropathy (primary prevention) in those with T1D and T2D. Though
uncontrolled observational studies suggest physical activity may treat diabetic
neuropathy and nephropathy, RCTs are necessary to confirm this. Other
observational studies suggest no effect of physical activity on either the
prevention or treatment of diabetic retinopathy in T1D subjects. No data are
available on sex differences or dose-response of physical activity.
Moderate-intensity physical activity appears safe for all individuals
with diabetes even those with existing diabetic microvascular complications,
although vigorous-intensity activity, high-impact exercise, or weight-bearing
exercise may possibly lead to adverse outcomes in those with existing
proliferative retinopathy, severe nephropathy with renal osteodystrophy, or
severe neuropathy, respectively. Exercise stress testing is not recommended
before starting a moderate-intensity exercise regimen and is of controversial
benefit before initiating a vigorous intensity aerobic exercise program.
Introduction
Persons with diabetes have a highly increased prevalence of
microvascular complications, which are associated with substantial morbidity.
In this section, the role of physical activity in preventing and treating
microvascular complications in those with T1D and T2D will be discussed. For
the purpose of this document, microvascular complications of diabetes are
defined to include neuropathy (based either on symptoms, physical examination,
or abnormal electromyogram findings consistent with this diagnosis),
nephropathy (defined as microalbuminuria, macroalbuminuria, or decreased
calculated glomerular filtration rate), and retinopathy (defined as
non-proliferative or proliferative retinopathy diagnosed by an ophthalmologist
using retinal photographs).
To date, no large RCTs have investigated the role of exercise training
or physical activity in preventing or treating diabetic microvascular
complications. One small RCT and some observational studies have suggested a
possible relationship between physical activity and both the primary prevention
and treatment (tertiary prevention) of diabetic microvascular complications.
One meta-analysis (119) has evaluated the impact of
physical activity on a surrogate intermediate marker (HbA1c) for progression to
diabetic microvascular complications, and showed convincingly that physical
activity interventions lower HbA1c. Because better glycemic control has been
shown to decrease the incidence of diabetic microvascular complications in
subjects with T1D (163) and T2D (164), it is possible that exercise training could reduce
microvascular complications solely due to its general improvement of glycemic
control. However, the overall lack of studies in this area means that the role
of physical activity in preventing microvascular complications remains
inconclusive. Specific gaps in the literature that warrant further research are
large studies to determine the exercise dose-response curve for prevention or
treatment of microvascular complications, and determining whether differences
exist by subject race/ethnicity, sex, T1D vs. T2D, or exercise modality.
The next three sections will summarize what is known regarding the role
of physical activity in preventing and treating 1) diabetic neuropathy, 2)
diabetic nephropathy, and 3) diabetic retinopathy. Safety concerns for exercise
in these populations also will be discussed.
Rationale
Observational studies provide most of the existing data, which are of
limited scope and quality, to determine the role of physical activity in
primary prevention of diabetic nephropathy, neuropathy, and retinopathy.
Observational studies of lesser quality (often uncontrolled) have been
performed to address the role of physical activity for treatment of diabetic
nephropathy, neuropathy, and retinopathy. To determine the safety of physical
activity with existing microvascular complications, small observational studies
have been performed and clinical standards of care also have been discussed
when appropriate to supplement the scarce amount of safety data.
Diabetic Neuropathy
One small RCT (165), one cross-sectional study (166), and one retrospective cohort study (167) have evaluated the impact of physical activity on
primary prevention of diabetic neuropathy (Table G3.A12 [PDF - 138 KB], which
summarize these studies). From these limited data, no firm conclusions may be
drawn but it does appear that physical activity may possibly have some role in
preventing diabetic neuropathy. The RCT data, although only based on 78
participants (73% with T2D), revealed a reduction in both motor and sensory
neuropathy from 4 years of moderate-intensity exercise despite no significant
weight loss (165). Of the 2 cross-sectional studies
performed in T1D subjects addressing neuropathy, one showed physical activity
significantly benefited males only (166), while the other
had no effect (167).
Treatment of Diabetic Neuropathy
No studies have
evaluated the use of physical activity to treat diabetic neuropathy. One study
evaluated 12 months of physical activity in conjunction with a dietary
intervention for prediabetic neuropathy (Table G3.A13 [PDF - 102 KB], which
summarize these studies), using a pre-post study design in 40 subjects with
prediabetes to show significant differences in nerve fiber density at the
proximal portion of the leg (P <0.05), and non-significant
improvement in neuropathic pain and nerve fiber density at the distal portion
of the leg (168).
With respect to diabetic ulcer prevention in a group with diabetic
neuropathy, no significant improvement in the surrogate outcome of dorsal foot
cutaneous perfusion was found after either a 10-week aerobic exercise (169) or 8-week resistance exercise program (170). Although significant differences were initially
described in dorsal foot cutaneous perfusion between physically active
individuals with T2D as compared with sedentary individuals with T2D who had a
higher mean HbA1c (171), no differences were evident when
this study was repeated with similar HbA1c levels between groups (172). This area requires further study.
Safety of Exercise With Diabetic Neuropathy
Three different aspects of safety of exercise with comorbid neuropathy
are at issue: (1) Safety of exercise with autonomic neuropathy, (2) Ulcer risk
with existing neuropathy, (3) Fall risk with existing neuropathy.
Safety of exercise with autonomic
neuropathy. Existing guidelines are not based on data and are therefore
are outside the scope of this chapter. Graham and Lasko-McCarthy and Sigal and
colleagues provide further information on this topic (112;173).
Ulcer risk with existing
neuropathy. Two studies observed an inverse relationship between
physical activity and ulcer incidence (174;175). However, 2 other studies have suggested that abrupt
increases in activity may increase the short-term risk of ulceration. Armstrong
and colleagues found a significantly greater coefficient of variation in the
group with recurrent ulcer (174) and Lemaster and
colleagues (175) found a significant unadjusted increased
risk of ulcer with increased short-term activity. Ulcer risk was increased with
greater intensity and duration of loading pressure on the feet while walking
(176;177) possibly showing a
clinical benefit to protective diabetic footwear in this population.
Risk of falls with existing
neuropathy. Several studies have evaluated the degree to which gait is
altered by diabetic neuropathy (suggesting attendant increased fall risk), with
one study showing a targeted intervention may improve balance in this
population. Dingwell and colleagues as well as other researchers have performed
studies showing decreased walking speeds or decreased gait variability (176;178-180) in those with diabetic peripheral neuropathy versus
non-diabetic controls. Giacomozzi and colleagues also showed those with
diabetic neuropathy and a prior foot ulcer had even greater gait variability
than those with neuropathy and no prior ulcer (176).
Mueller and colleagues showed that the peak torque generated during plantar
flexion and the range of motion of dorsiflexion at the ankle are strongly
correlated (r = 0.78) and contribute to the power generated from the ankle
joint during ambulation (181). These data suggest that
decreased ankle dorsiflexion range of motion and/or plantar flexion strength
are associated with decreased step length and speed during walking (181). Novak and colleagues (182)
reported that 30 individuals with T2D and associated diabetic neuropathy
described worse foot pain and walked shorter distances than subjects with T2D
without neuropathy and non-diabetic controls, with strong correlation between
pain level and walking distance (r = -0.45, P <0.001) (182).
The data presented here generally support the pragmatic exercise
precautions recommended in clinical practice guidelines (Table G3.A14 [PDF - 152 KB], which
summarize these studies). Those with severe peripheral
neuropathy should use non-weight bearing activities to avoid foot ulceration or
Charcot joint destruction (112;173), and all individuals with diabetes should use
appropriate footwear and inspect their feet daily to reduce injury risk (183).
Diabetic Nephropathy
Four cross-sectional studies (150;152;166;184) and 1 retrospective cohort study (167) have evaluated the impact of physical activity
on diabetic nephropathy prevention in subjects with T1D (Table G3.A12 [PDF - 138 KB]). These
data are not available in patients with T2D. From these limited data, no firm
conclusions may be drawn but they suggest physical activity may prevent
diabetic nephropathy. In 2 separate cross-sectional analyses of slightly
different subsets of a Finnish population with T1D, less physical activity was
associated with greater prevalence of nephropathy (150;152). A significant association was observed between greater
leisure-time physical activity and decreased nephropathy in men only, with no
increased risk in women with T1D (166). The other 2
observational studies performed showed neither harm nor benefit in prevention
of diabetic nephropathy (167;184).
Physical Activity To Treat Diabetic Nephropathy
A pre-post analysis (185) evaluated the effect of
3 weeks of physical activity and low-calorie diet in treating existing
nephropathy (Table
G3.A13 [PDF - 102 KB]) in subjects with T2D. Although albuminuria was reduced, the dietary
intervention and/or associated weight loss may have confounded these results.
These data are somewhat promising but inconclusive.
