 |

Molecular Profiling Can Accurately Diagnose Burkitt's Lymphoma
Researchers have identified genetic signatures for distinguishing between two types of lymphomas: Burkitt's and diffuse large-B-cell (DLBCL). This distinction is critical because patients with Burkitt's lymphoma require more intense chemotherapy than those with DLBCL. Burkitt's lymphoma is fatal if untreated, and accurate diagnosis will have a major effect on these patients' prognosis.
Burkitt's lymphoma and DLBCL cells look similar under the microscope, and diagnosis can be difficult using conventional pathological methods. A study in the June 8 New England Journal of Medicine describes how gene-expression microarray technology can improve diagnosis of Burkitt's lymphoma.
The study resulted from the collaboration between NCI investigators and the multinational team of researchers in the Lymphoma/Leukemia Molecular Profiling Project. A panel of expert hematopathologists began by reevaluating samples that had been previously diagnosed as Burkitt's lymphoma or atypical Burkitt's lymphoma using available pathological methods. They reclassified a number of samples (originally diagnosed as Burkitt's lymphoma) as DLBCL or high-grade lymphomas, thus demonstrating the difficulty in making an accurate diagnosis.
Read more 


Taking Pride in an Important Achievement
An important public health milestone was reached last week when FDA approved a vaccine that prevents infection by the two types of the human papillomavirus (HPV) responsible for up to 70 percent of cervical cancer cases worldwide, HPV 16 and HPV 18, as well as two other HPV types, HPV 6 and HPV 11, that cause benign genital warts.
Our nation's strong commitment and investment in cancer research at NCI led to this approval, something in which we all can take great pride. NCI investigators throughout the institute were involved in the discovery that HPV causes cervical cancer. Most prominent among NCI scientists working on this project were Drs. Douglas Lowy and John Schiller of the Center for Cancer Research (CCR). They also were instrumental in the discovery of the virus-like particle technology that led to the vaccine's development.
Called Gardasil and manufactured by Merck & Co., this vaccine will provide a significant boost to NCI's efforts to reduce the burden of disease by eliminating the need for invasive procedures to remove many precancerous lesions. But its impact will likely be greatest in developing countries where a lack of public health infrastructure and screening programs results in hundreds of thousands of lives lost each year to cervical cancer.
Read more 
 |
The NCI Cancer Bulletin is produced by the National Cancer Institute (NCI). NCI, which was established in 1937, leads the national effort to eliminate the suffering and death due to cancer. Through basic, clinical, and population-based biomedical research and training, NCI conducts and supports research that will lead to a future in which we can identify the environmental and genetic causes of cancer, prevent cancer before it starts, identify cancers that do develop at the earliest stage, eliminate cancers through innovative treatment interventions, and biologically control those cancers that we cannot eliminate so they become manageable, chronic diseases.

For more information on cancer, call 1-800-4-CANCER or visit http://www.cancer.gov.

NCI Cancer Bulletin staff can be reached at ncicancerbulletin@mail.nih.gov.
|
|
 |
 |

Molecular Profiling Can Accurately Diagnose Burkitt's Lymphoma
Researchers have identified genetic signatures for distinguishing between two types of lymphomas: Burkitt's and diffuse large-B-cell (DLBCL). This distinction is critical because patients with Burkitt's lymphoma require more intense chemotherapy than those with DLBCL. Burkitt's lymphoma is fatal if untreated, and accurate diagnosis will have a major effect on these patients' prognosis.
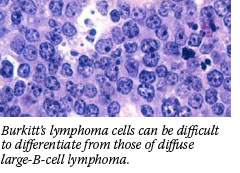 Burkitt's lymphoma and DLBCL cells look similar under the microscope, and diagnosis can be difficult using conventional pathological methods. A study in the June 8 New England Journal of Medicine describes how gene-expression microarray technology can improve diagnosis of Burkitt's lymphoma. Burkitt's lymphoma and DLBCL cells look similar under the microscope, and diagnosis can be difficult using conventional pathological methods. A study in the June 8 New England Journal of Medicine describes how gene-expression microarray technology can improve diagnosis of Burkitt's lymphoma.
