 |
|||||||||
skip to content |
|||||||||
First Live-Attenuated Vaccine for S. Iniae in Development

A juvenile hybrid striped bass used in vaccine studies. Credit: Jeff Locke
In preliminary trials, NOAA Sea Grant researchers have for the first time demonstrated the feasibility of using a live-attenuated vaccine to prevent the deadly Streptococcus iniae infection in fish.
The success raises the possibility of being able to inoculate hybrid striped bass, tilapia, rainbow trout and other cultured species orally through feed, instead of having to inject individual fish – a prohibitively labor-intensive process for American farms.
Besides the economic benefits, live-attenuated viruses also stimulate a more robust immune response, meaning that the vaccine offers better protection from infection, said John Buchanan, a former researcher at the University of California at San Diego.
There are currently two vaccines on the market for preventing S. iniae infections – AquaVac Garvetil and Norvax Strep Si. Both are classical vaccines based on exposing fish to killed versions of bacterial pathogens. However, neither is approved for use in the United States, Buchanan said. In addition, AquaVac is for use in tilapia only, and Norvax is most effective when fish are immersed in a 60-second dip initially; subsequent booster doses can be delivered orally.
The vaccine that Buchanan and UC San Diego pediatrics professor Victor Nizet are testing, in collaboration with Kent SeaTech, is based on mutating genes of the bacterial pathogen – not on killing the pathogen outright. These mutants have weakened virulence, but they can still infect fish, eliciting a strong adaptive immune response, in which antibodies to the real pathogen are created.

Carlo Milani, a former graduate student in the Nizet lab, injects a juvenile hybrid striped bass with S. iniae at Kent SeaTech’s research facility in San Diego, Calif. Credit: Jeff Locke

Sea Grant Trainee Jeff Locke injects a juvenile hybrid striped bass with S. iniae. Credit: Carlo Milani.
In the trials so far, their vaccine has been administered through injection, which means that each fish has to be given a shot. However, as Jim Carlberg, president of Kent SeaTech, emphasized: “The beauty of live-attenuated vaccines is that you have the potential to put the vaccine in feed.” The key is to be able to mutate a gene that does not wipe out the weakened pathogen’s ability to orally infect the animal.
“A vaccine that can be put in feed would have a huge potential advantage in cost,” Carlberg said.
“Oral delivery is the gold standard for aquaculture,” agreed Sea Grant Trainee Jeff Locke, a doctoral student with Nizet, who used to work at Kent SeaTech, a large hybrid striped bass farm in Southern California.
“S. iniae is a ubiquitous disease and a fairly chronic problem,” Carlberg said. “It has a huge economic impact on worldwide aquaculture.”
About 26 species of fish are susceptible to S. iniae, which causes meningitis in fish. Infected fish are anemic-looking, Buchanan, who is now the chief of finfish research at Aqua Bounty Technologies in San Diego, said. They can swim abnormally and have “popeye,” caused by swelling in the brain.
For U.S. farms, there is no satisfactory treatment for S. iniae. Infected fish can be fed antibiotics but sick fish often don’t eat, Carlberg said. To treat outbreaks, which tend to occur at facilities where warm-water fish are kept at high density in recirculating tanks, Kent SeaTech has developed its own killed vaccine for S. iniae. However, it too requires that fish be individually injected.
Carlberg estimates that an orally delivered, live-attenuated S. iniae vaccine would generate revenues of about $10 million to $15 million annually worldwide. The savings to the global aquaculture industry could be worth 10 times this amount, he said.
Nizet, Buchanan and Kent SeaTech have patented the technology that makes their live-attenuated vaccine possible and entered into discussions with pharmaceutical companies about possible licensing agreements.
Meanwhile, researchers with funding from California Sea Grant continue to study genes involved with pathogenesis. Locke, for example, has identified two new S. iniae genes whose mutated forms represent potential new vaccines.
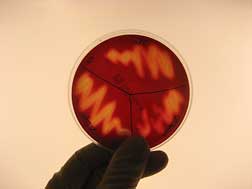
The bacterial pathogen Streptococcus iniae grows on a blood agar plate. S. iniae is closely related to the Strep A and B bacteria that commonly infect children. Credit: Jeff Locke
Scientists are hoping to present to a pharmaceutical partner a complete portfolio of potential mutants for development of a commercial product. “There may be one gene that contributes more to virulence than all the others,” Locke explained.
The USDA has already approved two other live-attenuated vaccines for aquaculture for other diseases, so there is optimism that a live-attenuated vaccine for S. iniae would clear regulatory hurdles.
Buchanan said that economics is the reason pharmaceutical companies have not pursued USDA approval for the two killed vaccines now used in Asia.
“The cost of getting the vaccine approved in the United States is more significant than the size of the market,” he said. “Farms are allowed to make their own vaccines with bacterial isolates from their own facilities. That is what Kent SeaTech does.”
An effective oral vaccine that could treat a variety of fish species would have greater value to American farms and therefore industry would pursue USDA approval, Buchanan said. Because the USDA is considered relatively strict, its nod would grease the regulatory process in nations that otherwise might be afraid of allowing a vaccine based on an infectious agent. “A killed vaccine is the norm,” he said.
Kent SeaTech is optimistic about the research outcomes. “I think there is an 80 percent chance that a real commercial product will come out of this research,” Carlberg said.
For more information, contact:
Victor Nizet
Professor of Pediatrics & Pharmacy
Chief, Division of Pediatric Pharmacology & Drug Discovery
UC San Diego School of Medicine
E. vnizet@ucsd.edu
Tel. (858) 534-7408
John Buchanan
Director of Finfish Research
Aqua Bounty Technologies
San Diego, Calif.
Tel. (858) 450-2972
Cell. (858) 334-5501
Jim Carlberg
President
Kent SeaTech Corp.
San Diego, Calif.
T. (858) 452-5765
Relevant Links:
Nizet laboratory, http://medicine.ucsd.edu/NizetLab
Kent SeaTech Corp., http://www.kentseatech.com/
|
California Sea Grant, the largest of 31 university-based programs, supports science-based management, conservation and enhancement of California's coastal and aquatic resources through research, extension and education. |
2/11/08
CLIMATE · OCEANS, GREAT LAKES, and COASTS · WEATHER
and AIR QUALITY
ABOUT US · RESEARCH
PROGRAMS · EDUCATION · HOME