Safety of Physical Activity With Existing Nephropathy
The relevant literature appears to show that exercise does not worsen
resting proteinuria (186-188). In a cohort of 373
subjects with T1D, a strong correlation between overnight albumin excretion
rate (AER) and post-exercise AER existed (r = 0.74, P <0.001), and
52% of subjects had an elevated overnight AER preceding an elevated
post-exercise AER (186). In a smaller cross-sectional
study, Groop and colleagues (187) showed exercise did not
increase protein excretion in 17 subjects newly diagnosed with T1D, but that 17
subjects with long-standing T1D had a significant increase in post-exercise
excretion of albumin, β2‑microglobulin, Kappa light chains, and IgG
independent of whether resting AER was elevated (n=7) or normal (n=10). A small
cohort study found no significant difference in time for nephropathy
progression in 6 subjects with "good" unrestricted physical activity as
compared with 7 subjects with "self-restricted" physical activity (188).
Despite hypothetical adverse effects of increased proteinuria
immediately after exercise (189), existing data show no
progression of nephropathy with exercise and, in fact, increasing physical
activity may decrease existing albuminuria, as described earlier in this
section (185;190;191). In the absence of primary data for other safety
considerations in those with diabetic nephropathy, a review of these issues is
outside the scope of this discussion, although guidelines exist (112;173).
Diabetic Retinopathy
One moderate-sized prospective cohort study (192),
and several cross-sectional (150;152;166;184;193) and retrospective (167;194) observational studies have evaluated the impact of
physical activity on diabetic retinopathy (Table G3.A12 [PDF - 138 KB]) in T1D.
These limited data suggest that physical activity does not influence the risk
of developing diabetic retinopathy. The moderately sized cohort study (192) observed no difference in the incidence of retinopathy
over 6 years in 606 T1D subjects with respect to current physical activity or
historical participation in team sports, in contrast to an earlier
cross-sectional analysis (193) in a subset of the same
cohort population where a decreased prevalence of retinopathy in women who
played team sports (OR 0.46, P <0.05) or who reported current
strenuous physical activity (OR 0.34, P <0.05) was previously
observed. Two cross-sectional analyses of slightly different subsets of a
Finnish population with T1D found no association between physical activity and
retinopathy (150;152) despite an
association between physical activity and less nephropathy in those same
studies (150;152). Of the 4 other
cross-sectional studies performed, none showed any benefit or harm of physical
activity in the prevention of diabetic retinopathy (166;167;184;194).
Treatment of Diabetic Retinopathy
A large cohort study reported no impact of self-reported current or
historical physical activity measurements on retinopathy in a large cohort of
T1D subjects with both non-proliferative and proliferative retinopathy at
baseline measurement (192).
Safety of Physical Activity With Existing Diabetic Retinopathy
Although existing data raise concerns about the plausible causality of
exercise-induced vitreous hemorrhages individuals with diabetic retinopathy,
existing data have not conclusively shown a risk of moderate-intensity exercise
in those with this condition (195).
The 2 prospective studies evaluating the safety of exercise in humans
with existing retinopathy have not shown an increased risk of retinopathy
progression or of vitreous hemorrhage in this population. The prospective
cohort study analysis by Cruickshanks and colleagues showed no risk of worsened
retinopathy in those with T1D who were more physically active over a 6-year
period as compared with their more sedentary counterparts, including a very
small subset of self-described weight lifters (192). A
pre-post exercise intervention study in 30 subjects with T1D or T2D and
existing proliferative diabetic retinopathy (90% or greater) or diabetic
macular edema observed no newly documented vitreous hemorrhages attributable to
a 12-week supervised exercise training program, although the study was
under-powered to definitively determine vitreous hemorrhage risk (196).
Given the preceding evidence, clinical providers have generally
recommended moderate-intensity exercise but advised against vigorous exercise
regimens for those with proliferative retinopathy (112;173;183;197) and
severe nonproliferative retinopathy (112) due to the
theoretical (yet unproven) increased risk for vitreous hemorrhage and retinal
detachments with vigorous exercise.
Cardiovascular Safety of Physical Activity With Existing Microvascular
Complications
Despite a lack of studies evaluating this practice, the most recent
published standards of care suggest that diabetic subjects with more than a 10%
10-year risk for CV disease by the United Kingdom Prospective Diabetes Study
risk calculator (198) should consider
exercise stress testing to screen for latent ischemia before initiating
vigorous aerobic exercise regimens that exceed the "demands of everyday living"
(199).
Question 6: Do Physical Activity and
Exercise Have a Role In Preventing Gestational Diabetes?
Conclusions
Although no RCTs have been performed to demonstrate that physical
activity can prevent gestational diabetes (GDM), data from observational
studies support that concept. Available studies suggest that approximately 30
minutes per day of moderate-intensity physical activity is likely a sufficient
dose to decrease the GDM risk (200). However, this
suggestion is based on relatively few studies, and further studies should
directly address the issue of dose-response.
Introduction
Gestational diabetes is defined as diabetes first identified during
pregnancy. Overall, prevalence rates of GDM have increased from 1.9% in
1989-1990 to 4.2% in 2003-2004, a relative increase of 122% (201). The prevalence of GDM is 17% in obese women, and
overweight women have a significantly greater risk of developing GDM than do
non-overweight women (202). It is estimated that up to
60% of women with GDM will develop T2D within 4 years of delivery (203). GDM can give rise to many adverse outcomes both to
mother and infant. It is associated with a greater likelihood of Caesarean
section deliveries and other birth complications (204).
Women with GDM also are more likely to have a difficult labor and delivery.
Babies of women with GDM are at increased risk of obesity and diabetes later in
life as well as other comorbid conditions at birth (205).
Given that women who develop GDM are at highly increased risk of
developing T2D, understanding how to prevent and treat GDM is very important.
The role of physical activity in preventing and treating GDM has not been as
well studied as for T2D. Indeed, no RCTs have assessed whether GDM can be
prevented by regular physical activity. However, observational epidemiologic
studies suggest overall that this may be the case (Table G3.A15 [PDF - 113 KB], which
summarize these studies).
Rationale
Data From Observational, Epidemiological Studies
Several studies have shown that physical activity is associated with a
significantly reduced risk of GDM (200). These studies
reported that increased levels of physical activity (assessed by questionnaire)
before pregnancy or during the first 20 weeks of pregnancy was associated with
reductions in risk of GDM. Overall the reduction in risk is about 50% when
active women are compared to inactive women.
Dose-Response Data
No RCTs have evaluated prospectively whether physical activity can
prevent GDM or what doses might be effective for such a response. Such trials
would be of great value to establish the role of exercise and physical activity
in GDM. Available studies suggest that approximately 30 minutes per day of
moderate intensity physical activity is likely a sufficient dose to decrease
the GDM risk (200). However, this suggestion is based on
relatively few studies, and further studies should directly address the issue
of dose-response.
Overall Summary and Conclusions
In summary, physical activity and exercise play a key role in preventing
and treating metabolic syndrome and T2D. The evidence for T2D are the clearest
because RCTs have been conducted to corroborate the findings of many
observational trials, although, as mentioned previously, 2 of the 3 RCTs
combined physical activity and diet in their lifestyle intervention. (The
post-hoc findings on effects of physical activity in the absence of weight
change, although consistent and strong, are therefore not considerd strong RCT
data but rather are equivalent to the quality of prospective cohort study
data.) The role of physical activity and exercise in treating T1D is still
being established. Current evidence suggests that benefits are likely, perhaps
most of all in the area of reducing mortality, CVD risk factors, and
microvascular complications. For both T1D and T2D, physical activity may
prevent the development of diabetic neuropathy and diabetic nephropathy.
Finally, it appears likely that physical activity and exercise may help prevent
and treat gestational diabetes although more research is needed to establish
these findings. The amount of exercise that appears to be the most well
accepted and documented across the conditions included in this section to date
is 30 minutes of moderate physical activity 5 days per week. However, it is
clear that benefits are obtained with even lower volumes of physical activity.
Walking is a beneficial form of physical activity and has been especially well
documented as effective in T2D (where it has been most extensively studied). In
the next section, the extensive research needs for further study in the area of
Metabolic Health are documented.
Research Needs
Although a considerable body of literature exists on the role of
physical activity in promoting and maintaining metabolic health, a number of
questions remain unanswered and require additional research:
- Available data indicate that regular physical activity is associated
with reduced risk of metabolic syndrome. However, it is not clear whether
physical activity and exercise can be used in treating or reversing metabolic
syndrome, and additional studies will help to clarify this issue.
- Research is needed in diverse populations to determine whether the
effects of physical activity across the range of metabolic health issues,
including metabolic syndrome, T2D, T1D, and gestational diabetes, differ with
race and ethnicity.
- Further examination of the effects of physical activity on metabolic
syndrome and T2D also is warranted to determine whether and how its effect
differ in youth and adults.
- Additional research evaluating dose-response patterns of exercise in
preventing diabetes and cardiovascular outcomes in diabetes would make a
valuable contribution to the metabolic health literature.
- RCTs are needed to examine the effects of exercise on treating T1D in
children and adults. Good cardiovascular outcome data in response to physical
activity in T1D is lacking and could potentially be obtained in adult-onset
T1D.