The study resulted from the collaboration between NCI investigators and the multinational team of researchers in the Lymphoma/Leukemia Molecular Profiling Project. A panel of expert hematopathologists began by reevaluating samples that had been previously diagnosed as Burkitt's lymphoma or atypical Burkitt's lymphoma using available pathological methods. They reclassified a number of samples (originally diagnosed as Burkitt's lymphoma) as DLBCL or high-grade lymphomas, thus demonstrating the difficulty in making an accurate diagnosis.
All samples were then analyzed using DNA gene-expression microarrays. All of the samples classified as Burkitt's lymphoma by the panel were validated as such by gene-expression analysis. However, several samples reclassified as DLBCL by the panel were shown to be Burkitt's by microarray analysis. Overall, the study suggests that 17 percent of the patients who were diagnosed with Burkitt's lymphoma by gene-expression analysis may have been misdiagnosed using standard pathological methods.
"The value of molecular profiling to accurately diagnosis Burkitt's lymphoma versus DLBCL will have a major impact on patients because the treatment for these two lymphomas is very different," said Dr. Louis Staudt, deputy chief of the Metabolism Branch at NCI's CCR and study co-leader. "If Burkitt's patients are treated with intensive therapy, there is roughly an 80-percent survival rate. However, if they are misdiagnosed with DLBCL, and treated with lower intensity chemotherapy, the survival rate is reversed to 20 percent or even less."
An editorial, by Drs. Nancy Lee Harris and Sandra J. Horning, concludes that gene-expression analysis identifies a characteristic genetic signature of Burkitt's lymphoma that clearly distinguishes it from DLBCL. Furthermore, they note, the microarray methods seem to "outperform" the expert pathologists.
"Although these results are experimental and the diagnostic techniques are a long way from use on patients, they show the usefulness of gene-expression microarrays for aiding the diagnosis and prognosis of these cancers," said Dr. Staudt. "In the future, knowing the genetic alterations in a cancer will allow us to tailor therapies to match the individual needs of each patient."
By Lynette Grouse
|
 |
 |

Taking Pride in an Important Achievement
An important public health milestone was reached last week when FDA approved a vaccine that prevents infection by the two types of the human papillomavirus (HPV) responsible for up to 70 percent of cervical cancer cases worldwide, HPV 16 and HPV 18, as well as two other HPV types, HPV 6 and HPV 11, that cause benign genital warts.
Our nation's strong commitment and investment in cancer research at NCI led to this approval, something in which we all can take great pride. NCI investigators throughout the institute were involved in the discovery that HPV causes cervical cancer. Most prominent among NCI scientists working on this project were Drs. Douglas Lowy and John Schiller of the Center for Cancer Research (CCR). They also were instrumental in the discovery of the virus-like particle technology that led to the vaccine's development.
Called Gardasil and manufactured by Merck & Co., this vaccine will provide a significant boost to NCI's efforts to reduce the burden of disease by eliminating the need for invasive procedures to remove many precancerous lesions. But its impact will likely be greatest in developing countries where a lack of public health infrastructure and screening programs results in hundreds of thousands of lives lost each year to cervical cancer.
There is much more to come in our efforts against cervical cancer. At the policy level, the Centers for Disease Control and Prevention's (CDC's) Advisory Committee on Immunization Practices (ACIP), an independent panel of scientific advisors that offers guidance to CDC on the development of immunization guidelines, is expected to make its recommendation on Gardasil's use at its next meeting on June 29.
Critical research also is ongoing. A second HPV vaccine that also protects against HPV 16 and 18, manufactured by GlaxoSmithKline and called Cervarix, is being tested in phase III clinical trials. And NCI's Division of Cancer Epidemiology and Genetics researchers, led by Drs. Alan Hildesheim and Mark Schiffman, in collaboration with investigators in Costa Rica, are conducting a clinical trial in that country to evaluate the safety, efficacy, and other performance characteristics of the HPV type 16 and 18 vaccine.
Continued research is essential because there are important questions that remain to be answered. For instance, although the studies conducted to date indicate that protection lasts at least 4 years, the ultimate duration of immunity is still unknown, so the vaccine trial participants will continue to be followed.
Meanwhile, next-generation HPV vaccines are under development. At the University of Colorado, with support from NCI's Division of Cancer Prevention's Rapid Access to Preventive Intervention Development, or RAPID, program, a vaccine is being developed that protects against HPV 16, the predominant contributor to cervical cancer among HPV types. The vaccine is stable at room temperature, meaning it would not have to be refrigerated for storage or distribution. Human clinical trials of the vaccine could begin in 2007.
Researchers also are developing a vaccine that protects against more HPV types, and NCI is collaborating with other researchers on therapeutic HPV vaccines to prevent cancer among women previously exposed to the virus.
The news of this approval allows us to stress the continued importance of cervical cancer screening. And it provides an opportunity to educate the public about the tremendous work cancer researchers are engaged in to develop preventive and therapeutic cancer vaccines.
The entire cancer community should be elated and proud. This approval is a watershed moment that highlights the very best of biomedical research: the translation of basic and population science into an intervention that will save hundreds of thousands of lives.
Dr. John E. Niederhuber
Acting Director,
National Cancer Institute
|
 |
 |

The Making of a Monoclonal Antibody
In cancer treatment today, three letters have infiltrated the oncology dialect: "mab," as in bevacizumab (Avastin), cetuximab (Erbitux), or trastuzumab (Herceptin). This suffix is reserved for monoclonal antibodies - agents that have become a growing component of the oncologist's arsenal.
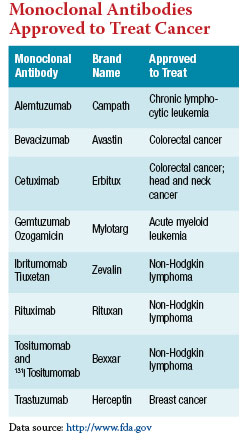
Currently, eight monoclonal antibodies, or MAbs, are approved by the FDA to treat cancer. And many more are being tested in phase II and III clinical trials for a variety of tumor types.
To understand how these drugs work, one must take a careful look at the human immune system. The immune system comprises a diverse collection of specialized cell types that circulate through the bloodstream and lymphatic system, and monitors the body for bacterial, viral, fungal, and parasitic invaders. The "innate" immune response provides the first line of defense, and includes cells such as granulocytes, macrophages, and dendritic cells, which are always ready to recognize and attack foreign particles in the body. For persistent or recurrent infections, the sophisticated "adaptive" immune response comes into play.
Dendritic cells (DCs) form the bridge between the innate immune response and the antibodies of the adaptive immune response. When DCs digest a pathogen, they save and display foreign proteins found on the surface of the pathogen - known as antigens - for the lymphocytes of the adaptive immune system to recognize. When a B lymphocyte (also called a B cell) recognizes an antigen displayed by a DC as foreign, it changes into a plasma cell that secretes antibodies - small proteins that recognize and bind to the antigen, either interfering with the invader's ability to attack the cells of the body or marking them for destruction by other immune cells.
Cancer cells exhibit many of the behaviors of foreign pathogens, such as invading and damaging healthy tissue. However, explains Dr. Robert Kreitman of NCI's CCR, "In general, the immune system recognizes cancer cells as self and does not attack them as foreign antigens." This is because they carry many of the same surface proteins as normal cells, which mark them as part of the body. The development of MAbs circumvented this obstacle.
To create an MAb, researchers first identify a protein that is overexpressed on the surface of cancer cells and that the cells depend on to survive or grow. Trastuzumab, for example, is used to treat breast cancers that overexpress a protein called HER2, and rituximab (Rituxan) is used to treat blood cancers that overexpress the protein CD20. The more important the protein is to the cancer cell, the more effective the MAb will be as a therapy.
"Successful targeting of the HER2 protein has been the reason why Herceptin has been so effective," explains Dr. Charles Geyer, the National Surgical Adjuvant Breast and Bowel Project's director of Medical Affairs. "HER2 gene amplification and the resultant abnormal protein production clearly play a very important, central role in the aggressive malignant phenotype of HER2-positive breast cancers, and Herceptin appears to shut down the activity of the HER2 protein."
Once a target protein is isolated, it is injected into mice. Unlike the human immune system, the mouse immune system recognizes the protein as foreign and produces antibodies to fight it. These mouse antibodies are then isolated, but cannot be used directly as a therapy because they would be recognized as foreign and attacked as invaders if injected into the human body. So they must first go through a process called humanization.