- Clinical studies in post-exercise hypoglycemia are needed to further
study the intermittent high-intensity exercise approach to prevention and to
compare extra carbohydrate versus lower insulin dosing approaches to treating
T2D.
- Research is needed on several issues related to gestational diabetes.
For example, RCTs are needed to determine whether physical activity can prevent
gestational diabetes. It also would be useful to have additional dose-response
data on the role of exercise and physical activity in treating gestational
diabetes.
Reference List
- Ford ES, Giles WH, Dietz WH. Prevalence of the
metabolic syndrome among US adults: findings from the third National Health and
Nutrition Examination Survey. JAMA 2002 Jan 16;287(3):356-9.
- American Diabetes Association. American Diabetes
Association website 2008 Available from
http://www.diabetes.org/home.jsp.

- National Institute of Diabetes and Digestive and
Kidney Diseases, National Diabetes Information Clearinghouse (U.S.). National
Diabetes Information Clearinghouse 2005 Available from
http://www.niddk.nih.gov/health/diabetes/ndic.htm.
- Vaidya D, Szklo M, Ding J, Tracy R, Liu K, Saad M,
Ouyang P. Agreement of two metabolic syndrome definitions and their association
with subclinical atherosclerosis: multi-ethnic study of atherosclerosis cross
sectional study. Metab Syndr.Relat Disord. 2007 Dec;5(4):343-52.
- Executive Summary of The Third Report of The
National Cholesterol Education Program (NCEP) Expert Panel on Detection,
Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment
Panel III). JAMA 2001 May 16;285(19):2486-97.
- Alberti KG, Zimmet PZ. Definition, diagnosis and
classification of diabetes mellitus and its complications. Part 1: diagnosis
and classification of diabetes mellitus provisional report of a WHO
consultation. Diabet.Med. 1998 Jul;15(7):539-53.
- Balkau B, Charles MA. Comment on the provisional
report from the WHO consultation. European Group for the Study of Insulin
Resistance (EGIR). Diabet.Med. 1999 May;16(5):442-3.
- Alberti KG, Zimmet P, Shaw J. The metabolic
syndrome--a new worldwide definition. Lancet 2005 Sep
24;366(9491):1059-62.
- Wareham NJ, Rennie KL. The assessment of physical
activity in individuals and populations: why try to be more precise about how
physical activity is assessed? Int.J.Obes.Relat Metab Disord. 1998 Aug;22 Suppl
2:S30-S38.
- Carroll S, Cooke CB, Butterly RJ. Metabolic
clustering, physical activity and fitness in nonsmoking, middle-aged men.
Med.Sci.Sports Exerc. 2000 Dec;32(12):2079-86.
- Irwin ML, Ainsworth BE, Mayer-Davis EJ, Addy CL,
Pate RR, Durstine JL. Physical activity and the metabolic syndrome in a
tri-ethnic sample of women. Obes.Res. 2002 Oct;10(10):1030-7.
- Ekelund U, Griffin SJ, Wareham NJ. Physical
activity and metabolic risk in individuals with a family history of type 2
diabetes. Diabetes Care 2007 Feb;30(2):337-42.
- Lakka TA, Laaksonen DE, Lakka HM, Mannikko N,
Niskanen LK, Rauramaa R, Salonen JT. Sedentary lifestyle, poor
cardiorespiratory fitness, and the metabolic syndrome. Med.Sci.Sports Exerc.
2003 Aug;35(8):1279-86.
- Rennie KL, McCarthy N, Yazdgerdi S, Marmot M,
Brunner E. Association of the metabolic syndrome with both vigorous and
moderate physical activity. Int.J.Epidemiol. 2003 Aug;32(4):600-6.
- Brage S, Wedderkopp N, Ekelund U, Franks PW,
Wareham NJ, Andersen LB, Froberg K. Features of the metabolic syndrome are
associated with objectively measured physical activity and fitness in Danish
children: the European Youth Heart Study (EYHS). Diabetes Care 2004
Sep;27(9):2141-8.
- Chuang SY, Chen CH, Chou P. Prevalence of
metabolic syndrome in a large health check-up population in Taiwan. J.Chin
Med.Assoc. 2004 Dec;67(12):611-20.
- Franks PW, Ekelund U, Brage S, Wong MY, Wareham
NJ. Does the association of habitual physical activity with the metabolic
syndrome differ by level of cardiorespiratory fitness? Diabetes Care 2004
May;27(5):1187-93.
- Panagiotakos DB, Pitsavos C, Chrysohoou C,
Skoumas J, Tousoulis D, Toutouza M, Toutouzas P, Stefanadis C. Impact of
lifestyle habits on the prevalence of the metabolic syndrome among Greek adults
from the ATTICA study. Am.Heart J. 2004 Jan;147(1):106-12.
- Rguibi M, Belahsen R. Metabolic syndrome among
Moroccan Sahraoui adult Women. Am.J.Hum.Biol. 2004 Sep;16(5):598-601.
- Villegas R, Creagh D, Hinchion R, O'Halloran D,
Perry IJ. Prevalence and lifestyle determinants of the metabolic syndrome.
Ir.Med.J. 2004 Nov;97(10):300-3.
- Zhu S, St-Onge MP, Heshka S, Heymsfield SB.
Lifestyle behaviors associated with lower risk of having the metabolic
syndrome. Metabolism 2004 Nov;53(11):1503-11.
- Adams RJ, Appleton S, Wilson DH, Taylor AW, Dal
GE, Chittleborough C, Gill T, Ruffin R. Population comparison of two clinical
approaches to the metabolic syndrome: implications of the new International
Diabetes Federation consensus definition. Diabetes Care 2005
Nov;28(11):2777-9.
- Bertrais S, Beyeme-Ondoua JP, Czernichow S, Galan
P, Hercberg S, Oppert JM. Sedentary behaviors, physical activity, and metabolic
syndrome in middle-aged French subjects. Obes.Res. 2005 May;13(5):936-44.
- Bo S, Gentile L, Ciccone G, Baldi C, Benini L,
Dusio F, Lucia C, Forastiere G, Nuti C, Cassader M, et al. The metabolic
syndrome and high C-reactive protein: prevalence and differences by sex in a
southern-European population-based cohort. Diabetes Metab Res.Rev. 2005
Nov;21(6):515-24.
- Dunstan DW, Salmon J, Owen N, Armstrong T, Zimmet
PZ, Welborn TA, Cameron AJ, Dwyer T, Jolley D, Shaw JE. Associations of TV
viewing and physical activity with the metabolic syndrome in Australian adults.
Diabetologia 2005 Nov;48(11):2254-61.
- Ford ES, Kohl HW, III, Mokdad AH, Ajani UA.
Sedentary behavior, physical activity, and the metabolic syndrome among U.S.
adults. Obes.Res. 2005 Mar;13(3):608-14.
- Lee WY, Jung CH, Park JS, Rhee EJ, Kim SW.
Effects of smoking, alcohol, exercise, education, and family history on the
metabolic syndrome as defined by the ATP III. Diabetes Res.Clin.Pract. 2005
Jan;67(1):70-7.
- Misra KB, Endemann SW, Ayer M. Leisure time
physical activity and metabolic syndrome in Asian Indian immigrants residing in
northern California. Ethn.Dis. 2005;15(4):627-34.
- Mohan V, Gokulakrishnan K, Deepa R, Shanthirani
CS, Datta M. Association of physical inactivity with components of metabolic
syndrome and coronary artery disease--the Chennai Urban Population Study (CUPS
no. 15). Diabet.Med. 2005 Sep;22(9):1206-11.
- Nakanishi N, Takatorige T, Suzuki K. Cigarette
smoking and the risk of the metabolic syndrome in middle-aged Japanese male
office workers. Ind.Health 2005 Apr;43(2):295-301.
- Onat A, Hergenc G, Keles I, Dogan Y, Turkmen S,
Sansoy V. Sex difference in development of diabetes and cardiovascular disease
on the way from obesity and metabolic syndrome. Metabolism 2005
Jun;54(6):800-8.
- Brien SE, Janssen I, Katzmarzyk PT.
Cardiorespiratory fitness and metabolic syndrome: US National Health and
Nutrition Examination Survey 1999-2002. Appl.Physiol Nutr.Metab 2007
Feb;32(1):143-7.
- Desai MY, Dalal D, Santos RD, Carvalho JA, Nasir
K, Blumenthal RS. Association of body mass index, metabolic syndrome, and
leukocyte count. Am.J.Cardiol. 2006 Mar 15;97(6):835-8.
- Hassinen M, Komulainen P, Lakka TA, Vaisanen SB,
Haapala I, Gylling H, Alen M, Schmidt-Trucksass A, Nissinen A, Rauramaa R.
Metabolic syndrome and the progression of carotid intima-media thickness in
elderly women. Arch.Intern.Med. 2006 Feb 27;166(4):444-9.
- Liu J, Young TK, Zinman B, Harris SB, Connelly
PW, Hanley AJ. Lifestyle variables, non-traditional cardiovascular risk
factors, and the metabolic syndrome in an Aboriginal Canadian population.