In humanization, the variable domain of the mouse antibody - the very small portion that recognizes the human protein - is removed and grafted onto a human antibody. When introduced back into the human body, the MAb recognizes and binds to the target protein, disrupting a vital signaling pathway in the cancer cell.
Some MAbs are further engineered to carry a toxin or radionuclide, to increase the damage done after the MAb binds to a cancer cell, such as 131 I tositumomab (Bexxar), which is used to treat B-cell lymphoma. Because the MAbs bind predominantly to cancer cells, very few normal cells are damaged either by the antibodies or by their toxic conjugates.
Another class of therapeutic drugs called small - molecule inhibitors also target proteins on the surface of cancer cells. But MAbs are distinct from these agents because they also may play a role in activating an immune response called antibody-dependent cellular cytotoxicity. Researchers are now trying to better understand how MAbs interact with the rest of the immune system and how they can best be used with other treatments to maximize their effectiveness as an anticancer therapy.
"I think that discovering better combinations with other agents and the timing of how to give them to get the best response will be the future," says Dr. Kreitman. "In the 1950s and 1960s, we had chemotherapy drugs, but we didn't know how to combine them, so diseases like Hodgkin's disease were not curable. But as people learned how to combine drugs and do it safely… diseases started becoming curable. I think the same will happen with monoclonal antibodies and other agents."
By Sharon Reynolds
|
 |
 |
 |
 |
Featured Meetings and Events |
 |
|
A calendar of scientific meetings and events sponsored by the National Institutes of Health (NIH) is available at http://calendar.nih.gov.
|
|
Younger Black Women Develop a More Lethal Type of Breast Cancer
The incidence of breast cancer in premenopausal African American women is lower than in their white counterparts, but they are more likely to die from the disease. New findings reported in the June 7 Journal of the American Medical Association determined for the first time the population prevalence of a basal-like subtype of cancer in this group, say researchers, which may help to explain their higher mortality.
The risk of the less treatable, more deadly basal-type breast cancer was 2.1 times greater in African Americans than others: 39 percent in premenopausal African Americans, falling to 14 percent after they reached menopause. Risk for non-African American women was 16 percent, both before and after menopause.
Dr. Lisa A. Carey of the University of North Carolina at Chapel Hill and colleagues found the pattern by analyzing a subgroup of 469 women participating in the Carolina Breast Cancer Study, a population-based case-control study designed to look at molecular and environmental determinants of breast cancer risk. Study participants were sorted into two groups: those identifying themselves as African Americans and all others.
Immunohistochemical analysis of tumors was used to identify four main subtypes of breast cancer, which were then adjusted by age, race, and stage of cancer. Compared with the least threatening type, all women in the study had an 80-percent increase in mortality with the basal-like cancer. When these cases were removed from the analysis, however, younger African American women still had the highest mortality, "which may reflect the impact on prognosis of access to care, treatment, or other differences," wrote the authors.
Bevacizumab plus Chemotherapy Promising for Recurrent
Brain Cancer
Malignant glioma (MG), a type of brain tumor, carries a grim prognosis. Adjuvant treatment with radiation therapy and the drug temozolomide (Temodar) provides a small increase in survival; however, the disease almost always returns. A phase II study, presented at the ASCO annual meeting, of the anti-angiogenesis agent bevacizumab (Avastin) combined with irinotecan showed promising results in patients with recurrent MG.
The investigators enrolled 32 patients who had previously received radiation therapy and chemotherapy for their cancer. Four patients had thrombotic complications from the treatment regimen, but no patients developed a central nervous system hemorrhage, which had originally been a concern with the use of bevacizumab. Four patients withdrew or were removed from the study due to other toxicities, and three withdrew to have surgery for other conditions.
The overall response rate was 63 percent, and the median progression-free survival (PFS) was 24 weeks, which was greater than the PFS seen in historical control groups. The median overall survival was 40 weeks. Dr. James Vredenburgh of Duke University Medical Center, who presented the data, explained that the likely reason for the combination's efficacy was that bevacizumab suppressed abnormal tumor blood-vessel growth. This decreased the interstitial pressure around the tumor, which in turn allowed more of the cytotoxic drug to actually reach the cancer cells. The investigators now hope to test bevacizumab as an addition to first-line therapy with radiation and temozolomide for patients with newly diagnosed MG.
Two Follow-Up CT Scans Adequate for Some Testicular Tumors
Computed tomography (CT) scans are an important part of surveillance for men after surgery (orchiectomy) for stage I nonseminomatous germ cell tumors (NSGCT) of the testes. Because CT scans are costly and deliver a substantial amount of radiation to the body, determining the minimum number of postoperative CT scans needed to safely follow patients has been of interest to researchers.
New data from a study presented at the ASCO annual meeting suggest that two postoperative CT scans are as safe for detecting relapse as five scans. Investigators from the United Kingdom's Medical Research Council randomly assigned 414 patients who elected to have only surveillance after surgery to follow-up routines containing either 2 CT scans (247 patients at 3 and 12 months after surgery) or 5 CT scans (167 patients at 3, 6, 9, 12, and 24 months after surgery). All other surveillance tests were performed with equal frequency between groups during monthly follow-up visits during the first year, every other month during the second year, and every 3 to 6 months thereafter.
At a median follow-up of 40 months, the investigators had detected 37 relapses (15 percent) in the 2-CT group and 33 (20 percent) in the 5-CT group. Recurrent tumors were approximately the same size at detection in both groups. "There is no clear advantage to more frequent CT scans in follow-up" of these patients, stated Dr. G.M. Mead, of the Mount Vernon Cancer Centre in Middlesex, England. "The two-CT-scan schedule can be considered a new standard."
BRCA Mutations Correlate with Risk Reduction after Surgery
Previous studies have suggested that risk-reducing salpingo-oophorectomy (removal of the ovaries and fallopian tubes) can prevent breast cancer in 53 to 68 percent of women who carry BRCA mutations, and prevent ovarian, fallopian tube, and peritoneal cancers in 71 to 96 percent of this group. However, these previous studies had a limited follow-up period, and did not look at whether women with BRCA1 and BRCA2 mutations derive the same benefit from risk-reducing surgery.
Data from a large prospective cohort study presented at the ASCO annual meeting suggest that women with BRCA2 mutations receive more protection from breast cancer from risk-reducing surgery than do women with BRCA1 mutations and that future studies should stratify patients by mutation type to prevent bias.
In the study, presented by Dr. Noah Kauff of Memorial Sloan-Kettering Cancer Center, 546 women who elected to undergo risk-reducing salpingo-oophorectomy (352 BRCA1 and 194 BRCA2) and 325 women who refused surgery (198 BRCA1 and 127 BRCA2) were followed for a mean of 40 months. Women with either BRCA1 or BRCA2 mutations who underwent surgery were less likely to develop gynecologic cancer and also had a lower likelihood of developing breast cancer, although the difference in breast cancer occurrence was only significant in women with BRCA2 mutations.
"There do... appear to be differences in the magnitude of protection against breast cancer conferred by risk-reducing salpingo-oophorectomy, perhaps caused by differences in the breast cancer phenotype between BRCA1 and BRCA2," stated Dr. Kauff. |
 |
 |

FDA Approves HPV Vaccine
The Food and Drug Administration (FDA) last week approved a vaccine that protects against the two types of human papillomavirus (HPV) that are responsible for 70 percent of all cases of cervical cancer. The approval covers the vaccine's use in females from ages 9 to 26.
The FDA action comes on the heels of a unanimous vote by the agency's Vaccines and Related Biological Products Advisory Committee that the vaccine, called Gardasil, is both safe and effective in the populations in which it was tested.
Manufactured by Merck & Co., Gardasil is a "quadrivalent" vaccine, meaning that it protects against four HPV types: 6, 11, 16, and 18. While the latter two types are responsible for the large majority of cervical cancer cases, types 6 and 11 are responsible for 90 percent of genital warts cases.
The approval for Gardasil, which is administered as a series of three injections over a 6-month period, includes its use for the prevention of cervical cancer and certain cervical, vulvular, and vaginal precancers called intraepithelial neoplasias.
In large international clinical trials testing Gardasil, it prevented nearly 100 percent of the precancerous cervical cell changes caused by the HPV types targeted by the vaccine for up to 4 years after vaccination.
These efficacy studies included females aged 15 to 26. Because of logistical and ethical concerns, Merck conducted trials in girls aged 9 to 15 only to measure their immune response to the vaccine. Participants in those studies had an even stronger immune response than the older participants in the efficacy trials, which the advisory committee and FDA agreed supported "bridging" or extending the effectiveness data to include this younger group.