Obesity.(Silver.Spring) 2006 Mar;14(3):500-8.
- Halldin M, Rosell M, de FU, Hellenius ML. The
metabolic syndrome: prevalence and association to leisure-time and work-related
physical activity in 60-year-old men and women. Nutr.Metab Cardiovasc.Dis. 2007
Jun;17(5):349-57.
- Pitsavos C, Panagiotakos DB, Chrysohoou C,
Kavouras S, Stefanadis C. The associations between physical activity,
inflammation, and coagulation markers, in people with metabolic syndrome: the
ATTICA study. Eur.J.Cardiovasc.Prev.Rehabil. 2005 Apr;12(2):151-8.
- Brouwer BG, Visseren FL, van der GY. The effect
of leisure-time physical activity on the presence of metabolic syndrome in
patients with manifest arterial disease. The SMART study. Am.Heart J. 2007
Dec;154(6):1146-52.
- Ekelund U, Brage S, Franks PW, Hennings S, Emms
S, Wareham NJ. Physical activity energy expenditure predicts progression toward
the metabolic syndrome independently of aerobic fitness in middle-aged healthy
Caucasians: the Medical Research Council Ely Study. Diabetes Care 2005
May;28(5):1195-200.
- Ekelund U, Franks PW, Sharp S, Brage S, Wareham
NJ. Increase in physical activity energy expenditure is associated with reduced
metabolic risk independent of change in fatness and fitness. Diabetes Care 2007
Aug;30(8):2101-6.
- Laaksonen DE, Lakka HM, Salonen JT, Niskanen LK,
Rauramaa R, Lakka TA. Low levels of leisure-time physical activity and
cardiorespiratory fitness predict development of the metabolic syndrome.
Diabetes Care 2002 Sep;25(9):1612-8.
- Carnethon MR, Loria CM, Hill JO, Sidney S, Savage
PJ, Liu K. Risk factors for the metabolic syndrome: the Coronary Artery Risk
Development in Young Adults (CARDIA) study, 1985-2001. Diabetes Care 2004
Nov;27(11):2707-15.
- Palaniappan L, Carnethon MR, Wang Y, Hanley AJ,
Fortmann SP, Haffner SM, Wagenknecht L. Predictors of the incident metabolic
syndrome in adults: the Insulin Resistance Atherosclerosis Study. Diabetes Care
2004 Mar;27(3):788-93.
- Ferreira I, Twisk JW, van MW, Kemper HC,
Stehouwer CD. Development of fatness, fitness, and lifestyle from adolescence
to the age of 36 years: determinants of the metabolic syndrome in young adults:
the amsterdam growth and health longitudinal study. Arch.Intern.Med. 2005 Jan
10;165(1):42-8.
- Holme I, Tonstad S, Sogaard AJ, Larsen PG, Haheim
LL. Leisure time physical activity in middle age predicts the metabolic
syndrome in old age: results of a 28-year follow-up of men in the Oslo study.
BMC.Public Health 2007;7(147):154.
- Carnethon MR, Gidding SS, Nehgme R, Sidney S,
Jacobs DR, Jr., Liu K. Cardiorespiratory fitness in young adulthood and the
development of cardiovascular disease risk factors. JAMA 2003 Dec
17;290(23):3092-100.
- Ferreira I, Henry RM, Twisk JW, van MW, Kemper
HC, Stehouwer CD. The metabolic syndrome, cardiopulmonary fitness, and
subcutaneous trunk fat as independent determinants of arterial stiffness: the
Amsterdam Growth and Health Longitudinal Study. Arch.Intern.Med. 2005 Apr
25;165(8):875-82.
- LaMonte MJ, Ainsworth BE, Durstine JL. Influence
of cardiorespiratory fitness on the association between C-reactive protein and
metabolic syndrome prevalence in racially diverse women. J.Womens Health
(Larchmt.) 2005 Apr;14(3):233-9.
- Platat C, Wagner A, Klumpp T, Schweitzer B, Simon
C. Relationships of physical activity with metabolic syndrome features and
low-grade inflammation in adolescents. Diabetologia 2006
Sep;49(9):2078-85.
- DuBose KD, Eisenmann JC, Donnelly JE. Aerobic
fitness attenuates the metabolic syndrome score in normal-weight,
at-risk-for-overweight, and overweight children. Pediatrics 2007
Nov;120(5):e1262-e1268.
- Kelishadi R, Razaghi EM, Gouya MM, Ardalan G,
Gheiratmand R, Delavari A, Motaghian M, Ziaee V, Siadat ZD, Majdzadeh R, et al.
Association of physical activity and the metabolic syndrome in children and
adolescents: CASPIAN Study. Horm.Res. 2007;67(1):46-52.
- Eisenmann JC, Welk GJ, Ihmels M, Dollman J.
Fatness, fitness, and cardiovascular disease risk factors in children and
adolescents. Med.Sci.Sports Exerc. 2007 Aug;39(8):1251-6.
- Eisenmann JC, Wickel EE, Welk GJ, Blair SN.
Relationship between adolescent fitness and fatness and cardiovascular disease
risk factors in adulthood: the Aerobics Center Longitudinal Study (ACLS).
Am.Heart J. 2005 Jan;149(1):46-53.
- Katzmarzyk PT, Leon AS, Wilmore JH, Skinner JS,
Rao DC, Rankinen T, Bouchard C. Targeting the metabolic syndrome with exercise:
evidence from the HERITAGE Family Study. Med.Sci.Sports Exerc. 2003
Oct;35(10):1703-9.
- Johnson JL, Slentz CA, Houmard JA, Samsa GP,
Duscha BD, Aiken LB, McCartney JS, Tanner CJ, Kraus WE. Exercise training
amount and intensity effects on metabolic syndrome (from Studies of a Targeted
Risk Reduction Intervention through Defined Exercise). Am.J.Cardiol. 2007 Dec
15;100(12):1759-66.
- Jurca R, LaMonte MJ, Church TS, Earnest CP,
Fitzgerald SJ, Barlow CE, Jordan AN, Kampert JB, Blair SN. Associations of
muscle strength and fitness with metabolic syndrome in men. Med.Sci.Sports
Exerc. 2004 Aug;36(8):1301-7.
- Jurca R, LaMonte MJ, Barlow CE, Kampert JB,
Church TS, Blair SN. Association of muscular strength with incidence of
metabolic syndrome in men. Med.Sci.Sports Exerc. 2005 Nov;37(11):1849-55.
- Wijndaele K, Duvigneaud N, Matton L, Duquet W,
Thomis M, Beunen G, Lefevre J, Philippaerts RM. Muscular strength, aerobic
fitness, and metabolic syndrome risk in Flemish adults. Med.Sci.Sports Exerc.
2007 Feb;39(2):233-40.
- Blair SN, Kampert JB, Kohl HW, III, Barlow CE,
Macera CA, Paffenbarger RS, Jr., Gibbons LW. Influences of cardiorespiratory
fitness and other precursors on cardiovascular disease and all-cause mortality
in men and women. JAMA 1996 Jul 17;276(3):205-10.
- Wei M, Gibbons LW, Kampert JB, Nichaman MZ, Blair
SN. Low cardiorespiratory fitness and physical inactivity as predictors of
mortality in men with type 2 diabetes. Ann.Intern.Med. 2000 Apr
18;132(8):605-11.
- Helmrich SP, Ragland DR, Leung RW, Paffenbarger
RS, Jr. Physical activity and reduced occurrence of non-insulin-dependent
diabetes mellitus. N.Engl.J.Med. 1991 Jul 18;325(3):147-52.
- Manson JE, Rimm EB, Stampfer MJ, Colditz GA,
Willett WC, Krolewski AS, Rosner B, Hennekens CH, Speizer FE. Physical activity
and incidence of non-insulin-dependent diabetes mellitus in women. Lancet 1991
Sep 28;338(8770):774-8.
- Hu FB, Sigal RJ, Rich-Edwards JW, Colditz GA,
Solomon CG, Willett WC, Speizer FE, Manson JE. Walking compared with vigorous
physical activity and risk of type 2 diabetes in women: a prospective study.
JAMA 1999 Oct 20;282(15):1433-9.
- Wannamethee SG, Shaper AG, Alberti KG. Physical
activity, metabolic factors, and the incidence of coronary heart disease and
type 2 diabetes. Arch.Intern.Med. 2000 Jul 24;160(14):2108-16.
- Okada K, Hayashi T, Tsumura K, Suematsu C, Endo
G, Fujii S. Leisure-time physical activity at weekends and the risk of Type 2
diabetes mellitus in Japanese men: the Osaka Health Survey. Diabet.Med. 2000
Jan;17(1):53-8.
- Folsom AR, Kushi LH, Hong CP. Physical activity
and incident diabetes mellitus in postmenopausal women. Am.J.Public Health 2000
Jan;90(1):134-8.
- Hu FB, Stampfer MJ, Solomon C, Liu S, Colditz GA,
Speizer FE, Willett WC, Manson JE. Physical activity and risk for
cardiovascular events in diabetic women. Ann.Intern.Med. 2001 Jan
16;134(2):96-105.