Merck is conducting several post-market studies, including those to further evaluate general safety and long-term effectiveness, and to monitor the pregnancy outcomes of women who received Gardasil but didn't know they were pregnant at the time. Merck is also conducting trials to evaluate the safety and effectiveness of Gardasil in males.
|
 |
 |

New Strategy for Treatment-Resistant Solid Tumors in Children
Name of the Trial
Phase I Study of Talabostat in Combination with Temozolomide or Carboplatin in Pediatric Patients with Relapsed or Refractory Solid Tumors, Including Brain Tumors (NCI-05-C-0239). See the protocol summary at http://cancer.gov/clinicaltrials/NCI-05-C-0239.
 Principal Investigator
Principal Investigator
Dr. Frank M. Balis, NCI CCR
Why This Trial Is Important
Children and adolescents with solid tumors that are resistant to standard chemotherapy or have recurred after treatment have a relatively poor prognosis because of the lack of alternative therapies.
However, new-generation targeted agents now in clinical trials can increase the effectiveness of conventional chemotherapy drugs. One such agent is talabostat, a drug first developed to enhance the recovery of normal blood cells after chemotherapy. In cancer models in animals, talabostat also induces the immune system to mount an antitumor response capable of shrinking tumors or slowing their growth, and augments tumor response to standard chemotherapy drugs.
How talabostat triggers an antitumor immune response is not completely understood, but it is known that the drug blocks an enzyme, fibroblast activation protein (FAP), produced by tumor-associated fibroblasts (cells present in the connective tissue, or stroma, surrounding solid tumors). Inhibition of FAP increases production of a variety of cytokines that boost the activity of immune-system cells.
This trial assesses the safety of talabostat in combination with chemotherapy in pediatric patients with cancer. Depending on tumor type and prior treatment, trial participants will receive temozolomide or carboplatin, both standard chemotherapy agents, followed by talabostat. Talabostat will be given by mouth once daily in gradually increasing doses in small subgroups of patients until the optimal dose is determined. Blockage of a blood-borne enzyme closely related to FAP will also be measured during treatment and will serve as an indicator of talabostat's effectiveness.
Who Can Join This Trial
Researchers will enroll 24 patients between the ages of 2 and 19 with solid tumors that have not responded to, or have recurred after, standard chemotherapy. See the list of eligibility criteria at http://www.cancer.gov/clinicaltrials/NCI-05-C-0239.
Study Site and Contact Information
The trial is taking place at the NIH Clinical Center in Bethesda, Md. For more information, contact the NCI Clinical Studies Support Center at 1-888-NCI-1937. The toll-free call is confidential.
An archive of "Featured Clinical Trial" columns is available at http://cancer.gov/clinicaltrials/ft-all-featured-trials.
|
 |
 |
NCI Program Helps Cancer Centers Meet Educational Needs
Demand for NCI educational materials has continually exceeded the supply. To help meet demand, NCI allows organizations to add extra quantities to the government's publication print orders through its Ride-On program. Organizations can take advantage of the government's lower printing costs and upfront charges, providing an economical way to obtain a supply of NCI publications.
More information is available online at http://ncipoet.org/promoToolsRideOn.cfm.
Childhood Cancer Media Seminar Scheduled
On June 15, from 10:00 a.m. to 1:00 p.m., NCI and the Children's Inn at NIH will host a media seminar on childhood cancer focusing on the stresses children and their families endure and the hope they can receive at a place like the Children's Inn, where recovery and treatment are linked and supported.
In anticipation of the PBS documentary, A Lion in the House, airing on June 21 and 22, the media seminar will offer journalists access to the movie producers and medical experts. To register for the seminar, contact Meredith Daly at the Children's Inn at 301-451-3075 or dalym@mail.nih.gov.
NIH Bench-to-Bedside Awards Announced
NIH has awarded nearly $4 million to fund 19 bench-to-bedside medical research projects designed to speed translation of promising laboratory discoveries into new medical treatments.
For the first time, applications for these awards were open to research teams made up of NIH intramural and extramural collaborators from medical schools, health care organizations, and private industry. All but 1 of the funded projects include extramural partners; 9 of the 19 projects involve researchers from 2 or more NIH institutes or centers.
Awards were made in four categories. A fifth category is cofounded by sponsoring institutes and, for the projects' extramural components, the NIH National Center for Research Resources. Project teams receive up to $200,000 over 2 years to support their work.
For details, go to http://www.cc.nih.gov/ccc/btb/awards.shtml.
Save the Date for Cancer Survivorship Research Conference
The third biennial cancer survivorship research conference, "Cancer Survivorship: Embracing the Future," will be held October 4-6 in Bethesda, Md.
Conference attendees will include researchers in biomedical and behavioral science, health care professionals, scientists, graduate students in health-related sciences, community-based advocates, state public health planners, and cancer survivors and their families.
Researchers from many fields will share information on innovative research advances, interventions, and methods, while all participants will have the opportunity to learn about the future directions of cancer survivorship research, advances in e-health and communications, challenges to follow-up care for cancer survivors, understanding and fulfilling the needs of cancer caregivers and families, and reviewing research and strategies to address health disparities in cancer survivorship.
More details on the conference - sponsored by the NCI's Office of Cancer Survivorship, the American Cancer Society, and their new partner, the Lance Armstrong Foundation - can be found at http://www.blsmeetings.net/Survivorship06/gen.htm.
|
 |
 |

San Juan Minority-Based Community Clinical Oncology Program (MBCCOP)
Principal Investigator: Dr. Luis Baez; Co-Principal Investigator: Dr. William Cáceres • Administrator: Doris Cuadrado • PMB #54 P.O. Box 70344, San Juan, PR 00936-8344 • Phone: 787-763-1296 or 787-758-7348 • Email: sjccop@prtc.net
 Background
Background
The San Juan MBCCOP received its initial funding from NCI in July 1990. The program comprises four main hospitals: San Juan VA Hospital (VA Caribbean Health Care System), San Juan Hospital, San Juan Oncology Hospital (also known as I. Gonzalez Martinez Hospital), and Ponce Oncology Hospital (also known as Andres Grillasca Hospital). It also includes four satellite private clinics across the island: Doctors Cancer Center in Manatí, Hato Rey Hematology Oncology Group in Hato Rey, Hematology Oncology Group in Bayamón, and the Primary Care Physicians Group.
Staff affiliated with the program include 23 oncologists, 7 surgeons, 2 pathologists, 5 radiation oncologists, 3 primary care physicians, 8 hematology-oncology fellows, 2 research fellows, 3 research nurses, and 9 study coordinators who have completed training on the protection of research subjects and good clinical practices.
At this time, the San Juan MBCCOP is a member of the Eastern Cooperative Oncology Group, National Surgical Adjuvant Breast and Bowel Project (NSABP), University of Texas M.D. Anderson Cancer Center, and Southwest Oncology Group (SWOG).
Community Characteristics
Since 1990, the San Juan MBCCOP has served approximately 2,000 patients in the context of treatment trials and cancer control trials. The local patient base is 99 percent minority based, 40 percent of whom have a health care plan through government benefits. According to the program staff, these patients are very reliable and highly committed to research protocols.
The San Juan MBCCOP has facilitated the participation of primary care physicians in the SWOG SELECT Trial, subsequently enrolling 1,456 patients in the study. It also participates in the Breast Cancer Prevention Trial, the STAR Trial, and NSABP adjuvant treatment trials, as well as pain management protocols that have highlighted the challenges associated with pain management among Hispanics who receive treatment for cancer. During this year, the program has exceeded the expected enrollment of patients in treatment trials by 40 percent.
Outreach Activities
To educate people about the opportunity of cancer clinical trials, the San Juan MBCCOP participates in health fairs at shopping malls, health care conferences, and physician education programs, as well as prevention clinics at hospitals and industrial manufacturing facilities. These activities have helped the program sites to recruit more patients to the trials in which they participate.
Other Key Facts
The San Juan MBCCOP works very closely with the San Juan Hospital and San Juan VA Hospital fellows, who evaluate inpatient and outpatient subjects for clinical trial eligibility and randomization. This activity is done under close supervision during five clinics per week held at the participating hospitals.
On March 15, 2006, Principal Investigator Dr. Luis Baez received the Association of Community Cancer Centers' Community Clinical Scientist Award, presented to him by former NCI Director Dr. Andrew C. von Eschenbach.
|
 |
|