- Hu G, Qiao Q, Silventoinen K, Eriksson JG,
Jousilahti P, Lindstrom J, Valle TT, Nissinen A, Tuomilehto J. Occupational,
commuting, and leisure-time physical activity in relation to risk for Type 2
diabetes in middle-aged Finnish men and women. Diabetologia 2003
Mar;46(3):322-9.
- Weinstein AR, Sesso HD, Lee IM, Cook NR, Manson
JE, Buring JE, Gaziano JM. Relationship of physical activity vs body mass index
with type 2 diabetes in women. JAMA 2004 Sep 8;292(10):1188-94.
- Hsia J, Wu L, Allen C, Oberman A, Lawson WE,
Torrens J, Safford M, Limacher MC, Howard BV. Physical activity and diabetes
risk in postmenopausal women. Am.J.Prev.Med. 2005 Jan;28(1):19-25.
- Lynch J, Helmrich SP, Lakka TA, Kaplan GA, Cohen
RD, Salonen R, Salonen JT. Moderately intense physical activities and high
levels of cardiorespiratory fitness reduce the risk of non-insulin-dependent
diabetes mellitus in middle-aged men. Arch.Intern.Med. 1996 Jun
24;156(12):1307-14.
- Wei M, Gibbons LW, Mitchell TL, Kampert JB, Lee
CD, Blair SN. The association between cardiorespiratory fitness and impaired
fasting glucose and type 2 diabetes mellitus in men. Ann.Intern.Med. 1999 Jan
19;130(2):89-96.
- Sui X, Hooker SP, Lee IM, Church TS, Colabianchi
N, Lee CD, Blair SN. A prospective study of cardiorespiratory fitness and risk
of type 2 diabetes in women. Diabetes Care 2008 Mar;31(3):550-5.
- Bassuk SS, Manson JE. Epidemiological evidence
for the role of physical activity in reducing risk of type 2 diabetes and
cardiovascular disease. J.Appl.Physiol 2005 Sep;99(3):1193-204.
- Jeon CY, Lokken RP, Hu FB, van Dam RM. Physical
activity of moderate intensity and risk of type 2 diabetes: a systematic
review. Diabetes Care 2007 Mar;30(3):744-52.
- Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, Hu
ZX, Lin J, Xiao JZ, Cao HB, et al. Effects of diet and exercise in preventing
NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes
Study. Diabetes Care 1997 Apr;20(4):537-44.
- Eriksson J, Lindstrom J, Valle T, Aunola S,
Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Lauhkonen M,
Lehto P, et al. Prevention of Type II diabetes in subjects with impaired
glucose tolerance: the Diabetes Prevention Study (DPS) in Finland. Study design
and 1-year interim report on the feasibility of the lifestyle intervention
programme. Diabetologia 1999 Jul;42(7):793-801.
- Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT,
Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta
A, Rastas M, et al. Prevention of type 2 diabetes mellitus by changes in
lifestyle among subjects with impaired glucose tolerance. N.Engl.J.Med. 2001
May 3;344(18):1343-50.
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman
RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2
diabetes with lifestyle intervention or metformin. N.Engl.J.Med. 2002 Feb
7;346(6):393-403.
- Hamman RF, Wing RR, Edelstein SL, Lachin JM, Bray
GA, Delahanty L, Hoskin M, Kriska AM, Mayer-Davis EJ, Pi-Sunyer X, et al.
Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes
Care 2006 Sep;29(9):2102-7.
- Laaksonen DE, Lindstrom J, Lakka TA, Eriksson JG,
Niskanen L, Wikstrom K, Aunola S, Keinanen-Kiukaanniemi S, Laakso M, Valle TT,
et al. Physical activity in the prevention of type 2 diabetes: the Finnish
diabetes prevention study. Diabetes 2005 Jan;54(1):158-65.
- Lindstrom J, Ilanne-Parikka P, Peltonen M, Aunola
S, Eriksson JG, Hemio K, Hamalainen H, Harkonen P, Keinanen-Kiukaanniemi S,
Laakso M, et al. Sustained reduction in the incidence of type 2 diabetes by
lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study.
Lancet 2006 Nov 11;368(9548):1673-9.
- Schneider SH, Amorosa LF, Khachadurian AK,
Ruderman NB. Studies on the mechanism of improved glucose control during
regular exercise in type 2 (non-insulin-dependent) diabetes. Diabetologia 1984
May;26(5):355-60.
- Regensteiner JG, Bauer TA, Reusch JE, Brandenburg
SL, Sippel JM, Vogelsong AM, Smith S, Wolfel EE, Eckel RH, Hiatt WR. Abnormal
oxygen uptake kinetic responses in women with type II diabetes mellitus.
J.Appl.Physiol 1998 Jul;85(1):310-7.
- Poirier P, Bogaty P, Garneau C, Marois L,
Dumesnil JG. Diastolic dysfunction in normotensive men with well-controlled
type 2 diabetes: importance of maneuvers in echocardiographic screening for
preclinical diabetic cardiomyopathy. Diabetes Care 2001 Jan;24(1):5-10.
- Regensteiner JG, Groves BM, Bauer TA, Reusch JEB,
Smith SC, Wolfel EE. Recently diagnosed type 2 diabetes mellitus adversely
affects cardiac function during exercise. Diabetes 2002;51(Suppl 2):A59.
- Bauer TA, Reusch JE, Levi M, Regensteiner JG.
Skeletal muscle deoxygenation after the onset of moderate exercise suggests
slowed microvascular blood flow kinetics in type 2 diabetes. Diabetes Care 2007
Nov;30(11):2880-5.
- Devlin JT, Hirshman M, Horton ED, Horton ES.
Enhanced peripheral and splanchnic insulin sensitivity in NIDDM men after
single bout of exercise. Diabetes 1987 Apr;36(4):434-9.
- Thomas DE, Elliott EJ, Naughton GA. Exercise for
type 2 diabetes mellitus. Cochrane.Database.Syst.Rev. 2006;3:CD002968.
- Brandenburg SL, Reusch JE, Bauer TA, Jeffers BW,
Hiatt WR, Regensteiner JG. Effects of exercise training on oxygen uptake
kinetic responses in women with type 2 diabetes. Diabetes Care 1999
Oct;22(10):1640-6.
- Wilmore JH, Green JS, Stanforth PR, Gagnon J,
Rankinen T, Leon AS, Rao DC, Skinner JS, Bouchard C. Relationship of changes in
maximal and submaximal aerobic fitness to changes in cardiovascular disease and
non-insulin-dependent diabetes mellitus risk factors with endurance training:
the HERITAGE Family Study. Metabolism 2001 Nov;50(11):1255-63.
- Gaillard TR, Sherman WM, Devor ST, Kirby TE, Osei
K. Importance of aerobic fitness in cardiovascular risks in sedentary
overweight and obese African-American women. Nurs.Res. 2007
Nov;56(6):407-15.
- National Institute of Diabetes and Digestive and
Kidney Disease (NIDDK), NIH. Diabetes Prevention Program 2002 Available from
http://diabetes.niddk.nih.gov/dm/pubs/preventionprogram/.
- Orchard TJ, Temprosa M, Goldberg R, Haffner S,
Ratner R, Marcovina S, Fowler S. The effect of metformin and intensive
lifestyle intervention on the metabolic syndrome: the Diabetes Prevention
Program randomized trial. Ann.Intern.Med. 2005 Apr 19;142(8):611-9.
- Ramachandran A, Snehalatha C, Mary S, Mukesh B,
Bhaskar AD, Vijay V. The Indian Diabetes Prevention Programme shows that
lifestyle modification and metformin prevent type 2 diabetes in Asian Indian
subjects with impaired glucose tolerance (IDPP-1). Diabetologia 2006
Feb;49(2):289-97.
- Kosaka K, Noda M, Kuzuya T. Prevention of type 2
diabetes by lifestyle intervention: a Japanese trial in IGT males. Diabetes
Res.Clin.Pract. 2005 Feb;67(2):152-62.
- Rodriguez BL, Fujimoto WY, Mayer-Davis EJ,
Imperatore G, Williams DE, Bell RA, Wadwa RP, Palla SL, Liu LL, Kershnar A, et
al. Prevalence of cardiovascular disease risk factors in U.S. children and
adolescents with diabetes: the SEARCH for diabetes in youth study. Diabetes
Care 2006 Aug;29(8):1891-6.
- Gungor N, Thompson T, Sutton-Tyrrell K, Janosky
J, Arslanian S. Early signs of cardiovascular disease in youth with obesity and
type 2 diabetes. Diabetes Care 2005 May;28(5):1219-21.
- Ribeiro MM, Silva AG, Santos NS, Guazzelle I,
Matos LN, Trombetta IC, Halpern A, Negrao CE, Villares SM. Diet and exercise
training restore blood pressure and vasodilatory responses during physiological
maneuvers in obese children. Circulation 2005 Apr 19;111(15):1915-23.
- Nassis GP, Papantakou K, Skenderi K,
Triandafillopoulou M, Kavouras SA, Yannakoulia M, Chrousos GP, Sidossis LS.
Aerobic exercise training improves insulin sensitivity without changes in body
weight, body fat, adiponectin, and inflammatory markers in overweight and obese
girls. Metabolism 2005 Nov;54(11):1472-9.
- Carrel AL, Clark RR, Peterson SE, Nemeth BA,
Sullivan J, Allen DB. Improvement of fitness, body composition, and insulin
sensitivity in overweight children in a school-based exercise program: a
randomized, controlled study. Arch.Pediatr.Adolesc.Med. 2005
Oct;159(10):963-8.
- Nwobu CO, Johnson CC. Targeting obesity to
reduce the risk for type 2 diabetes and other co-morbidities in African
American youth: a review of the literature and recommendations for prevention.
Diab.Vasc.Dis.Res. 2007 Dec;4(4):311-9.
- Sallis JF, McKenzie TL, Alcaraz JE, Kolody B,
Faucette N, Hovell MF. The effects of a 2-year physical education program
(SPARK) on physical activity and fitness in elementary school students. Sports,
Play and Active Recreation for Kids. Am.J.Public Health 1997
Aug;87(8):1328-34.
- Pangrazi RP, Beighle A, Vehige T, Vack C. Impact
of Promoting Lifestyle Activity for Youth (PLAY) on children's physical
activity. J.Sch Health 2003 Oct;73(8):317-21.
- McGavock J, Sellers E, Dean H. Physical activity
for the prevention and management of youth-onset type 2 diabetes mellitus:
focus on cardiovascular complications. Diab.Vasc.Dis.Res. 2007
Dec;4(4):305-10.
- Crespo CJ, Smit E, Troiano RP, Bartlett SJ,
Macera CA, Andersen RE. Television watching, energy intake, and obesity in US
children: results from the third National Health and Nutrition Examination
Survey, 1988-1994. Arch.Pediatr.Adolesc.Med. 2001 Mar;155(3):360-5.
- Berkey CS, Rockett HR, Gillman MW, Colditz GA.
One-year changes in activity and in inactivity among 10- to 15-year-old boys
and girls: relationship to change in body mass index. Pediatrics 2003 Apr;111(4
Pt 1):836-43.
- Zeitler P, Epstein L, Grey M, Hirst K, Kaufman
F, Tamborlane W, Wilfley D. Treatment options for type 2 diabetes in
adolescents and youth: a study of the comparative efficacy of metformin alone
or in combination with rosiglitazone or lifestyle intervention in adolescents
with type 2 diabetes. Pediatr.Diabetes 2007 Apr;8(2):74-87.
- Castaneda C, Layne JE, Munoz-Orians L, Gordon
PL, Walsmith J, Foldvari M, Roubenoff R, Tucker KL, Nelson ME. A randomized
controlled trial of resistance exercise training to improve glycemic control in
older adults with type 2 diabetes. Diabetes Care 2002 Dec;25(12):2335-41.
- Dunstan DW, Daly RM, Owen N, Jolley D, De Court,
Shaw J, Zimmet P. High-intensity resistance training improves glycemic control
in older patients with type 2 diabetes. Diabetes Care 2002
Oct;25(10):1729-36.
- Sigal RJ, Kenny GP, Boule NG, Wells GA,
Prud'homme D, Fortier M, Reid RD, Tulloch H, Coyle D, Phillips P, et al.
Effects of aerobic training, resistance training, or both on glycemic control
in type 2 diabetes: a randomized trial. Ann.Intern.Med. 2007 Sep
18;147(6):357-69.
- Sigal RJ, Kenny GP, Wasserman DH,
Castaneda-Sceppa C, White RD. Physical activity/exercise and type 2 diabetes: a
consensus statement from the American Diabetes Association. Diabetes Care 2006
Jun;29(6):1433-8.
- Flood L, Constance A. Diabetes and exercise
safety. Am.J.Nurs. 2002 Jun;102(6):47-55.
- Blair SN, Kohl HW, III, Paffenbarger RS, Jr.,
Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A
prospective study of healthy men and women. JAMA 1989 Nov
3;262(17):2395-401.
- Gregg EW, Gerzoff RB, Caspersen CJ, Williamson
DF, Narayan KM. Relationship of walking to mortality among US adults with
diabetes. Arch.Intern.Med. 2003 Jun 23;163(12):1440-7.
- Tanasescu M, Leitzmann MF, Rimm EB, Hu FB.
Physical activity in relation to cardiovascular disease and total mortality
among men with type 2 diabetes. Circulation 2003 May 20;107(19):2435-9.
- Hu G, Eriksson J, Barengo NC, Lakka TA, Valle
TT, Nissinen A, Jousilahti P, Tuomilehto J. Occupational, commuting, and
leisure-time physical activity in relation to total and cardiovascular
mortality among Finnish subjects with type 2 diabetes. Circulation 2004 Aug
10;110(6):666-73.
- Hu G, Jousilahti P, Barengo NC, Qiao Q, Lakka
TA, Tuomilehto J. Physical activity, cardiovascular risk factors, and mortality
among Finnish adults with diabetes. Diabetes Care 2005 Apr;28(4):799-805.
- Snowling NJ, Hopkins WG. Effects of different
modes of exercise training on glucose control and risk factors for
complications in type 2 diabetic patients: a meta-analysis. Diabetes Care 2006
Nov;29(11):2518-27.
- Kelley GA, Kelley KS. Effects of aerobic
exercise on lipids and lipoproteins in adults with type 2 diabetes: a
meta-analysis of randomized-controlled trials. Public Health 2007
Sep;121(9):643-55.
- Zoppini G, Targher G, Zamboni C, Venturi C,
Cacciatori V, Moghetti P, Muggeo M. Effects of moderate-intensity exercise
training on plasma biomarkers of inflammation and endothelial dysfunction in
older patients with type 2 diabetes. Nutr.Metab Cardiovasc.Dis. 2006
Dec;16(8):543-9.
- Kelley DE. Action for health in diabetes: the
look AHEAD clinical trial. Curr.Diab.Rep. 2002 Jun;2(3):207-9.
- Ryan DH, Espeland MA, Foster GD, Haffner SM,
Hubbard VS, Johnson KC, Kahn SE, Knowler WC, Yanovski SZ. Look AHEAD (Action
for Health in Diabetes): design and methods for a clinical trial of weight loss
for the prevention of cardiovascular disease in type 2 diabetes. Control
Clin.Trials 2003 Oct;24(5):610-28.
- Wadden TA, West DS, Delahanty L, Jakicic J,
Rejeski J, Williamson D, Berkowitz RI, Kelley DE, Tomchee C, Hill JO, et al.
The Look AHEAD study: a description of the lifestyle intervention and the
evidence supporting it. Obesity.(Silver.Spring) 2006 May;14(5):737-52.
- Shinji S, Shigeru M, Ryusei U, Mitsuru M,
Shigehiro K. Adherence to a home-based exercise program and incidence of
cardiovascular disease in type 2 diabetes patients. Int.J.Sports Med. 2007
Oct;28(10):877-9.
- Church TS, LaMonte MJ, Barlow CE, Blair SN.
Cardiorespiratory fitness and body mass index as predictors of cardiovascular
disease mortality among men with diabetes. Arch.Intern.Med. 2005 Oct
10;165(18):2114-20.
- Seyoum B, Estacio RO, Berhanu P, Schrier RW.
Exercise capacity is a predictor of cardiovascular events in patients with type
2 diabetes mellitus. Diab.Vasc.Dis.Res. 2006 Dec;3(3):197-201.
- McAuley PA, Myers JN, Abella JP, Tan SY,
Froelicher VF. Exercise capacity and body mass as predictors of mortality among
male veterans with type 2 diabetes. Diabetes Care 2007 Jun;30(6):1539-43.
- Trichopoulou A, Psaltopoulou T, Orfanos P,
Trichopoulos D. Diet and physical activity in relation to overall mortality
amongst adult diabetics in a general population cohort. J.Intern.Med. 2006
Jun;259(6):583-91.
- Smith TC, Wingard DL, Smith B, Kritz-Silverstein
D, Barrett-Connor E. Walking decreased risk of cardiovascular disease mortality
in older adults with diabetes. J.Clin.Epidemiol. 2007 Mar;60(3):309-17.
- Batty GD, Shipley MJ, Marmot M, Smith GD.
Physical activity and cause-specific mortality in men with Type 2
diabetes/impaired glucose tolerance: evidence from the Whitehall study.
Diabet.Med. 2002 Jul;19(7):580-8.
- Gill JM, Malkova D. Physical activity, fitness
and cardiovascular disease risk in adults: interactions with insulin resistance
and obesity. Clin.Sci.(Lond) 2006 Apr;110(4):409-25.
- Boule NG, Kenny GP, Haddad E, Wells GA, Sigal
RJ. Meta-analysis of the effect of structured exercise training on
cardiorespiratory fitness in Type 2 diabetes mellitus. Diabetologia 2003
Aug;46(8):1071-81.
- Liese AD, D'Agostino RB, Jr., Hamman RF, Kilgo
PD, Lawrence JM, Liu LL, Loots B, Linder B, Marcovina S, Rodriguez B, et al.
The burden of diabetes mellitus among US youth: prevalence estimates from the
SEARCH for Diabetes in Youth Study. Pediatrics 2006 Oct;118(4):1510-8.
- Moy CS, Songer TJ, LaPorte RE, Dorman JS, Kriska
AM, Orchard TJ, Becker DJ, Drash AL. Insulin-dependent diabetes mellitus,
physical activity, and death. Am.J.Epidemiol. 1993 Jan 1;137(1):74-81.
- Williams KV, Erbey JR, Becker D, Arslanian S,
Orchard TJ. Can clinical factors estimate insulin resistance in type 1
diabetes? Diabetes 2000 Apr;49(4):626-32.
- Schauer IE, Bergman SJ, Maahs DM, Kretowski A,
Eckel RH, Rewers M. Insulin sensitivity and free fatty acid supression differ
in persons with type 1 diabetes compared to non-diabetic controls: the cacti
study. Diabetes 2008;In Press.
- Wallberg-Henriksson H, Gunnarsson R, Henriksson
J, DeFronzo R, Felig P, Ostman J, Wahren J. Increased peripheral insulin
sensitivity and muscle mitochondrial enzymes but unchanged blood glucose
control in type I diabetics after physical training. Diabetes 1982
Dec;31(12):1044-50.
- Zinman B, Zuniga-Guajardo S, Kelly D. Comparison
of the acute and long-term effects of exercise on glucose control in type I
diabetes. Diabetes Care 1984 Nov;7(6):515-9.
- Wallberg-Henriksson H, Gunnarsson R, Rossner S,
Wahren J. Long-term physical training in female type 1 (insulin-dependent)
diabetic patients: absence of significant effect on glycaemic control and
lipoprotein levels. Diabetologia 1986 Jan;29(1):53-7.
- Huttunen NP, Lankela SL, Knip M, Lautala P, Kaar
ML, Laasonen K, Puukka R. Effect of once-a-week training program on physical
fitness and metabolic control in children with IDDM. Diabetes Care 1989
Nov;12(10):737-40.
- Roberts L, Jones TW, Fournier PA. Exercise
training and glycemic control in adolescents with poorly controlled type 1
diabetes mellitus. J.Pediatr.Endocrinol.Metab 2002 May;15(5):621-7.
- Ramalho AC, de Lourdes LM, Nunes F, Cambui Z,
Barbosa C, Andrade A, Viana A, Martins M, Abrantes V, Aragao C, et al. The
effect of resistance versus aerobic training on metabolic control in patients
with type-1 diabetes mellitus. Diabetes Res.Clin.Pract. 2006
Jun;72(3):271-6.
- Harmer AR, Chisholm DJ, McKenna MJ, Morris NR,
Thom JM, Bennett G, Flack JR. High-intensity training improves plasma glucose
and acid-base regulation during intermittent maximal exercise in type 1
diabetes. Diabetes Care 2007 May;30(5):1269-71.
- Peterson CM, Jones RL, Esterly JA, Wantz GE,
Jackson RL. Changes in basement membrane thickening and pulse volume
concomitant with improved glucose control and exercise in patients with
insulin-dependent diabetes mellitus. Diabetes Care 1980 Sep;3(5):586-9.
- Campaigne BN, Gilliam TB, Spencer ML, Lampman
RM, Schork MA. Effects of a physical activity program on metabolic control and
cardiovascular fitness in children with insulin-dependent diabetes mellitus.
Diabetes Care 1984 Jan;7(1):57-62.
- Bak JF, Jacobsen UK, Jorgensen FS, Pedersen O.
Insulin receptor function and glycogen synthase activity in skeletal muscle
biopsies from patients with insulin-dependent diabetes mellitus: effects of
physical training. J.Clin.Endocrinol.Metab 1989 Jul;69(1):158-64.
- Durak EP, Jovanovic-Peterson L, Peterson CM.
Randomized crossover study of effect of resistance training on glycemic
control, muscular strength, and cholesterol in type I diabetic men. Diabetes
Care 1990 Oct;13(10):1039-43.
- Ligtenberg PC, Blans M, Hoekstra JB, van dT, I,
Erkelens DW. No effect of long-term physical activity on the glycemic control
in type 1 diabetes patients: a cross-sectional study. Neth.J.Med. 1999
Aug;55(2):59-63.
- Waden J, Tikkanen H, Forsblom C, Fagerudd J,
Pettersson-Fernholm K, Lakka T, Riska M, Groop PH. Leisure time physical
activity is associated with poor glycemic control in type 1 diabetic women: the
FinnDiane study. Diabetes Care 2005 Apr;28(4):777-82.
- Herbst A, Kordonouri O, Schwab KO, Schmidt F,
Holl RW. Impact of physical activity on cardiovascular risk factors in children
with type 1 diabetes: a multicenter study of 23,251 patients. Diabetes Care
2007 Aug;30(8):2098-100.
- Waden J, Thorn LM, Forsblom C, Lakka T,
Saraheimo M, Rosengard-Barlund M, Heikkila O, Wessman M, Turunen JA, Parkkonen
M, et al. Leisure-time physical activity is associated with the metabolic
syndrome in type 1 diabetes: effect of the PPARgamma Pro12Ala polymorphism: the
FinnDiane Study. Diabetes Care 2007 Jun;30(6):1618-20.
- Lehmann R, Kaplan V, Bingisser R, Bloch KE,
Spinas GA. Impact of physical activity on cardiovascular risk factors in IDDM.
Diabetes Care 1997 Oct;20(10):1603-11.
- Zinman B, Ruderman N, Campaigne BN, Devlin JT,
Schneider SH. Physical activity/exercise and diabetes. Diabetes Care 2004
Jan;27 Suppl 1:S58-S62.
- Tsalikian E, Kollman C, Tamborlane WB, Beck RW,
Fiallo-Scharer R, Fox L, Janz KF, Ruedy KJ, Wilson D, Xing D, et al. Prevention
of hypoglycemia during exercise in children with type 1 diabetes by suspending
basal insulin. Diabetes Care 2006 Oct;29(10):2200-4.
- Rabasa-Lhoret R, Bourque J, Ducros F, Chiasson
JL. Guidelines for premeal insulin dose reduction for postprandial exercise of
different intensities and durations in type 1 diabetic subjects treated
intensively with a basal-bolus insulin regimen (ultralente-lispro). Diabetes
Care 2001 Apr;24(4):625-30.
- Grimm JJ, Ybarra J, Berne C, Muchnick S, Golay
A. A new table for prevention of hypoglycaemia during physical activity in type
1 diabetic patients. Diabetes Metab 2004 Nov;30(5):465-70.
- Guelfi KJ, Jones TW, Fournier PA. The decline in
blood glucose levels is less with intermittent high-intensity compared with
moderate exercise in individuals with type 1 diabetes. Diabetes Care 2005
Jun;28(6):1289-94.
- Bussau VA, Ferreira LD, Jones TW, Fournier PA.
The 10-s maximal sprint: a novel approach to counter an exercise-mediated fall
in glycemia in individuals with type 1 diabetes. Diabetes Care 2006
Mar;29(3):601-6.
- Bussau VA, Ferreira LD, Jones TW, Fournier PA. A
10-s sprint performed prior to moderate-intensity exercise prevents early
post-exercise fall in glycaemia in individuals with type 1 diabetes.
Diabetologia 2007 Sep;50(9):1815-8.
- Guelfi KJ, Ratnam N, Smythe GA, Jones TW,
Fournier PA. Effect of intermittent high-intensity compared with continuous
moderate exercise on glucose production and utilization in individuals with
type 1 diabetes. Am.J.Physiol Endocrinol.Metab 2007 Mar;292(3):E865-E870.
- Guelfi KJ, Jones TW, Fournier PA. New insights
into managing the risk of hypoglycaemia associated with intermittent
high-intensity exercise in individuals with type 1 diabetes mellitus:
implications for existing guidelines. Sports Med. 2007;37(11):937-46.
- The effect of intensive treatment of diabetes on
the development and progression of long-term complications in insulin-dependent
diabetes mellitus. The Diabetes Control and Complications Trial Research Group.
N.Engl.J.Med. 1993 Sep 30;329(14):977-86.
- Intensive blood-glucose control with
sulphonylureas or insulin compared with conventional treatment and risk of
complications in patients with type 2 diabetes (UKPDS 33). UK Prospective
Diabetes Study (UKPDS) Group. Lancet 1998 Sep 12;352(9131):837-53.
- Balducci S, Iacobellis G, Parisi L, Di BN,
Calandriello E, Leonetti F, Fallucca F. Exercise training can modify the
natural history of diabetic peripheral neuropathy. J.Diabetes Complications
2006 Jul;20(4):216-23.
- Kriska AM, LaPorte RE, Patrick SL, Kuller LH,
Orchard TJ. The association of physical activity and diabetic complications in
individuals with insulin-dependent diabetes mellitus: the Epidemiology of
Diabetes Complications Study--VII. J.Clin.Epidemiol. 1991;44(11):1207-14.
- Orchard TJ, Dorman JS, Maser RE, Becker DJ,
Ellis D, LaPorte RE, Kuller LH, Wolfson SK, Jr., Drash AL. Factors associated
with avoidance of severe complications after 25 yr of IDDM. Pittsburgh
Epidemiology of Diabetes Complications Study I. Diabetes Care 1990
Jul;13(7):741-7.
- Smith AG, Russell J, Feldman EL, Goldstein J,
Peltier A, Smith S, Hamwi J, Pollari D, Bixby B, Howard J, et al. Lifestyle
intervention for pre-diabetic neuropathy. Diabetes Care 2006
Jun;29(6):1294-9.
- Colberg SR, Parson HK, Nunnold T, Holton DR,
Swain DP, Vinik AI. Change in cutaneous perfusion following 10 weeks of aerobic
training in Type 2 diabetes. J.Diabetes Complications 2005
Sep;19(5):276-83.
- Colberg SR, Parson HK, Nunnold T, Herriott MT,
Vinik AI. Effect of an 8-week resistance training program on cutaneous
perfusion in type 2 diabetes. Microvasc.Res. 2006 Mar;71(2):121-7.
- Colberg SR, Stansberry KB, McNitt PM, Vinik AI.
Chronic exercise is associated with enhanced cutaneous blood flow in type 2
diabetes. J.Diabetes Complications 2002 Mar;16(2):139-45.
- Colberg SR, Parson HK, Holton DR, Nunnold T,
Vinik AI. Cutaneous blood flow in type 2 diabetic individuals after an acute
bout of maximal exercise. Diabetes Care 2003 Jun;26(6):1883-8.
- Graham C, Lasko-McCarthey P. Exercise options
for persons with diabetic complications. Diabetes Educ. 1990
May;16(3):212-20.
- Armstrong DG, Lavery LA, Holtz-Neiderer K,
Mohler MJ, Wendel CS, Nixon BP, Boulton AJ. Variability in activity may precede
diabetic foot ulceration. Diabetes Care 2004 Aug;27(8):1980-4.
- Lemaster JW, Reiber GE, Smith DG, Heagerty PJ,
Wallace C. Daily weight-bearing activity does not increase the risk of diabetic
foot ulcers. Med.Sci.Sports Exerc. 2003 Jul;35(7):1093-9.
- Giacomozzi C, Caselli A, Macellari V, Giurato L,
Lardieri L, Uccioli L. Walking strategy in diabetic patients with peripheral
neuropathy. Diabetes Care 2002 Aug;25(8):1451-7.
- Kanade RV, van Deursen RW, Harding K, Price P.
Walking performance in people with diabetic neuropathy: benefits and threats.
Diabetologia 2006 Aug;49(8):1747-54.
- Dingwell JB, Cusumano JP, Sternad D, Cavanagh
PR. Slower speeds in patients with diabetic neuropathy lead to improved local
dynamic stability of continuous overground walking. J.Biomech. 2000
Oct;33(10):1269-77.
- Dingwell JB, Cavanagh PR. Increased variability
of continuous overground walking in neuropathic patients is only indirectly
related to sensory loss. Gait.Posture. 2001 Jul;14(1):1-10.
- Dingwell JB, Kang HG, Marin LC. The effects of
sensory loss and walking speed on the orbital dynamic stability of human
walking. J.Biomech. 2007;40(8):1723-30.
- Mueller MJ, Minor SD, Schaaf JA, Strube MJ,
Sahrmann SA. Relationship of plantar-flexor peak torque and dorsiflexion range
of motion to kinetic variables during walking. Phys.Ther. 1995
Aug;75(8):684-93.
- Novak P, Burger H, Marincek C, Meh D. Influence
of foot pain on walking ability of diabetic patients. J.Rehabil.Med. 2004
Nov;36(6):249-52.
- Wallberg-Henriksson H, Rincon J, Zierath JR.
Exercise in the management of non-insulin-dependent diabetes mellitus. Sports
Med. 1998 Jan;25(1):25-35.
- Samanta A, Burden AC, Jagger C. A comparison of
the clinical features and vascular complications of diabetes between migrant
Asians and Caucasians in Leicester, U.K. Diabetes Res.Clin.Pract. 1991
Dec;14(3):205-13.
- Hotta O, Taguma Y, Mitsuoka M, Takeshita K,
Takahashi H. Urinary albumin excretion in patients with non-insulin-dependent
diabetes mellitus in an early microalbuminuric stage. Nephron
1991;58(1):23-6.
- Garg SK, Chase HP, Shapiro H, Harris S, Osberg
IM. Exercise versus overnight albumin excretion rates in subjects with type 1
diabetes. Diabetes Res.Clin.Pract. 1995 Apr;28(1):51-5.
- Groop L, Stenman S, Groop PH, Makipernaa A,
Teppo AM. The effect of exercise on urinary excretion of different size
proteins in patients with insulin-dependent diabetes mellitus. Scand.J.Clin.Lab
Invest 1990 Sep;50(5):525-32.
- Matsuoka K, Nakao T, Atsumi Y, Takekoshi H.
Exercise regimen for patients with diabetic nephropathy. J.Diabet.Complications
1991 Apr;5(2-3):98-100.
- Morgensen CE. Nephropathy: early. In: Ruderman
N, Devlin JT, Schneider SH, et al., editors. Handbook of Exercise in Diabetes.
Alexandria, VA: American Diabetes Association; 2002. p. 433-49.
- Fredrickson SK, Ferro TJ, Schutrumpf AC.
Disappearance of microalbuminuria in a patient with type 2 diabetes and the
metabolic syndrome in the setting of an intense exercise and dietary program
with sustained weight reduction. Diabetes Care 2004 Jul;27(7):1754-5.
- Lazarevic G, Antic S, Vlahovic P, Djordjevic V,
Zvezdanovic L, Stefanovic V. Effects of aerobic exercise on microalbuminuria
and enzymuria in type 2 diabetic patients. Ren Fail. 2007;29(2):199-205.
- Cruickshanks KJ, Moss SE, Klein R, Klein BE.
Physical activity and the risk of progression of retinopathy or the development
of proliferative retinopathy. Ophthalmology 1995 Aug;102(8):1177-82.
- Cruickshanks KJ, Moss SE, Klein R, Klein BE.
Physical activity and proliferative retinopathy in people diagnosed with
diabetes before age 30 yr. Diabetes Care 1992 Oct;15(10):1267-72.
- LaPorte RE, Dorman JS, Tajima N, Cruickshanks
KJ, Orchard TJ, Cavender DE, Becker DJ, Drash AL. Pittsburgh Insulin-Dependent
Diabetes Mellitus Morbidity and Mortality Study: physical activity and diabetic
complications. Pediatrics 1986 Dec;78(6):1027-33.
- Anderson B, Jr. Activity and diabetic vitreous
hemorrhages. Ophthalmology 1980 Mar;87(3):173-5.
- Bernbaum M, Albert SG, Cohen JD, Drimmer A.
Cardiovascular conditioning in individuals with diabetic retinopathy. Diabetes
Care 1989 Nov;12(10):740-2.
- Albert SG, Bernbaum M. Exercise for patients
with diabetic retinopathy. Diabetes Care 1995 Jan;18(1):130-2.
- Stevens RJ, Kothari V, Adler AI, Stratton IM.
The UKPDS risk engine: a model for the risk of coronary heart disease in Type
II diabetes (UKPDS 56). Clin.Sci.(Lond) 2001 Dec;101(6):671-9.
- Summary of revisions for the 2006 Clinical
Practice Recommendations. Diabetes Care 2006 Jan;29 Suppl 1:S3.
- Dempsey JC, Butler CL, Williams MA. No need for
a pregnant pause: physical activity may reduce the occurrence of gestational
diabetes mellitus and preeclampsia. Exerc.Sport Sci.Rev. 2005
Jul;33(3):141-9.
- Getahun D, Nath C, Ananth CV, Chavez MR, Smulian
JC. Gestational diabetes in the United States: temporal trends 1989 through
2004. Am.J.Obstet.Gynecol. 2008 Feb 15.
- Linne Y. Effects of obesity on women's
reproduction and complications during pregnancy. Obes.Rev. 2004
Aug;5(3):137-43.
- Kelly C, Booth GL. Diabetes in Canadian Women.
BMC.Womens Health 2004 Aug 25;4 Suppl 1:S16.
- Jovanovic L. What is so bad about a big baby?
Diabetes Care 2001 Aug;24(8):1317-8.
- Schaefer-Graf UM, Kleinwechter H. Diagnosis and
new approaches in the therapy of gestational diabetes mellitus. Curr.Diabetes
Rev. 2006 Aug;2(3):343-52.
top of page
Note: Documents in PDF format require the
Adobe Acrobat Reader®.
If you experience problems with PDF documents, please
download the latest version of the Reader®.
Continue to G4. Energy
Back to G2. Cardiorespiratory Health
Back to Physical Activity Guidelines Advisory Committee Report
|